Influence of SnO2 Content on the Humidity Dependent Impedance of the MgFe2O4-Fe2O3-SnO2 Compound
Abstract
1. Introduction
2. Materials and Methods
3. Results
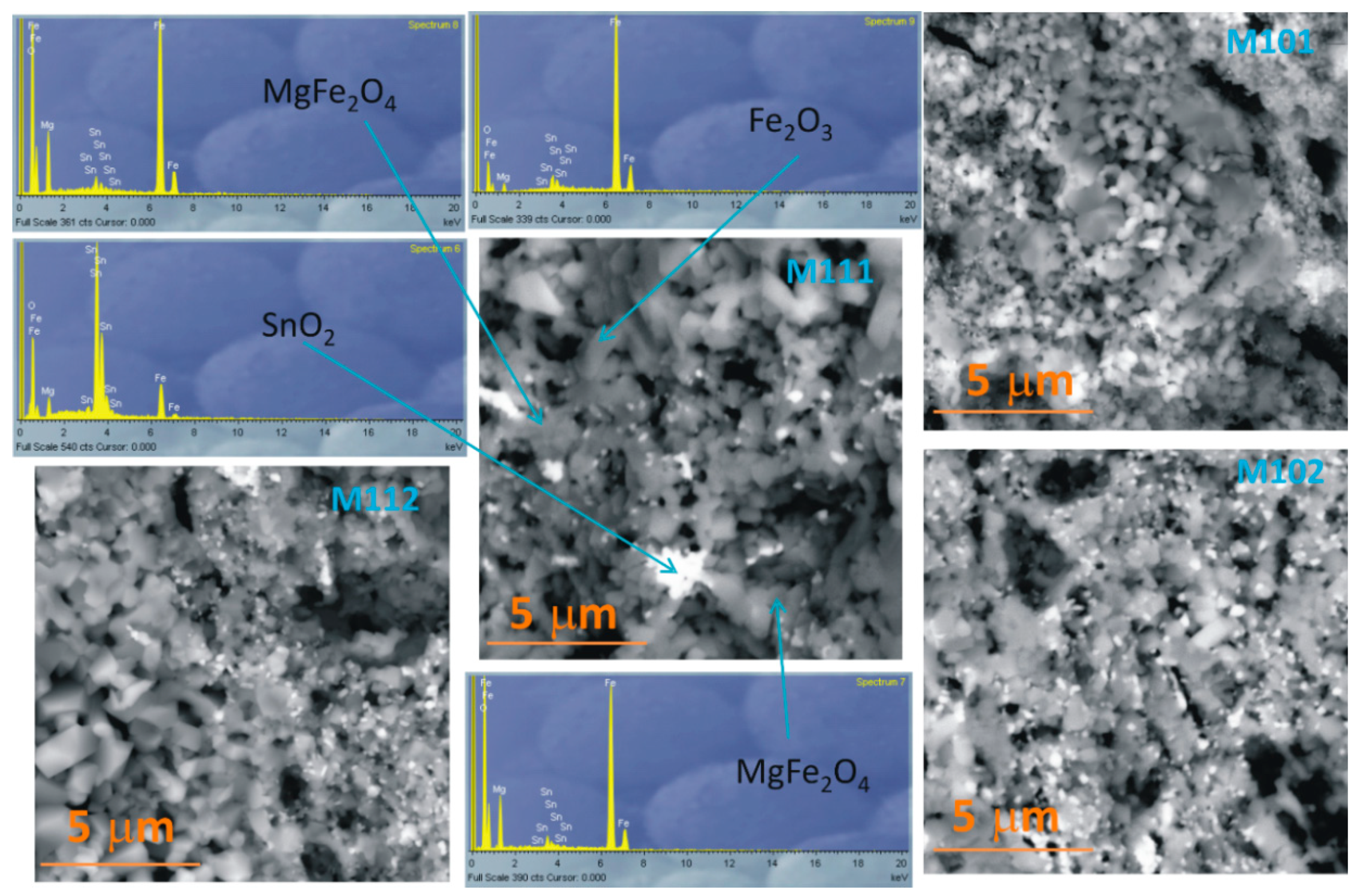
3.1. Structural and Morphological Analysis
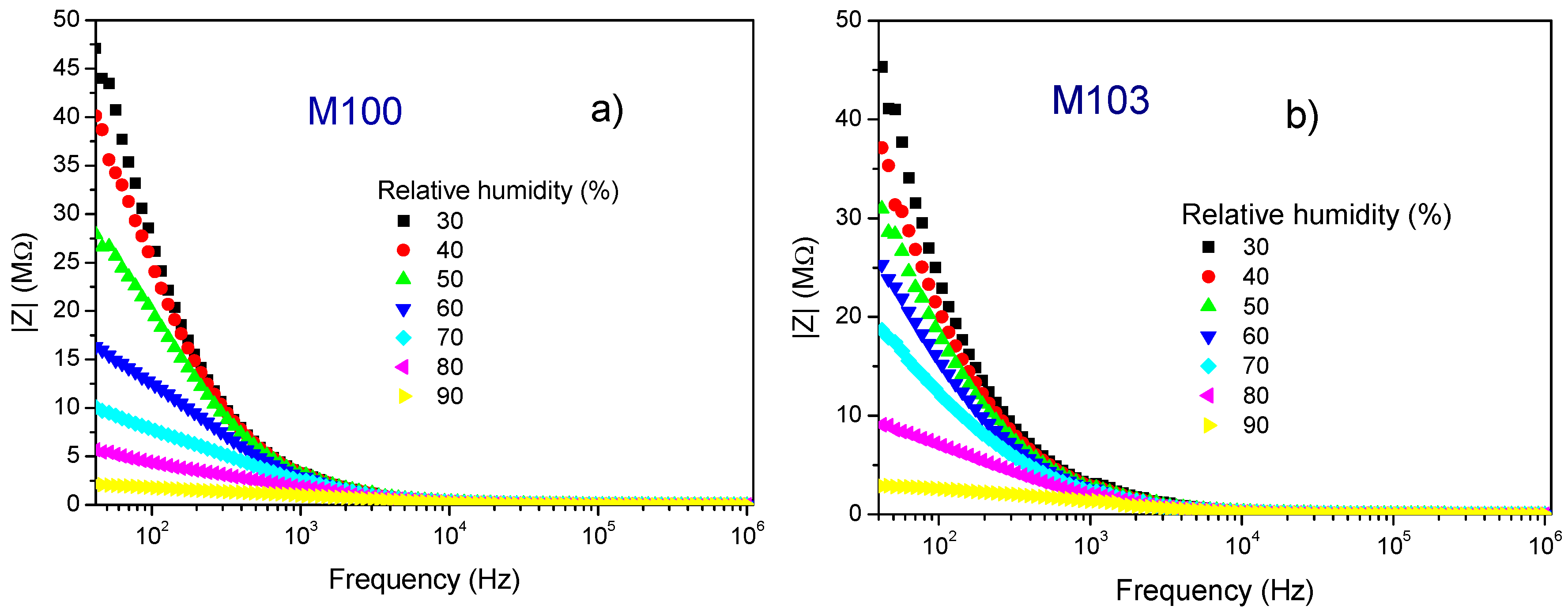
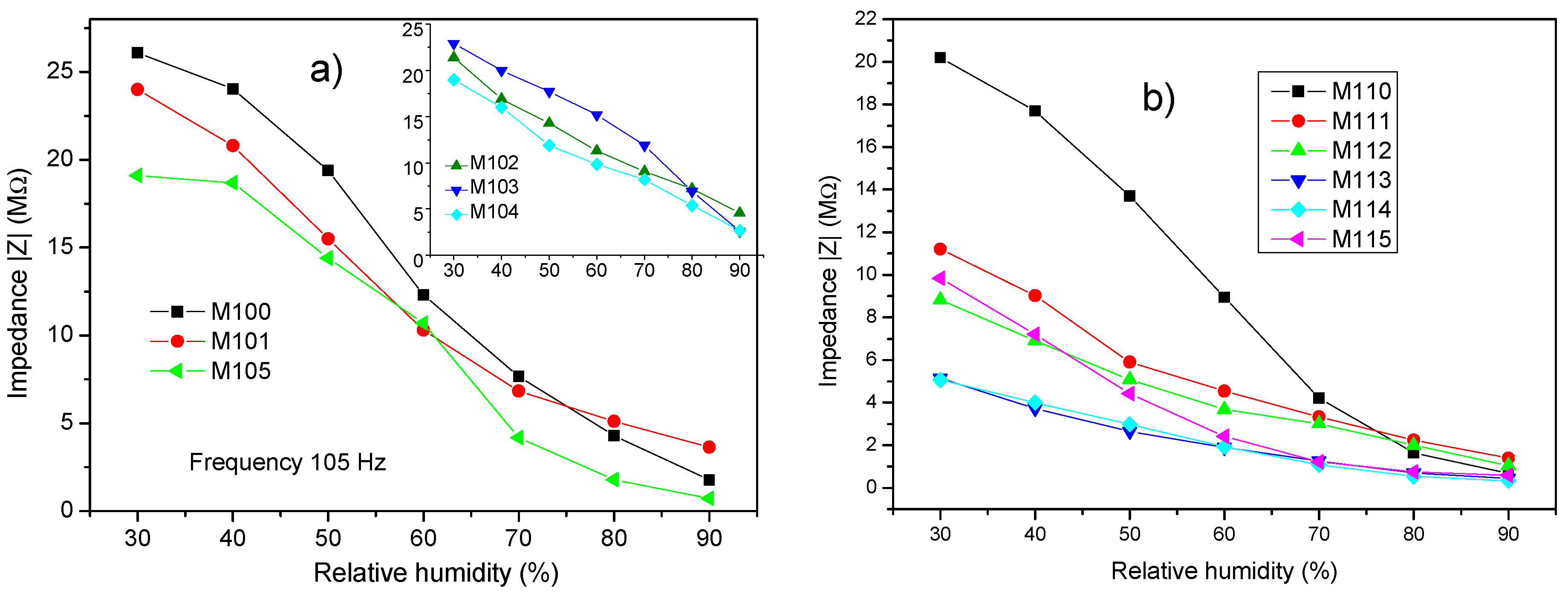
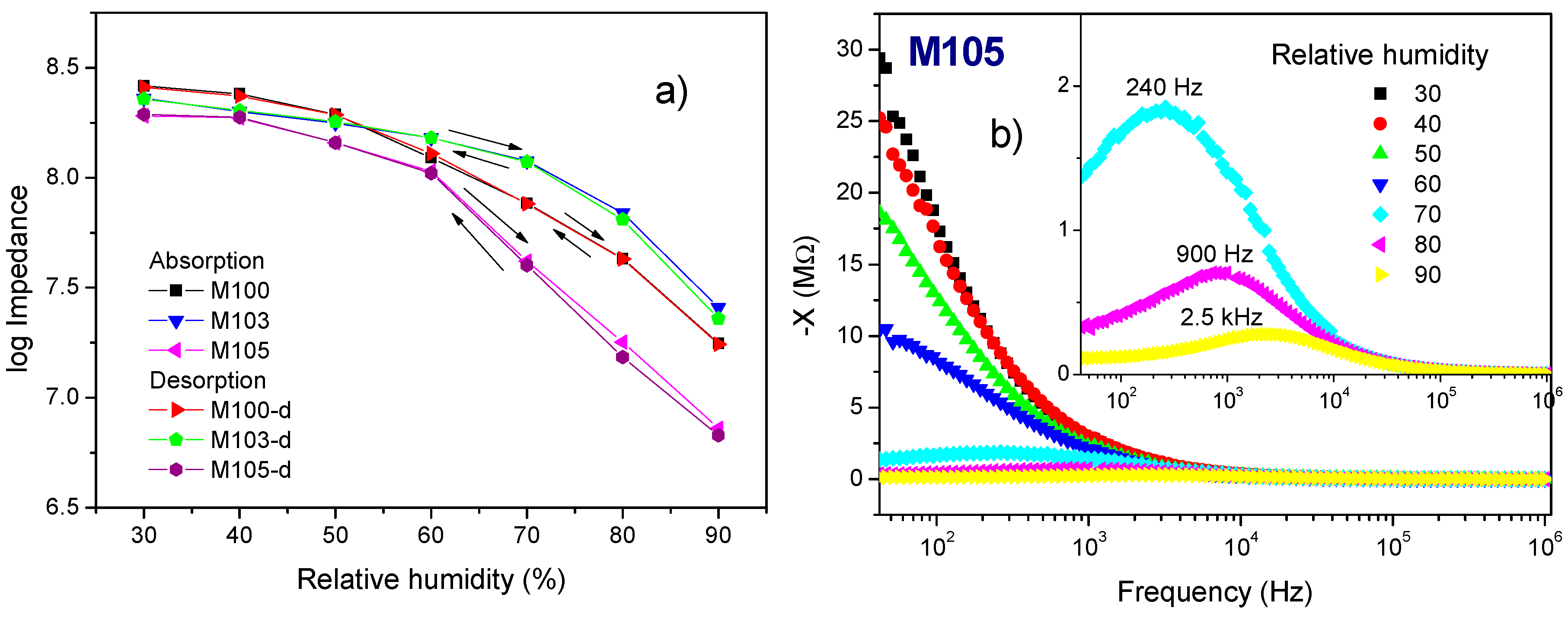
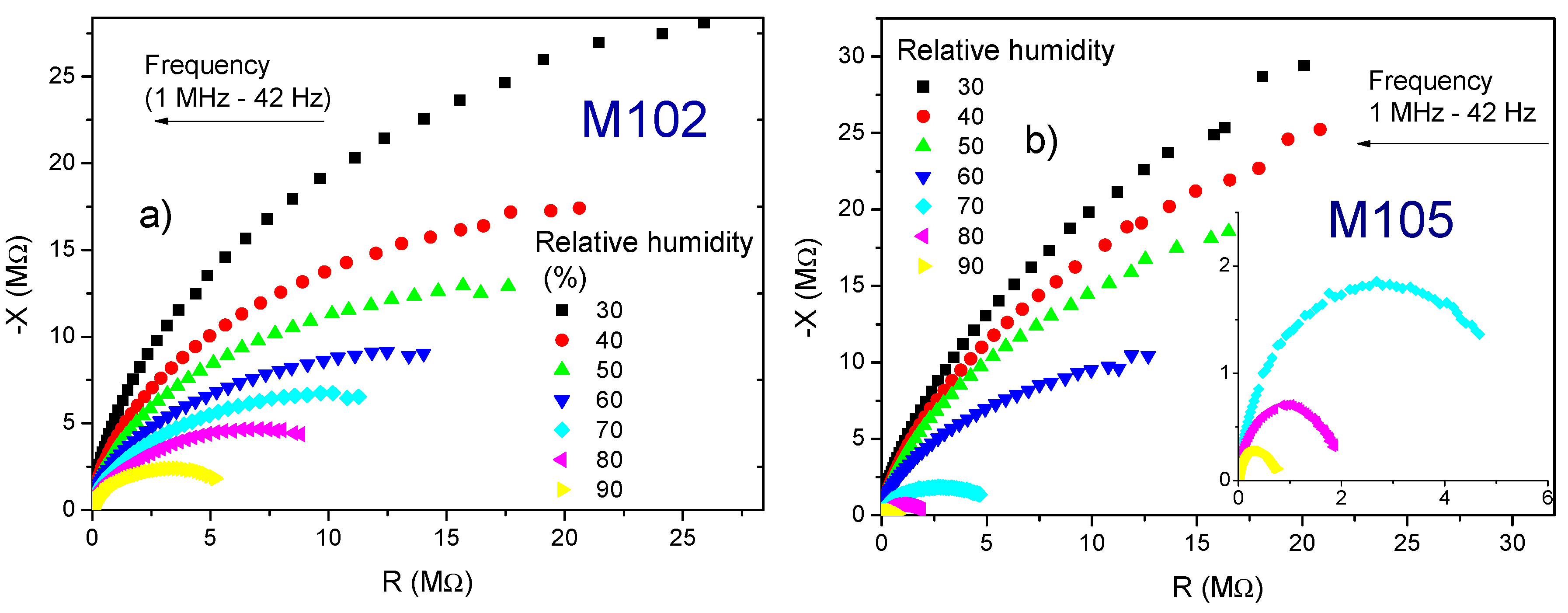
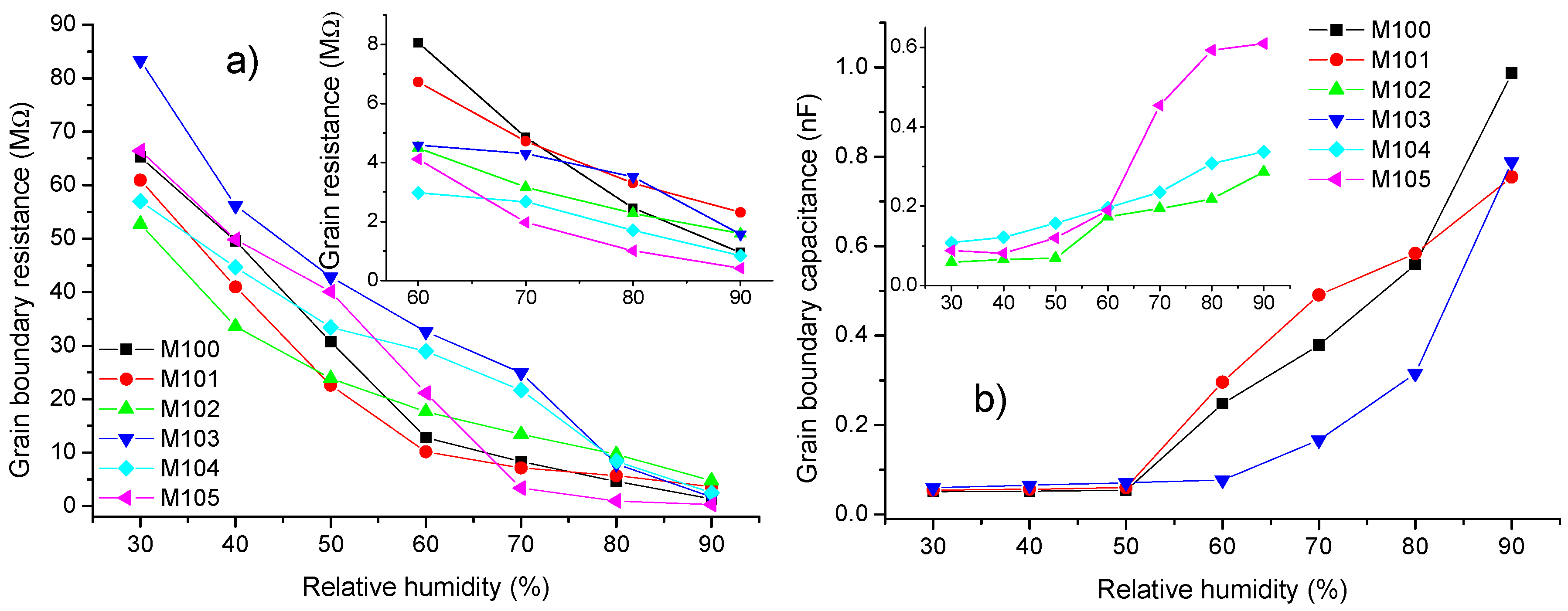
3.2. Impedance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shah, J.; Kotnala, R. Humidity sensing exclusively by physisorption of water vapors on magnesium ferrite. Sens. Actuators B Chem. 2012, 171, 832–837. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hashemi, B.; Janghorban, K. α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci. Mater. Electron. 2015, 27, 3109–3144. [Google Scholar] [CrossRef]
- Ponce, M.A.; Bueno, P.R.; Varela, J.; Castro, M.; Aldao, C.M. Impedance spectroscopy analysis of SnO2 thick-films gas sensors. J. Mater. Sci. Mater. Electron. 2007, 19, 1169–1175. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Hariharan, V.; Sekar, C. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 2011, 31, 840–844. [Google Scholar] [CrossRef]
- Malagù, C.; Carotta, M.; Giberti, A.; Guidi, V.; Martinelli, G.; Ponce, M.A.; Castro, M.; Aldao, C.M. Two mechanisms of conduction in polycrystalline SnO2. Sens. Actuators B Chem. 2009, 136, 230–234. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Druc, A.; Borhan, A.; Diaconu, A.; Iordan, A.; Nedelcu, G.; Leontie, L.; Palamaru, M. How cobalt ions substitution changes the structure and dielectric properties of magnesium ferrite? Ceram. Int. 2014, 40, 13573–13578. [Google Scholar] [CrossRef]
- Rezlescu, N.; Doroftei, C.; Rezlescu, E.; Popa, P. Structure and humidity sensitive electrical properties of the Sn4+ and/or Mo6+ substituted Mg ferrite. Sens. Actuators B Chem. 2006, 115, 589–595. [Google Scholar] [CrossRef]
- Petrila, I.; Tudorache, F. Humidity sensor applicative material based on copper-zinc-tungsten spinel ferrite. Mater. Lett. 2013, 108, 129–133. [Google Scholar] [CrossRef]
- Kotnala, R.; Shah, J.; Singh, B.; Kishan, H.; Singh, S.; Dhawan, S.; Sengupta, A. Humidity response of Li-substituted magnesium ferrite. Sens. Actuators B Chem. 2008, 129, 909–914. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Reginamary, L.; Jeyaraj, B.; Dayalan, A.; Nagaraja, K.S. Nanocrystalline spinel NixCu0.8-xZn0.2Fe2O4: A novel material for humidity sensing. J. Mater. Sci. 2012, 47, 3529–3534. [Google Scholar] [CrossRef]
- Shah, J.; Kotnala, R.; Singh, B.; Kishan, H. Microstructure-dependent humidity sensitivity of porous MgFe2O4–CeO2 ceramic. Sens. Actuators B Chem. 2007, 128, 306–311. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Lukovic, M.D.; Vasiljevic, Z.Z.; Labus, N.; Aleksić, O.S. Humidity sensing potential of Fe2TiO5—pseudobrookite. J. Mater. Sci. Mater. Electron. 2018, 29, 9227–9238. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthus mechamism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Shah, J.; Arora, M.; Purohit, L.; Kotnala, R.K. Significant increase in humidity sensing characteristics of praseodymium doped magnesium ferrite. Sens. Actuators A Phys. 2011, 167, 332–337. [Google Scholar] [CrossRef]
- Rao, P.; Chikate, R.C.; Bhagwat, S. Highly responsive and stable Y 3+ doped NiMg–ferrite thick films as an efficient humidity sensor. New J. Chem. 2016, 40, 1720–1728. [Google Scholar] [CrossRef]
- Tudorache, F. Investigations on microstructure, electrical and magnetic properties of copper spinel ferrite with WO 3 addition for applications in the humidity sensors. Superlattices Microstruct. 2018, 116, 131–140. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Arachchige, H.M.; Sisman, O.; Comini, E. Metal oxide -based heterostructures for gas sensors-A review. Anal. Chim. Acta 2018, 1–23. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G. EIS Spectrum Analyzer. Available online: http://www.abc.chemistry.bsu.by (accessed on 25 November 2016).
- Nikolic, M.V.; Lukovic, M.D.; Labus, N.J. Influence of humidity on complex impedance and dielectric properties of iron manganite (FeMnO3). J. Mater. Sci. Mater. Electron. 2019, 30, 12399–12405. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Sekulic, D.L.; Vasiljevic, Z.Z.; Lukovic, M.D.; Pavlovic, V.; Aleksic, O.S. Dielectric properties, complex impedance and electrical conductivity of Fe2TiO5 nanopowder compacts and bulk samples at elevated temperatures. J. Mater. Sci. Mater. Electron. 2016, 28, 4796–4806. [Google Scholar] [CrossRef]
- Mukherjee, K.; Bharti, D.; Majumder, S.B. Solution synthesis and kinetic analyses of the gas sensing characteristics of magnesium ferrite particles. Sens. Actuators B Chem. 2010, 146, 91–97. [Google Scholar] [CrossRef]
- Sabarilakshmi, M.; Janaki, K. Effect of Mg concentration on structural, optical and humidity sensing performance of SnO2 nanoparticles prepared by one step facile route. J. Mater. Sci. Mater. Electron. 2017, 28, 8101–8107. [Google Scholar] [CrossRef]
- Orlandi, M.; Mazzi, A.; Arban, G.; Bazzanella, N.; Rudatis, P.; Caramori, S.; Patel, N.; Fernandes, R.; Bignozzi, C.A.; Miotello, A. On the effect of Sn-doping in hematite anodes for oxygen evolution. Electrochim. Acta 2016, 214, 345–353. [Google Scholar] [CrossRef]
- Dutta, S.; Choudhary, R.N.P.; Sinha, P.K.; Thakur, A.K. Microstructural studies of (PbLa)(ZrTi)O3 ceramics using complex impedance spectroscopy. J. Appl. Phys. 2004, 96, 1607. [Google Scholar] [CrossRef]
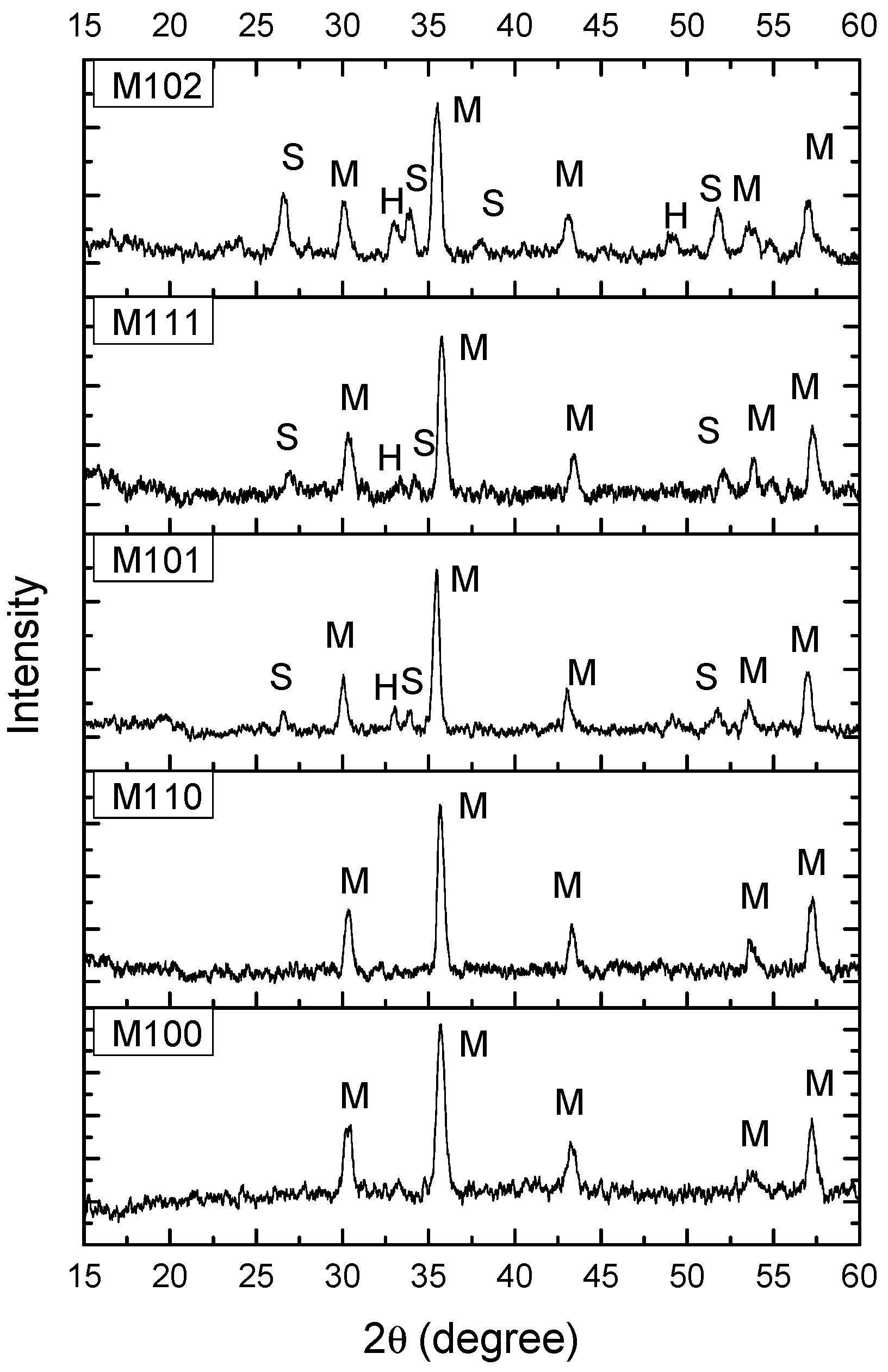
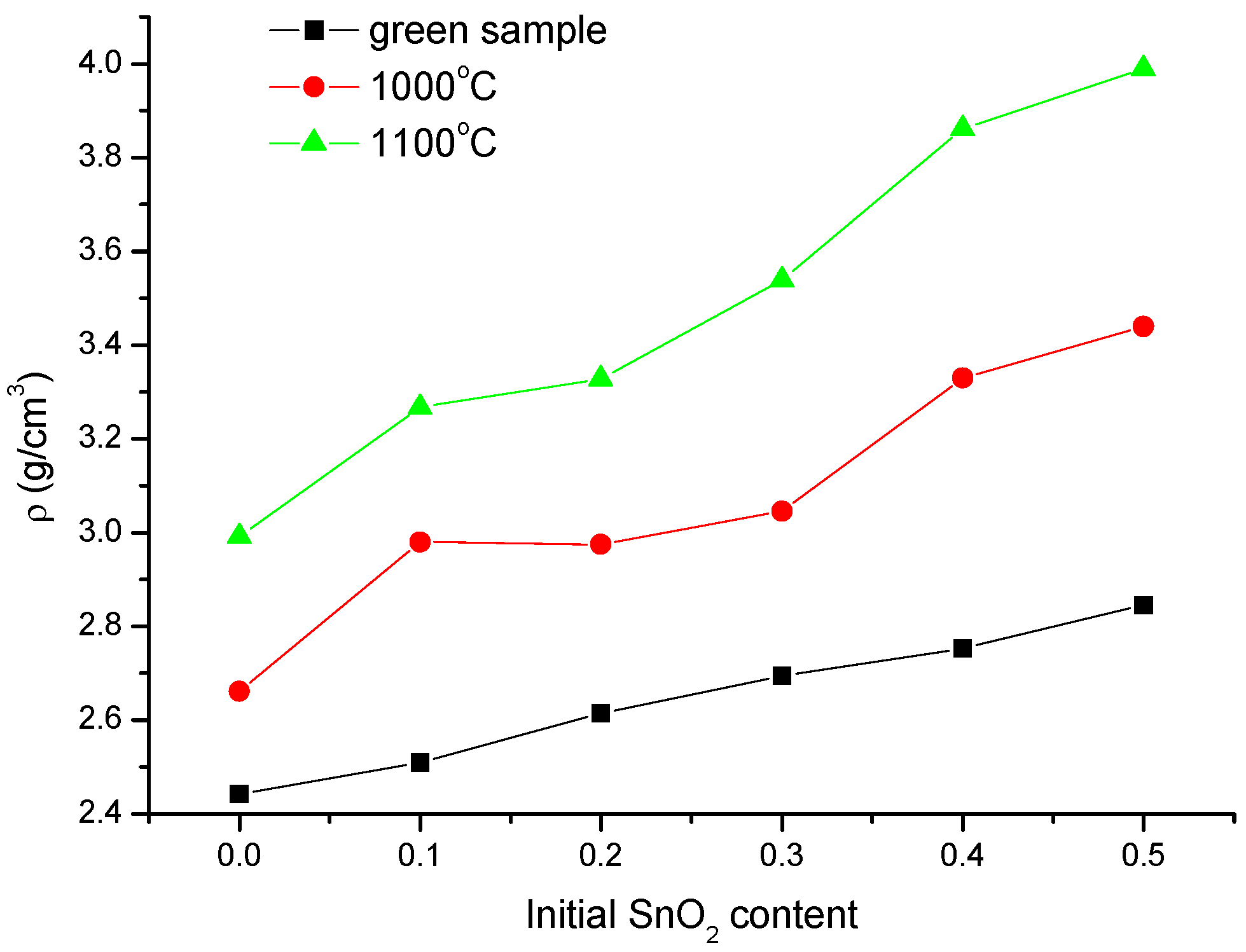
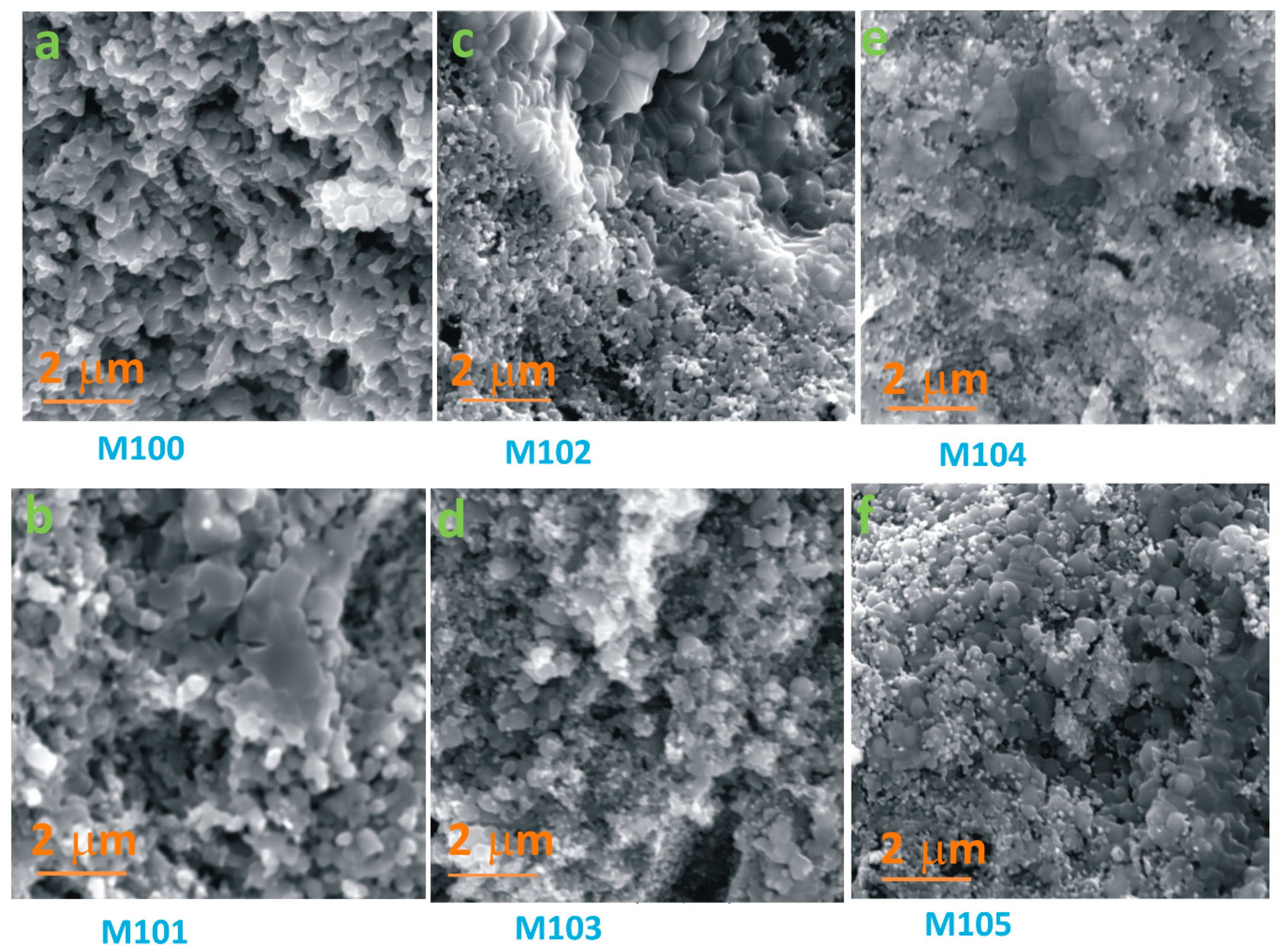
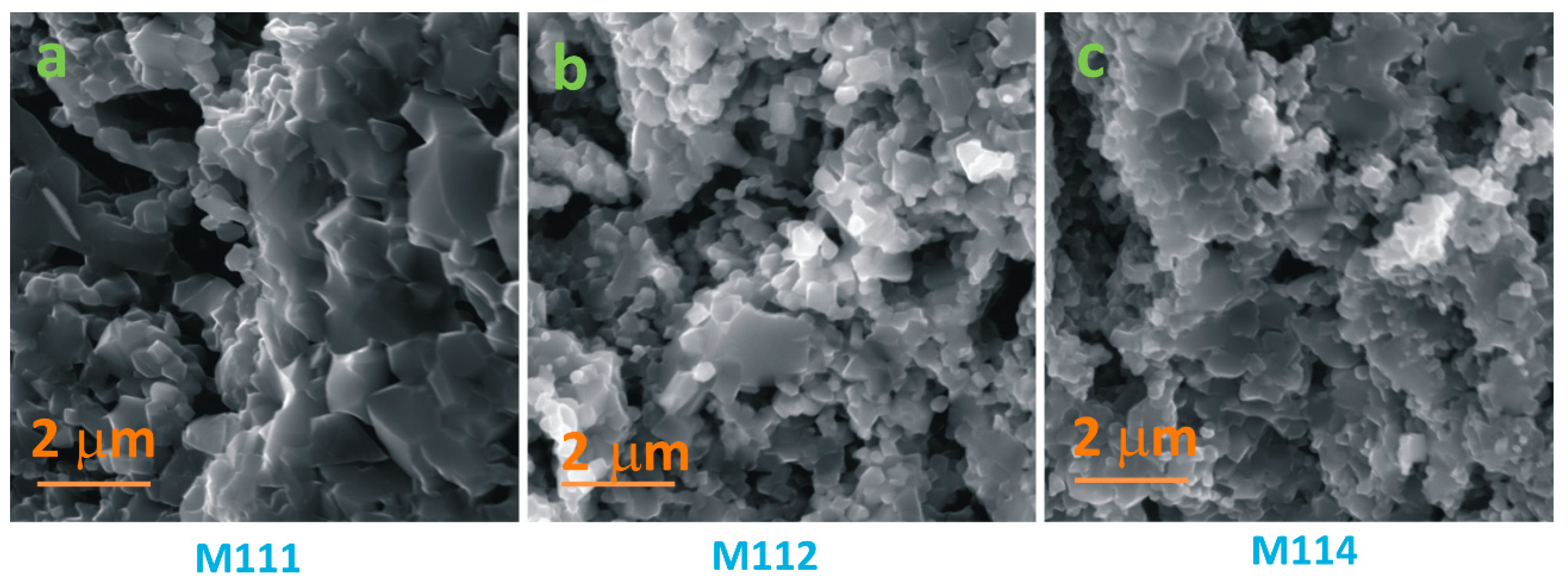
| Sample | RH 30–90% (MΩ/%RH) | RH 40–90% (MΩ/%RH) |
|---|---|---|
| M100 | 0.384 ± 0.0989 | 0.419 ± 0.0524 |
| M101 | 0.391 ± 0.0541 | 0.406 ± 0.0467 |
| M102 | 0.335 ± 0.0630 | 0.313 ± 0.0325 |
| M103 | 0.290 ± 0.0331 | 0.290 ± 0.0370 |
| M104 | 0.296 ± 0.0328 | 0.295 ± 0.0366 |
| M105 | 0.263 ± 0.1198 | 0.308 ± 0.0544 |
| M110 | 0.341 ± 0.0535 | 0.359 ± 0.0330 |
| M111 | 0.207 ± 0.0359 | 0.205 ± 0.0398 |
| M112 | 0.160 ± 0.0268 | 0.154 ± 0.0244 |
| M113 | 0.106 ± 0.0233 | 0.099 ± 0.0178 |
| M114 | 0.097 ± 0.0105 | 0.095 ± 0.0108 |
| M115 | 0.222 ± 0.0467 | 0.214 ± 0.0472 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolic, M.V.; Lukovic, M.D. Influence of SnO2 Content on the Humidity Dependent Impedance of the MgFe2O4-Fe2O3-SnO2 Compound. Chemosensors 2020, 8, 39. https://doi.org/10.3390/chemosensors8020039
Nikolic MV, Lukovic MD. Influence of SnO2 Content on the Humidity Dependent Impedance of the MgFe2O4-Fe2O3-SnO2 Compound. Chemosensors. 2020; 8(2):39. https://doi.org/10.3390/chemosensors8020039
Chicago/Turabian StyleNikolic, Maria Vesna, and Miloljub D. Lukovic. 2020. "Influence of SnO2 Content on the Humidity Dependent Impedance of the MgFe2O4-Fe2O3-SnO2 Compound" Chemosensors 8, no. 2: 39. https://doi.org/10.3390/chemosensors8020039
APA StyleNikolic, M. V., & Lukovic, M. D. (2020). Influence of SnO2 Content on the Humidity Dependent Impedance of the MgFe2O4-Fe2O3-SnO2 Compound. Chemosensors, 8(2), 39. https://doi.org/10.3390/chemosensors8020039





