Electrochemical Evaluation of Laccase Activity in Must
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Measurements of Laccase Activity Using Electrochemistry
2.3. Measurement of Laccase Activity Using Spectrophotometry
2.4. Preparation of Grape Musts
3. Results
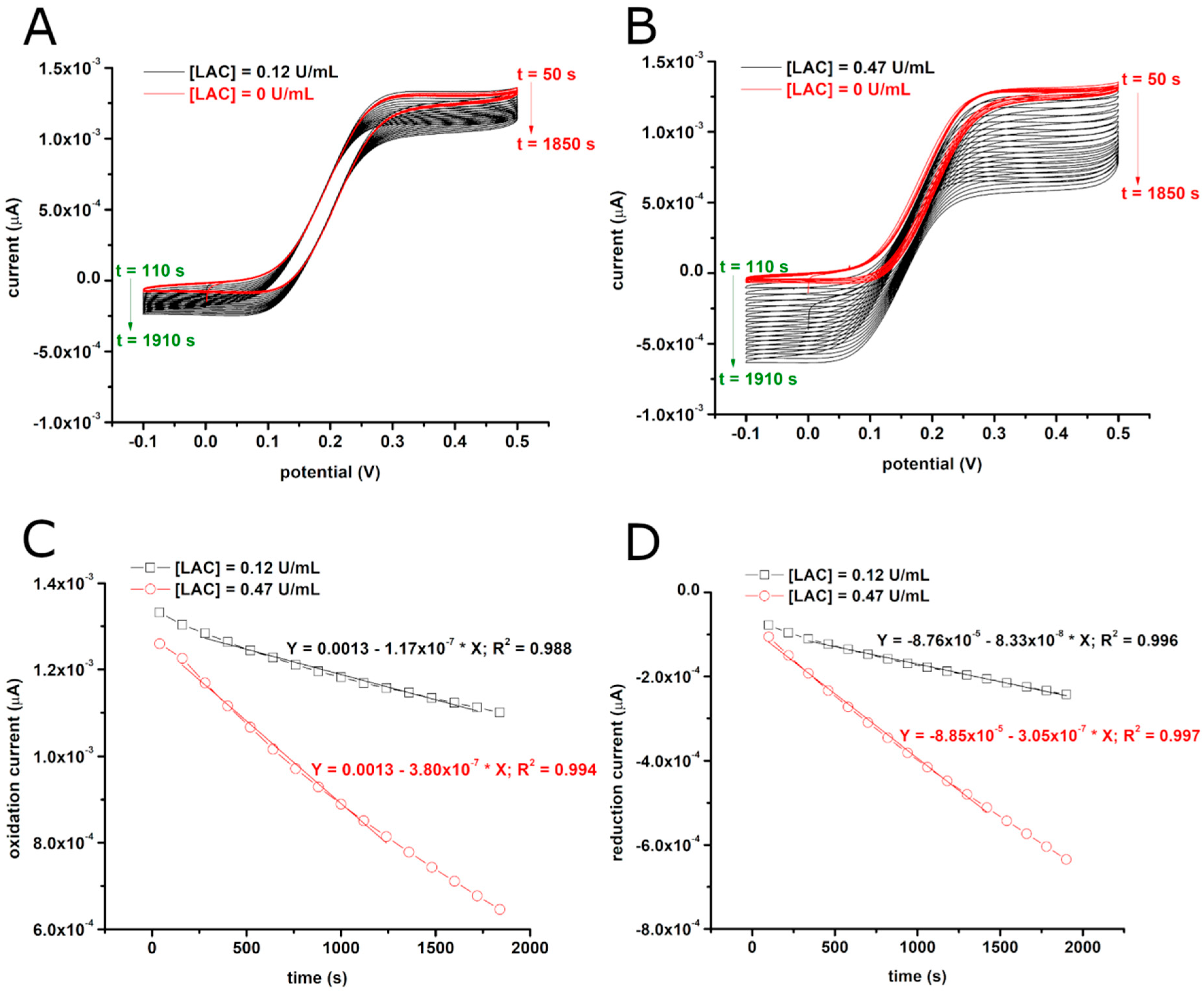
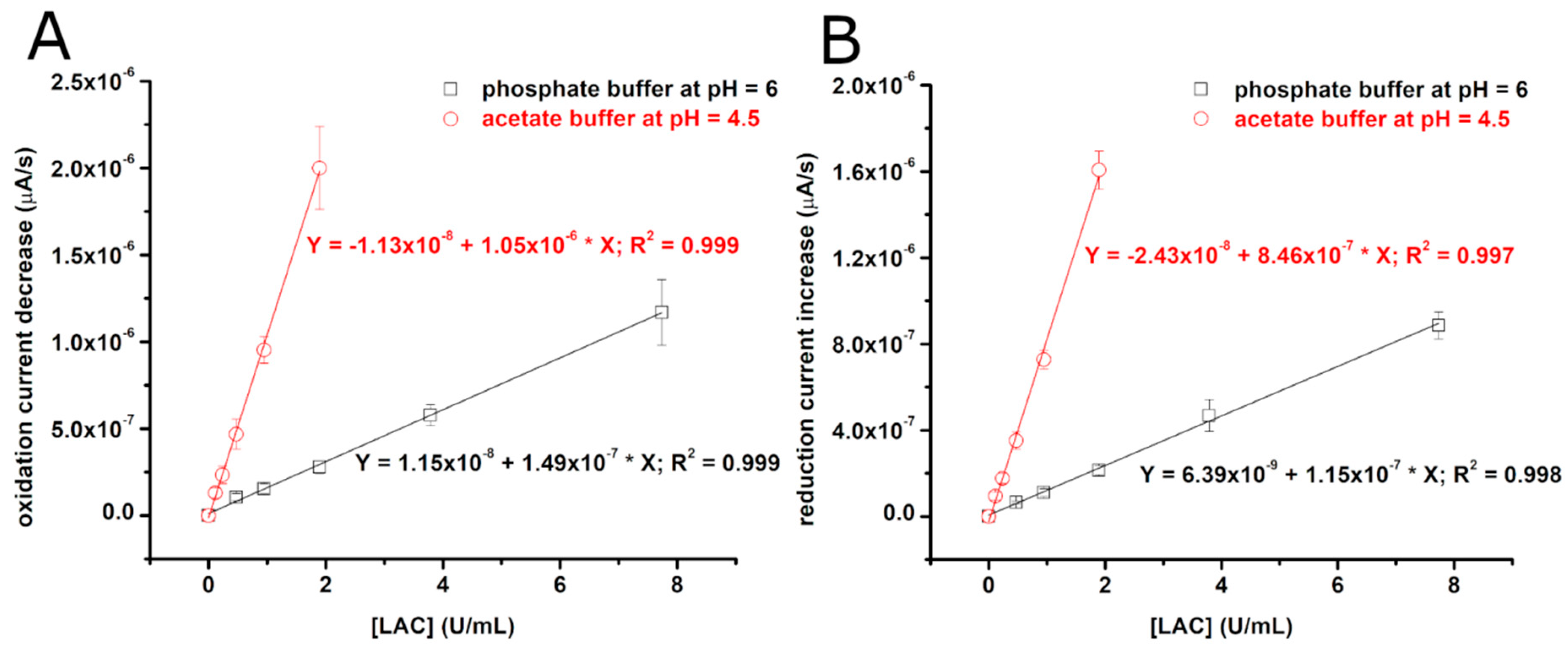
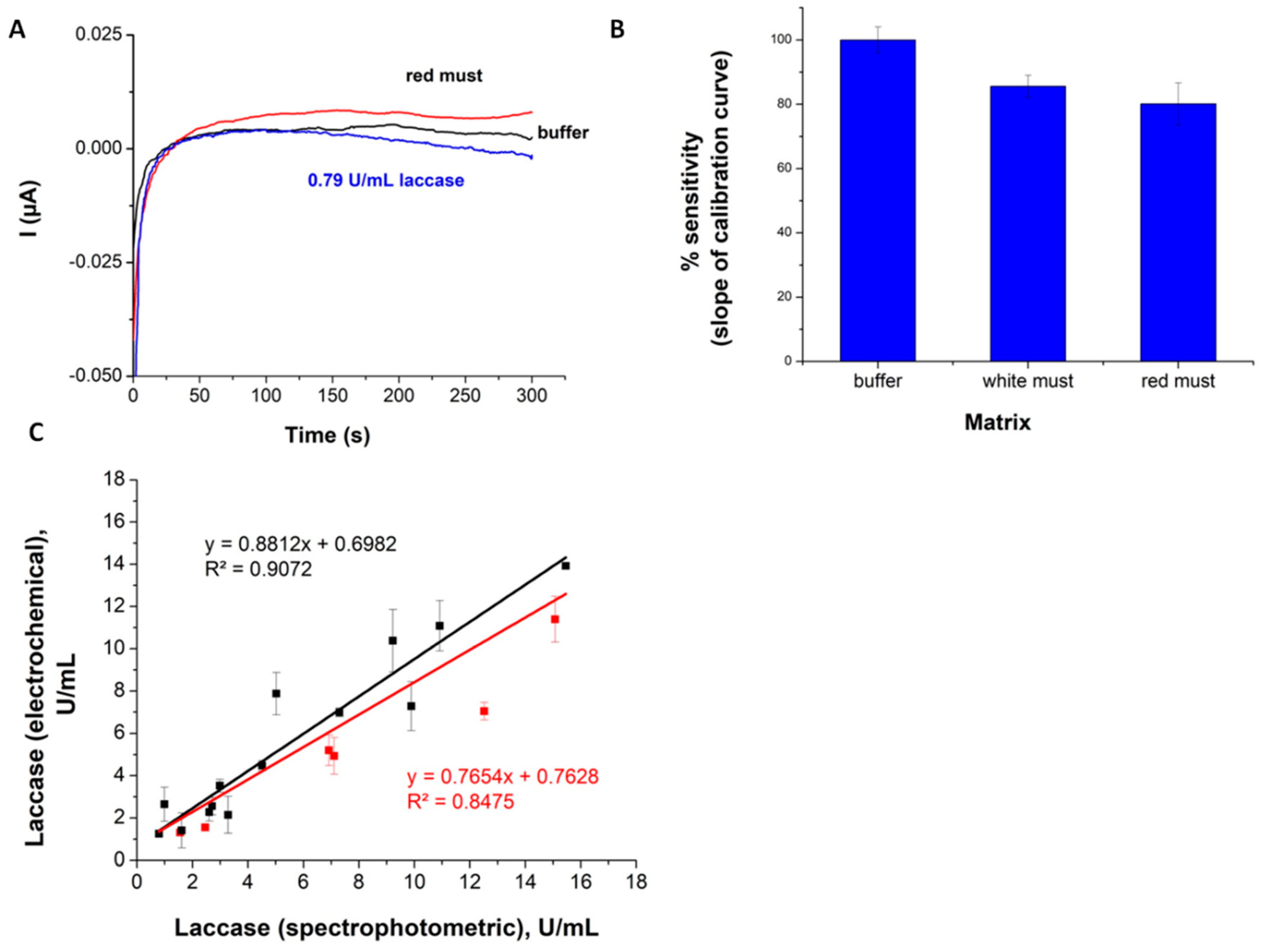
3.1. Electrochemical Evaluation of Laccase Activity Using CFMEs
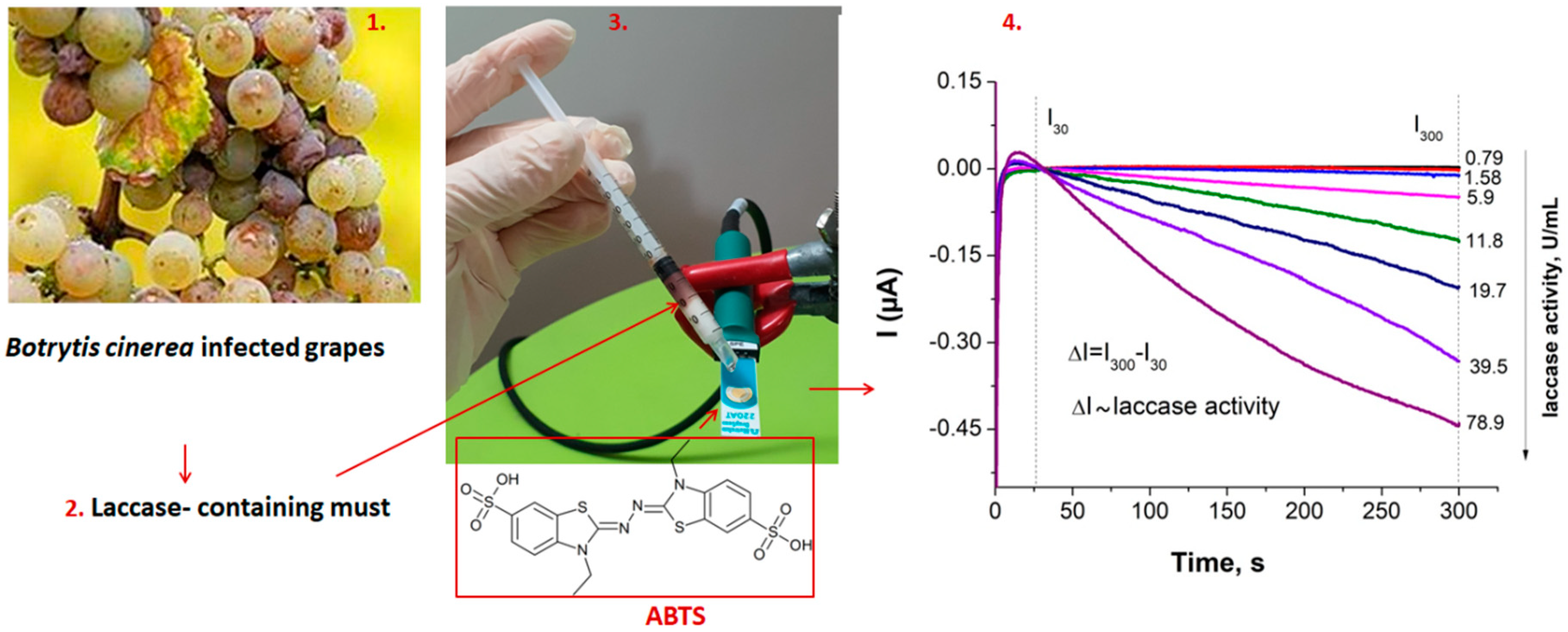
3.2. Evaluation of Laccase Activity Using SPAuEs
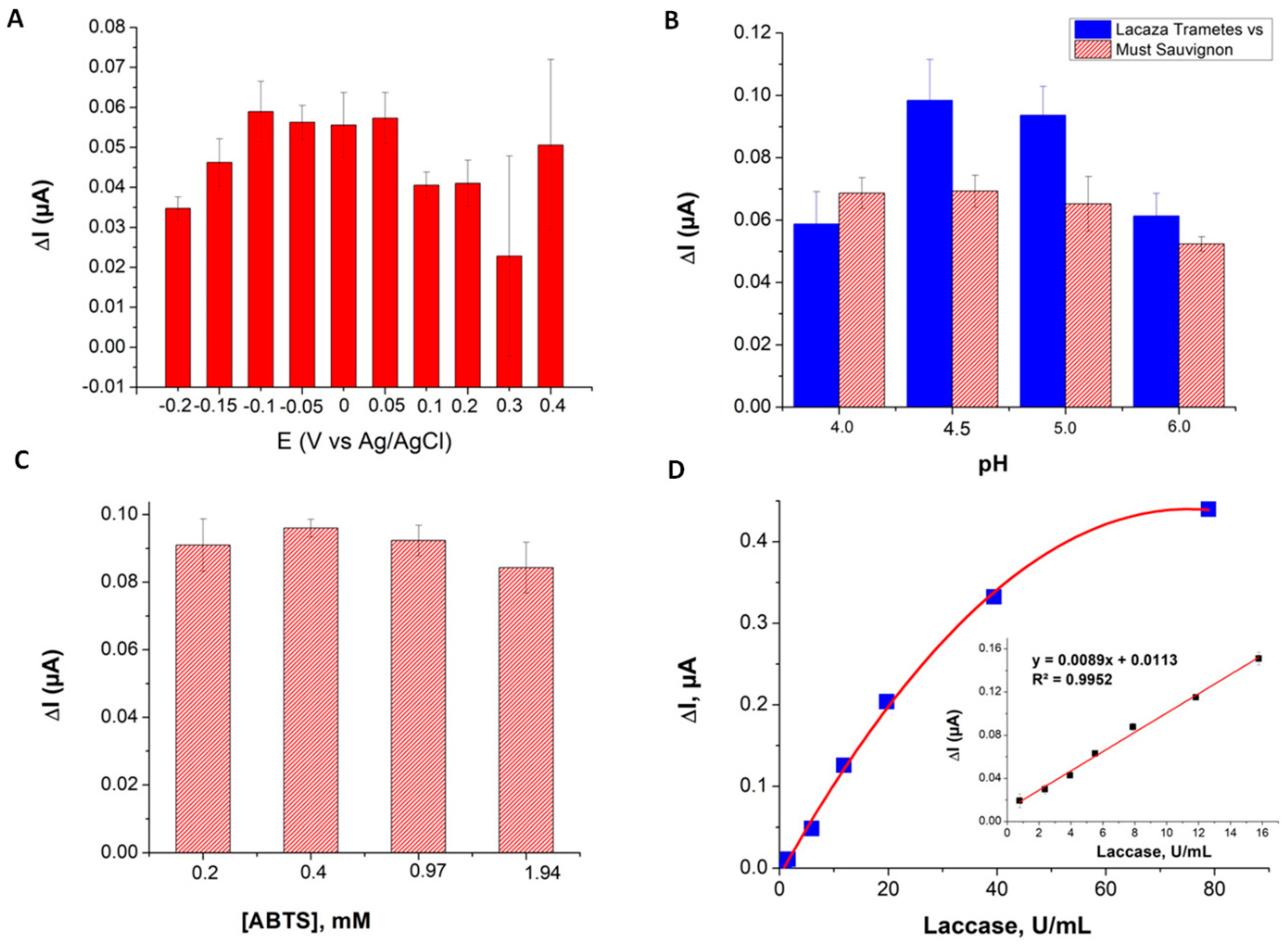
3.2.1. Optimization of The Experimental Conditions and Calibration
3.2.2. Investigation of The Grape Must Matrix Effects
3.2.3. Accuracy of The Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Diguta, C.F.; Rousseaux, S.; Weidmann, S.; Bretin, N.; Vincent, B.; Guilloux-Benatier, M.; Alexandre, H. Development of a qPCR assay for specific quantification of Botrytis cinerea on grapes. FEMS Microbiol. Lett. 2010, 313, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewey, F.M.R. Grant-Downton, Botrytis-Biology, Detection and Quantification. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–34. [Google Scholar]
- Binder, M. Development of a Botrytis Specific Immunosensor: Towards Using PCR Species Identification; University of Cranfield: Cranfield, UK, 2014. [Google Scholar]
- Bilkiss, M.; Shiddiky, M.J.A.; Ford, R. Advanced Diagnostic Approaches for Necrotrophic Fungal Pathogens of Temperate Legumes With a Focus on Botrytis spp. Front. Microbiol. 2019, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Wang, Y.; Wee, E.J.H.; Botella, J.R.; Trau, M. Field Demonstration of a Multiplexed Point-of-Care Diagnostic Platform for Plant Pathogens. Anal. Chem. 2016, 88, 8074–8081. [Google Scholar] [CrossRef]
- Grassin, C.; Dubourdieu, D. Quantitative determination ofBotrytis laccase in musts and wines by the syringaldazine test. J. Sci. Food Agric. 1989, 48, 369–376. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Grassin, C.; Deruche, C.; Ribéreau-Gayon, P. Mise au point d’une mesure rapide de l’activité laccase dans les moûts et dans les vins par la méthode a la syringaldazine. Application à l’appréciation de l’état sanitaire des vendanges. OENO One 1984, 18, 237–252. [Google Scholar] [CrossRef]
- Cuadrado, M.U.; Pérez-Juan, P.M.; De Castro, M.L.; Gómez-Nieto, M.Á. A fully automated method for in real time determination of laccase activity in wines. Anal. Chim. Acta 2005, 553, 99–104. [Google Scholar] [CrossRef]
- Detection Kit, Study of Botrytis Cinerea and Grape Juice Oxidability, Dolmar, Haro, Spain. Product information. Available online: https://www.laboratorysuppliesandreagents.com.au/test-kits/dolmar-laccase-test-kits/ (accessed on 30 October 2020).
- Laffort Botrytest Kit Laffort, France. Product Information. Available online: https://laffort.com/wp-content/uploads/FP/FP_EN_botrytest.pdf (accessed on 30 October 2020).
- Australian Wine Research Institute. Development of an Alternative Method for Laccase, AWRI Annual Report 2013. pp. 23–24. Available online: https://www.awri.com.au/flip/2014-annual-report/files/assets/basic-html/page24.html (accessed on 30 October 2020).
- SAFEGRAPE Consortium. Final Publishable Summary Report, Biosensor Based Instrumentation to be Used in Vineyards and Wineries for Fast and Sensitive Detection of Botrytis Cinerea (Grey Rot) in Grapes (SAFEGRAPE), 2011. Available online: https://cordis.europa.eu/project/id/232453/reporting (accessed on 30 October 2020).
- Zouari, N.; Romette, J.-L.; Thomas, D. A continuous-flow method for the rapid determination of sanitary quality of grape must at industrial scales. J. Chem. Technol. Biotechnol. 1988, 41, 243–248. [Google Scholar] [CrossRef]
- Zouari, N.; Romette, J.-L.; Thomas, D. Continuous-flow estimation of laccase activity in rotten grape juice by a computerized electrode. J. Chem. Technol. Biotechnol. 2007, 40, 195–201. [Google Scholar] [CrossRef]
- MyBioSource, San Diego, USA, Laccase Assay Kit, Micromethod Catalog No: MBS779938, 2020. Available online: https://www.mybiosource.com/assay-kits/laccase/779938 (accessed on 30 October 2020).
- BioVision Inc. Laccase Activity Assay Kit (Colorimetric), Catalog #: K2038 2020. Available online: https://www.biovision.com/laccase-activity-assay-kit-colorimetric.html (accessed on 30 October 2020).
- Rochelet, M.; Solanas, S.; Betelli, L.; Neuwirth, C.; Vienney, F.; Hartmann, A. Amperometric detection of extended-spectrum β-lactamase activity: Application to the characterization of resistant E. coli strains. Analyst 2015, 140, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- Betelli, L.; Neuwirth, C.; Solanas, S.; Chantemesse, B.; Vienney, F.; Hartmann, A.; Rochelet, M. A voltammetric test for the rapid discrimination of β-lactamase-producing Enterobacteriaceae in blood cultures. Talanta 2018, 184, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Timchalk, C.; Lin, Y. Carbon Nanotube-Based Electrochemical Sensor for Assay of Salivary Cholinesterase Enzyme Activity: An Exposure Biomarker of Organophosphate Pesticides and Nerve Agents. Environ. Sci. Technol. 2008, 42, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, K.; Gao, N.; Wei, S.; Liu, G.; Chai, Y.; Yang, M. Colorimetric and electrochemical determination of the activity of protein kinase based on retarded particle growth due to binding of phosphorylated peptides to DNA—Capped silver nanoclusters. Microchim. Acta 2016, 183, 2933–2939. [Google Scholar] [CrossRef]
- Pan, R.; Xu, M.; Jiang, D.; Burgess, J.D.; Chen, H. Nanokit for single-cell electrochemical analyses. Proc. Natl. Acad. Sci. USA 2016, 113, 11436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sappia, L.; Felice, B.; Sanchez, M.A.; Martí, M.; Madrid, R.; Pividori, M.I. Electrochemical sensor for alkaline phosphatase as biomarker for clinical and in vitro applications. Sens. Actuators B Chem. 2019, 281, 221–228. [Google Scholar] [CrossRef]
- Fernández-Sánchez, C.; Tzanov, T.; Gübitz, G.M.; Cavaco-Paulo, A. Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 2002, 58, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, L.M.; Santos, M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Flow injection based methods for fast screening of antioxidant capacity. Talanta 2009, 77, 1559–1566. [Google Scholar] [CrossRef]
- Cinquanta, L.; Albanese, D.; de Curtis, F.; Malvano, F.; Crescitelli, A.; di Matteo, M. Rapid Assessment of Gray Mold (Botrytis cinerea) Infection in Grapes with a Biosensor System. Am. J. Enol. Vitic. 2015, 66, 502. [Google Scholar]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta (BBA) Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Klis, M.; Rogalski, J.; Bilewicz, R. Voltammetric determination of catalytic reaction parameters of laccase based on electrooxidation of hydroquinone and ABTS. Bioelectrochemistry 2007, 71, 2–7. [Google Scholar] [CrossRef]
- Munteanu, R.-E.; Stǎnicǎ, L.; Gheorghiu, M.; Gáspár, S. Measurement of the Extracellular pH of Adherently Growing Mammalian Cells with High Spatial Resolution Using a Voltammetric pH Microsensor. Anal. Chem. 2018, 90, 6899–6905. [Google Scholar] [CrossRef]
- Hu, K.; Liu, Y.-L.; Oleinick, A.; Mirkin, M.V.; Huang, W.-H.; Amatore, C. Nanoelectrodes for intracellular measurements of reactive oxygen and nitrogen species in single living cells. Curr. Opin. Electrochem. 2020, 22, 44–50. [Google Scholar] [CrossRef]
- Solís-Oba, M.; Ugalde-Saldívar, V.M.; González, I.; Viniegra-González, G. An electrochemical–spectrophotometrical study of the oxidized forms of the mediator 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) produced by immobilized laccase. J. Electroanal. Chem. 2005, 579, 59–66. [Google Scholar] [CrossRef]
- Coulter, A. Laccase and rot: Is it there or is it not? In Australian and New Zealand Grapegrower and Winemaker; Winetitles Pty Ltd.: Broadview, Australia, 2012; pp. 69–72. [Google Scholar]
- Dewey, F.; Yohalem, D. Detection, Quantification and Immunolocalisation of Botrytis species. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2007; pp. 181–194. [Google Scholar]
- Dewey, F.; Hill, M.R. Descenzo, Quantification of Botrytis and laccase in winegrapes. Am. J. Enol. Vitic. 2008, 59, 47–54. [Google Scholar]
- Fernández-Baldo, M.A.; Messina, G.A.; Sanz, M.I.; Raba, J. Screen-printed immunosensor modified with carbon nanotubes in a continuous-flow system for the Botrytis cinerea determination in apple tissues. Talanta 2009, 79, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Baldo, M.A.; Messina, G.A.; Sanz, M.I.; Raba, J. Microfluidic Immunosensor with Micromagnetic Beads Coupled to Carbon-Based Screen-Printed Electrodes (SPCEs) for Determination of Botrytis cinerea in Tissue of Fruits. J. Agric. Food Chem. 2010, 58, 11201–11206. [Google Scholar] [CrossRef] [PubMed]
| Sample No. | Must Type | Spiked Laccase (u/mL) | Recovered Laccase, Based on Oxidation Currents | Recovered Laccase, Based on Reduction Currents | ||
|---|---|---|---|---|---|---|
| Found Laccase (U/mL) | Recovery (%) | Found Laccase (U/mL) | Recovery (%) | |||
| 1 | Red | 2.6 | 2.21 | 85 | 2.05 | 78.8 |
| 2 | 5.2 | 3.77 | 72.5 | 3.54 | 68.1 | |
| 3 | 2.6 | 1.70 | 65.4 | 1.87 | 71.9 | |
| 4 | 2.6 | 1.73 | 66.5 | 1.83 | 70.4 | |
| 5 | White | 2.6 | 2.02 | 77.7 | 2.3 | 88.5 |
| 6 | 2.6 | 2.72 | 104.6 | 2.63 | 101.2 | |
| 7 | 2.6 | 2.55 | 98.1 | 2.54 | 97.7 | |
| 8 | 5.2 | 4.65 | 89.4 | 4.28 | 82.3 | |
| 9 | 5.2 | 4.86 | 93.5 | 4.09 | 78.7 | |
| Must Type | Spiked Laccase, u/mL | Spectrophotometric Method | Electrochemical Sensor | ||
|---|---|---|---|---|---|
| Laccase Activity, U/mL | Recovery (%) | Laccase Activity, U/mL | Recovery (%) | ||
| White | 3.95 | 4.51 ± 0.11 | 114.4 ± 2.8 | 4.47 ± 0.24 | 113.4 ± 6.0 |
| 7.89 | 7.30 ± 0.24 | 92.5 ± 3.0 | 6.99 ± 0.21 | 88.6 ± 2.7 | |
| 11.8 | 10.91 ± 0.09 | 92.2 ± 0.7 | 11.09 ± 1.19 | 93.7 ± 10.1 | |
| 15.8 | 15.5 ± 1.7 | 98.0 ± 11.0 | 13.9 ± 0.10 | 88.3 ± 0.7 | |
| Red | 2.37 | 2.45 ± 0.07 | 103.7 ± 3.0 | 1.56 ± 0.10 | 66.0 ± 4.3 |
| 5.52 | 7.11 ± 0.14 | 128.7 ± 2.6 | 4.93 ± 0.87 | 89.3 ± 15.7 | |
| 7.89 | 6.92 ± 0.36 | 87.7 ± 4.5 | 5.20 ± 0.72 | 65.9 ± 9.2 | |
| 9.47 | 12.51 ± 0.25 | 132.2 ± 2.7 | 7.05 ± 0.41 | 74.4 ± 4.3 | |
| 15.8 | 15.07 ± 0.83 | 95.5 ± 5.3 | 11.39 ± 1.09 | 72.2 ± 6.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gáspár, S.; Brinduse, E.; Vasilescu, A. Electrochemical Evaluation of Laccase Activity in Must. Chemosensors 2020, 8, 126. https://doi.org/10.3390/chemosensors8040126
Gáspár S, Brinduse E, Vasilescu A. Electrochemical Evaluation of Laccase Activity in Must. Chemosensors. 2020; 8(4):126. https://doi.org/10.3390/chemosensors8040126
Chicago/Turabian StyleGáspár, Szilveszter, Elena Brinduse, and Alina Vasilescu. 2020. "Electrochemical Evaluation of Laccase Activity in Must" Chemosensors 8, no. 4: 126. https://doi.org/10.3390/chemosensors8040126
APA StyleGáspár, S., Brinduse, E., & Vasilescu, A. (2020). Electrochemical Evaluation of Laccase Activity in Must. Chemosensors, 8(4), 126. https://doi.org/10.3390/chemosensors8040126







