Sensing and Interaction of His-Tagged CA19-9 Antigen with Graphene-Modified Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochemical Measurements
2.2. Screen-Printed Electrodes Modified with Graphene Oxide (GO) or Thermally Reduced Graphene Oxide (TRGO)
2.3. Isothermal Titration Calorimetry (ITC)
3. Results and Discussion
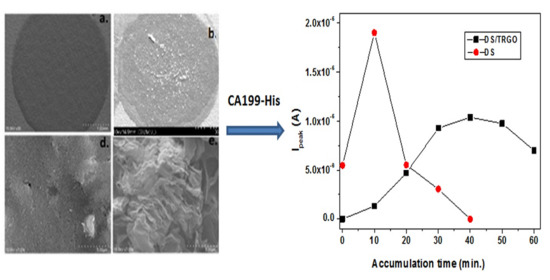
3.1. Morphological Characterization of the Electrodes
3.2. Thermodynamic Analysis by Isothermal Titration Calorimetry (ITC)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klug, T.L.; LeDonne, N.C.; Greber, T.F.; Zurawski, V.R., Jr. Purification and composition of a novel gastrointestinal tumor-associated glycoprotein expressing sialylated lacto-N-fucopentaose II (Ca19-9). Cancer Res. 1988, 48, 1505–1511. [Google Scholar] [PubMed]
- Yang, F.; Yang, Z.; Zhuo, Y.; Chai, Y.; Yuan, R. Ultrasensitive electrochemical immunosensor for carbohydrate antigen 19-9 using Au/porous graphene nanocomposite as platform and Au@Pd core/shell bimetallic functionalized graphene nanocomposites as signal enhancers. Biosens. Bioelectron. 2015, 66, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ju, H. Electrochemical and chemiluminescent immunosensors for tumor markers. Biosens. Bioelectron. 2005, 20, 1461–1470. [Google Scholar] [CrossRef]
- Ching, C.K.; Rhodes, J.M. Enzyme-linked PNA lectin binding assay compared with CA19-9 and CEA radioimmunoassay as a diagnostic blood test for pancreatic cancer. Br. J. Cancer 1989, 59, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (Ca19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, Y.; Baryeh, K.; Takalkar, S.; Lund, M.; Zheng, X.; Liu, G. Lateral flow assay for carbohydrate antigen 19-9 in whole blood by using magnetized carbon nanotubes. Microchim. Acta 2017, 184, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, M.; Hu, B.; Guo, C.; Song, Y.; Jia, Q.; He, L.; Zhang, Z.; Fang, S. Bimetallic cerium and ferric oxide nanoparticles embedded within mesoporous carbon matrix: Electrochemical immunosensor for sensitive detection of carbohydrate antigen 19-9. Biosens. Bioelectron. 2019, 135, 22–29. [Google Scholar] [CrossRef]
- Wang, L.; Shan, J.; Feng, F.; Ma, Z. Novel redox species polyaniline derivative-Au/Pt as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen 199. Anal. Chim. Acta 2016, 91, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Li, X.; Wu, Z.; Wang, M.; Hua, M.; Yang, Y. The preparation of label-free electrochemical immunosensor based on the Pt-Au alloy nanotube array for detection of human chorionic gonadotrophin. Clin. Chim. Acta 2011, 412, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Liu, Y.; Xue, Y.; Wang, A.J.; Wu, L.; Feng, J.J. L-Proline bio-inspired synthesis of AuPt nanocalliandras as sensing platform for label-free electrochemical immunoassay of carbohydrateantigen 19-9. Sens. Actuators B 2017, 250, 61–68. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.J.; Liu, W.D.; Jiang, L.Y.; Wang, A.J. A novel label-free electrochemical immunosensor based on the enhanced catalytic currents of oxygen reduction by AuAg hollow nanocrystals for detecting carbohydrate antigen 199. Biosens. Bioelectron. 2017, 96, 152–158. [Google Scholar] [CrossRef]
- Rong, Q.; Feng, F.; Man, Z. Metal ions doped chitosan–poly(acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 2016, 75, 148–154. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, D.; Chu, C.; Ma, Z. Label-assisted chemical adsorption triggered conversion of electroactivity of sensing interface to achieve the Ag/AgCl process for ultrasensitive detection of CA 19-9. Anal. Chim. Acta 2020, 1093, 43–51. [Google Scholar] [CrossRef]
- Ibanez-Redin, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Rebolho Batista Arantes, L.M.; Melendezd, M.E.; Carvalhod, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Malfoy, B.; Reynaud, J.A. Electrochemical investigations of amino acids at solid electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1980, 114, 213–223. [Google Scholar] [CrossRef]
- Chen, L.; Chang, C.; Chang, H. Electrochemical oxidation of histidine at an anodic oxidized boron-doped diamond electrode in neutral solution. Electrochim. Acta 2008, 53, 2883–2889. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Peptide methionine sulfoxide reductase A (Msra): Direct electrochemical oxidation on carbon electrodes. Bioelectrochemistry 2013, 89, 11–18. [Google Scholar] [CrossRef]
- Ley, C.; Holtmann, D.; Mangold, K.M.; Schrader, J. Immobilization of histidine-tagged proteins on electrodes. Colloids Surf. B 2011, 88, 539–551. [Google Scholar] [CrossRef]
- Thielges, M.C.; Chung, J.K.; Axup, J.Y.; Fayer, M.D. The influence of histidine tag attachment on picosecond protein dynamics. Biochemistry 2011, 50, 5799–5805. [Google Scholar] [CrossRef] [Green Version]
- Varghese, N.; Mogera, U.; Govindaraj, A.; Das, A.; Maiti, P.K.; Sood, A.K.; Rao, C.N.R. Binding of DNA nucleobases and nucleosides with graphene. ChemPhysChem 2009, 10, 206–210. [Google Scholar] [CrossRef]
- Ranganathan, S.V.; Halvorsen, K.; Myers, C.A.; Robertson, N.M.; Yigit, M.V.; Chen, A.A. Complex thermodynamic behavior of single-stranded nucleic acid adsorption to graphene surfaces. Langmuir 2016, 32, 6028–6034. [Google Scholar] [CrossRef] [PubMed]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Coros, M.; Pogacean, F.; Turza, A.; Dan, M.; Berghian-Grosan, C.; Pana, I.O.; Pruneanu, S. Green synthesis, characterization and potential application of reduced graphene oxide. Physica E 2020, 119, 113971. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Edsall, J.T.; Wyman, J. Biophysical Chemistry, Thermodynamics, Electrostatics, and the Biological Significance of the Properties of Matter, Chapter 8; Academic Press: Cambridge, MA, USA, 1958. [Google Scholar]
- Ogura, K.; Kobayashi, M.; Nakayama, M.; Miho, Y. In-situ FTIR studies on the electrochemical oxidation of histidine and tyrosine. J. Electroanal. Chem. 1999, 463, 218–223. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mic, M.; Varodi, C.; Pogacean, F.; Socaci, C.; Coros, M.; Stefan-van Staden, R.-I.; Pruneanu, S. Sensing and Interaction of His-Tagged CA19-9 Antigen with Graphene-Modified Electrodes. Chemosensors 2020, 8, 112. https://doi.org/10.3390/chemosensors8040112
Mic M, Varodi C, Pogacean F, Socaci C, Coros M, Stefan-van Staden R-I, Pruneanu S. Sensing and Interaction of His-Tagged CA19-9 Antigen with Graphene-Modified Electrodes. Chemosensors. 2020; 8(4):112. https://doi.org/10.3390/chemosensors8040112
Chicago/Turabian StyleMic, Mihaela, Codruta Varodi, Florina Pogacean, Crina Socaci, Maria Coros, Raluca-Ioana Stefan-van Staden, and Stela Pruneanu. 2020. "Sensing and Interaction of His-Tagged CA19-9 Antigen with Graphene-Modified Electrodes" Chemosensors 8, no. 4: 112. https://doi.org/10.3390/chemosensors8040112
APA StyleMic, M., Varodi, C., Pogacean, F., Socaci, C., Coros, M., Stefan-van Staden, R.-I., & Pruneanu, S. (2020). Sensing and Interaction of His-Tagged CA19-9 Antigen with Graphene-Modified Electrodes. Chemosensors, 8(4), 112. https://doi.org/10.3390/chemosensors8040112