Paracetamol Sensing with a Pencil Lead Electrode Modified with Carbon Nanotubes and Polyvinylpyrrolidone
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Preparation of the Pencil Graphite Electrode
2.4. Modification of the Working Electrodes
2.5. Electrochemical Measurement of the PGE
3. Results and Discussion
3.1. Effect of the Electrode Architecture
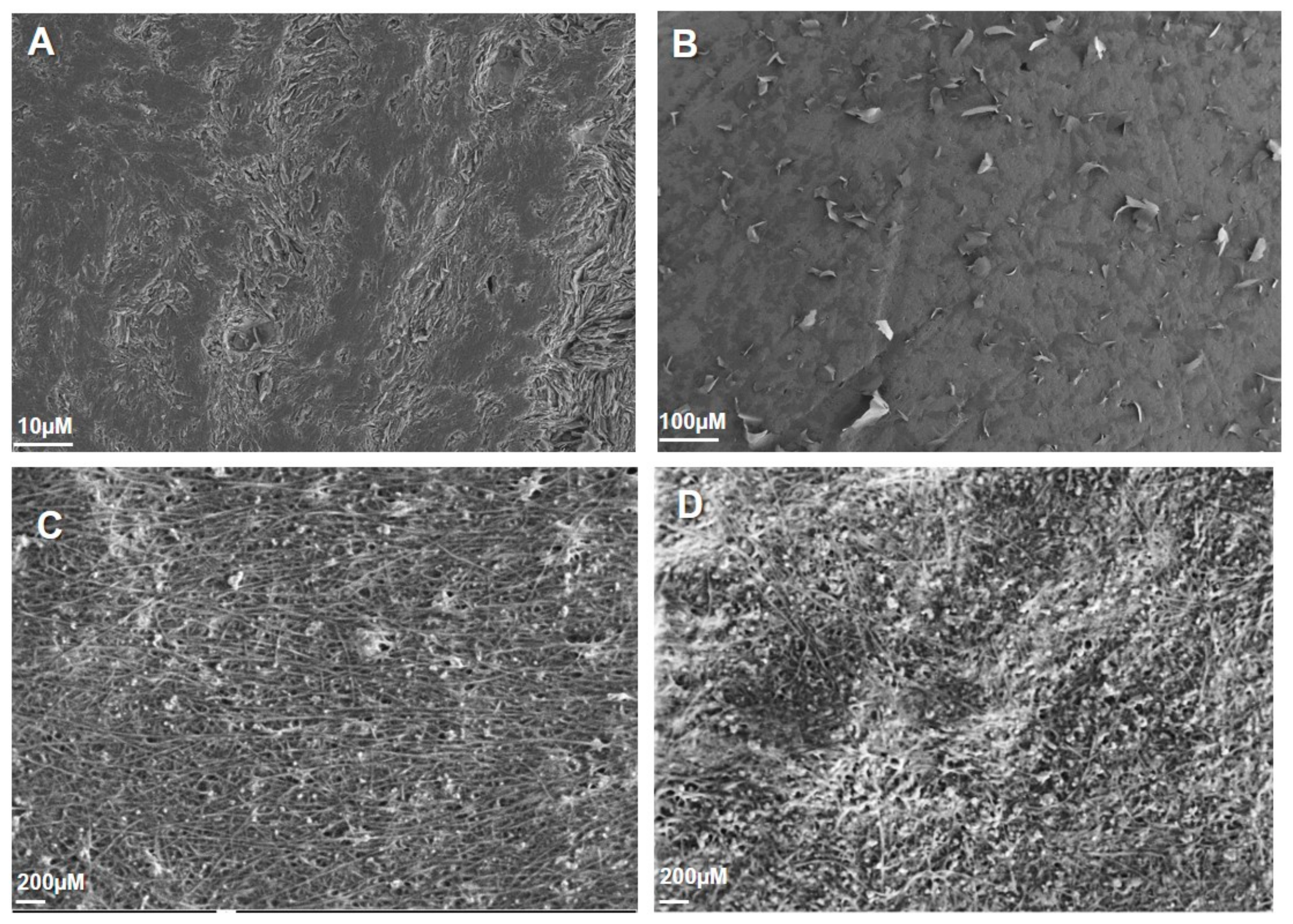
3.2. Surface Morphology of Bare PGE, PVP/PGE, SWCNT/PGE, and PVP/SWCNT/PGE
3.3. Electrochemical Behavior of Paracetamol
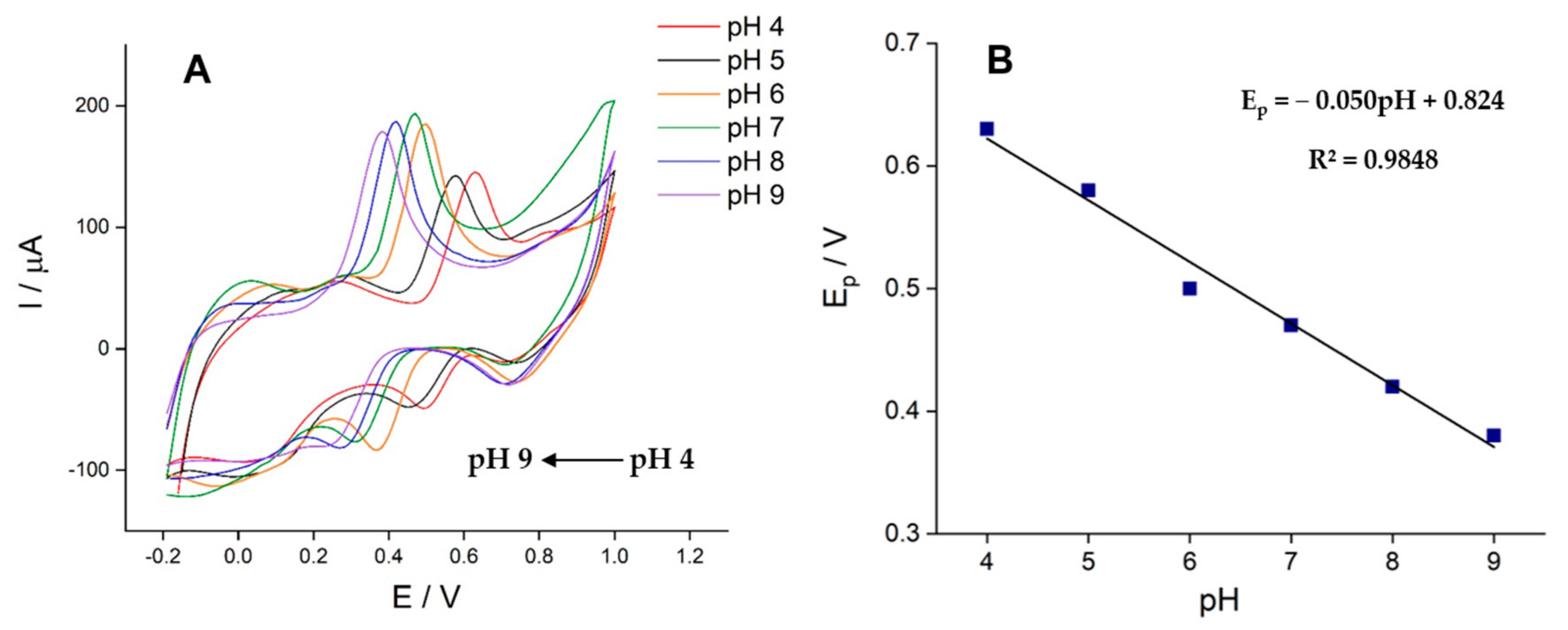
3.4. Effect of pH
3.5. Effect of Scan Rate
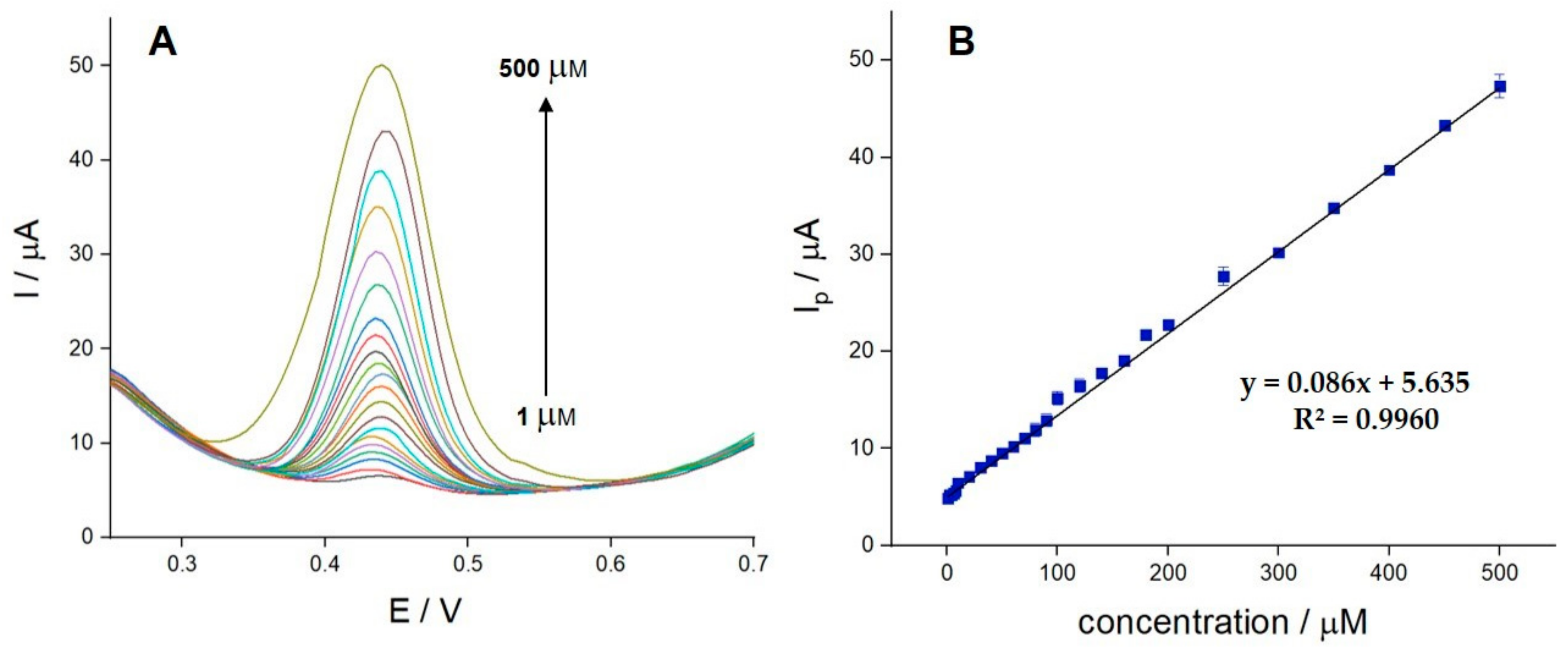
3.6. Determination of Paracetamol on the PVP/SWCNT/PGE
3.7. Interference Study
3.8. Reproducibility, Reusability, and Storage Stability
3.9. Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayram, E.; Akyilmaz, E. Development of a new microbial biosensor based on conductive polymer/multiwalled carbon nanotube and its application to paracetamol determination. Sens. Actuators B 2016, 233, 409–418. [Google Scholar] [CrossRef]
- Sheen, C.L.; Dillon, J.F.; Bateman, D.N.; Simpson, K.J.; Macdonald, T.M. Paracetamol toxicity: Epidemiology, prevention and costs to the health-care system. QJM Int. J. Med. 2002, 95, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Antunes, S.C.; Santos, J.; Martins, L.; Castro, B.B. Toxic potential of paracetamol to freshwater organisms: A headache to environmental regulators? Ecotoxicol. Environ. Saf. 2014, 107, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, P.; Caroli, S.; Caracciolo, A.B. Pharmaceuticals as priority water contaminants. Toxicol. Environ. Chem. 2010, 92, 549–565. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- De Voogt, P.; Janex-Habibi, M.-L.; Sacher, F.; Puijker, L.; Mons, M. Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci. Technol. 2009, 59, 39–46. [Google Scholar] [CrossRef]
- Montaseri, H.; Forbes, P.B.C. Analytical techniques for the determination of acetaminophen: A review. TrAC Trends Anal. Chem. 2018, 108, 122–134. [Google Scholar] [CrossRef]
- Sirajuddin; Khaskheli, A.R.; Shah, A.; Bhanger, M.I.; Niaz, A.; Mahesar, S. Simpler spectrophotometric assay of paracetamol in tablets and urine samples. Spectrochim. Acta A 2007, 68, 747–751. [Google Scholar] [CrossRef]
- Easwaramoorthy, D.; Yu, Y.-C.; Huang, H.-J. Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal. Chim. Acta 2001, 439, 95–100. [Google Scholar] [CrossRef]
- Kumar, K.G.; Letha, R. Determination of Paracetamol in pure form and in dosage forms using N,N-dibromo dimethylhydantoin. J. Pharm. Biomed. Anal. 1997, 15, 1725–1728. [Google Scholar] [CrossRef]
- Migowska, N.; Caban, M.; Stepnowski, P.; Kumirska, J. Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography–mass spectrometry and gas chromatography with electron capture detection. Sci. Total Environ. 2012, 441, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Pugajeva, I.; Rusko, J.; Perkons, I.; Lundanes, E.; Bartkevics, V. Determination of pharmaceutical residues in wastewater using high performance liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2017, 133, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.P.; Aguiar, J.P.; Fernandes, A.I.; Pinto, J.F. Quantification of theophylline or paracetamol in milk matrices by high-performance liquid chromatography. J. Pharm. Anal. 2017, 7, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.B.; Jariwala, F.B.; Pavlov, J.; Yang, Z.; Mahr, J.A.; Oviedo, M. Direct detection and identification of active pharmaceutical ingredients in intact tablets by helium plasma ionization (HePI) mass spectrometry. J. Pharm. Anal. 2014, 4, 166–172. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemical behavior and voltammetric determination of paracetamol on Nafion/TiO2–graphene modified glassy carbon electrode. Colloids Surf. B 2011, 85, 289–292. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Amini, R. Nanostructured hexacyanoferrate intercalated Ni/Al layered double hydroxide modified electrode as a sensitive electrochemical sensor for paracetamol determination. Electroanalysis 2017, 29, 635–642. [Google Scholar] [CrossRef]
- El Bouabi, Y.; Farahi, A.; Labjar, N.; El Hajjaji, S.; Bakasse, M.; El Mhammedi, M.A. Square wave voltammetric determination of paracetamol at chitosan modified carbon paste electrode: Application in natural water samples, commercial tablets and human urines. Mater. Sci. Eng. C 2016, 58, 70–77. [Google Scholar] [CrossRef]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in electrochemical (bio)sensors for monitoring wine production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef]
- Kawde, A.-N.; Baig, N.; Sajid, M. Graphite pencil electrodes as electrochemical sensors for environmental analysis: A review of features, developments, and applications. RSC Adv. 2016, 6, 91325–91340. [Google Scholar] [CrossRef]
- Annu; Sharma, S.; Jain, R.; Raja, A.N. Review—pencil graphite electrode: An emerging sensing material. J. Electrochem. Soc. 2019, 167, 037501. [Google Scholar] [CrossRef]
- Sağlam, Ö.; Dilgin, D.G.; Ertek, B.; Dilgin, Y. Differential pulse voltammetric determination of eugenol at a pencil graphite electrode. Mater. Sci. Eng. C 2016, 60, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Pattar, V.P.; Nandibewoor, S.T. Staircase voltammetric determination of 2-thiouracil in pharmaceuticals and human biological fluids at polyaniline and polypyrrole film modified sensors. Sens. Actuators A 2016, 250, 40–47. [Google Scholar] [CrossRef]
- Saleh, G.A.; Askal, H.F.; Refaat, I.H.; Naggar, A.H.; Abdel-aal, F.A.M. Adsorptive square wave voltammetric determination of the antiviral drug valacyclovir on a novel sensor of copper microparticles–modified pencil graphite electrode. Arab. J. Chem. 2016, 9, 143–151. [Google Scholar] [CrossRef]
- Eksin, E.; Zor, E.; Erdem, A.; Bingol, H. Electrochemical monitoring of biointeraction by graphene-based material modified pencil graphite electrode. Biosens. Bioelectron. 2017, 92, 207–214. [Google Scholar] [CrossRef]
- Blay, V.; Galian, R.E.; Muresan, L.M.; Pankratov, D.; Pinyou, P.; Zampardi, G. Research frontiers in energy-related materials and applications for 2020–2030. Adv. Sustain. Syst. 2020, 4, 1900145. [Google Scholar] [CrossRef]
- Torrinha, Á.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Araújo, A.N. Biosensing based on pencil graphite electrodes. Talanta 2018, 190, 235–247. [Google Scholar] [CrossRef]
- Lawal, A.T. Synthesis and utilization of carbon nanotubes for fabrication of electrochemical biosensors. Mater. Res. Bull. 2016, 73, 308–350. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Alhans, R.; Singh, A.; Singhal, C.; Narang, J.; Wadhwa, S.; Mathur, A. Comparative analysis of single-walled and multi-walled carbon nanotubes for electrochemical sensing of glucose on gold printed circuit boards. Mater. Sci. Eng. C 2018, 90, 273–279. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Morais, S. New generation of electrochemical sensors based on multi-walled carbon nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Arul, P.; John, S.A. Size controlled synthesis of Ni-MOF using polyvinylpyrrolidone: New electrode material for the trace level determination of nitrobenzene. J. Electroanal. Chem. 2018, 829, 168–176. [Google Scholar] [CrossRef]
- Afrasiabi garekani, H.; Ghazi, A. Increasing the aqueous solubility of acetaminophen in the presence of polyvinylpyrrolidone and investigation of the mechanisms involved. Drug Dev. Ind. Pharm. 2003, 29, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-Y.; Qi, S.; Craig, D.Q.M. An investigation into the influence of drug–polymer interactions on the miscibility, processability and structure of polyvinylpyrrolidone-based hot melt extrusion formulations. Int. J. Pharm. 2015, 496, 95–106. [Google Scholar] [CrossRef]
- Siswana, M.P.; Ozoemena, K.I.; Nyokong, T. Electrocatalysis of asulam on cobalt phthalocyanine modified multi-walled carbon nanotubes immobilized on a basal plane pyrolytic graphite electrode. Electrochim. Acta 2006, 52, 114–122. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2000. [Google Scholar]
- Chang, B.Y.; Park, S.M. Electrochemical Impedance Spectroscopy. Annu. Rev. Anal. Chem. 2010, 3, 207–229. [Google Scholar] [CrossRef]
- David, I.G.; Litescu, S.C.; Popa, D.E.; Buleandra, M.; Iordache, L.; Albu, C.; Alecu, A.; Penu, R.L. Voltammetric analysis of naringenin at a disposable pencil graphite electrode—application to polyphenol content determination in citrus juice. Anal. Methods 2018, 10, 5763–5772. [Google Scholar] [CrossRef]
- Navratil, R.; Kotzianova, A.; Halouzka, V.; Opletal, T.; Triskova, I.; Trnkova, L.; Hrbac, J. Polymer lead pencil graphite as electrode material: Voltammetric, XPS and Raman study. J. Electroanal. Chem. 2016, 783, 152–160. [Google Scholar] [CrossRef]
- Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and characterization of polyvinylpyrrolidone/cellulose nanocrystals composites. Nanomaterials 2018, 8, 1011. [Google Scholar] [CrossRef] [PubMed]
- Zidan, M.; Tan, W.; Abdullah, A.; Goh, J. Electrochemical oxidation of paracetamol mediated by nanoparticles bismuth oxide modified glassy carbon electrode. Int. J. Electrochem. Sci. 2011, 279–288. [Google Scholar]
- Ghadimi, H.; Tehrani, R.M.A.; Ali, A.S.M.; Mohamed, N.; Ab Ghani, S. Sensitive voltammetric determination of paracetamol by poly (4-vinylpyridine)/multiwalled carbon nanotubes modified glassy carbon electrode. Anal. Chim. Acta 2013, 765, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pinyou, P.; Blay, V.; Muresan, L.M.; Noguer, T. Enzyme-modified electrodes for biosensors and biofuel cells. Mater. Horiz. 2019, 6, 1336–1358. [Google Scholar] [CrossRef]
- Yaman, Y.T.; Abaci, S. Sensitive adsorptive voltammetric method for determination of bisphenol A by gold nanoparticle/polyvinylpyrrolidone-modified pencil graphite electrode. Sensors 2016, 16, 756. [Google Scholar] [CrossRef]
- Manikandan, R.R.; Deepa, P.N.; Narayanan, S.S. Fabrication and characterization of poly 2-napthol orange film modified electrode and its application to selective detection of dopamine. J. Solid State Electrochem. 2017, 21, 3567–3578. [Google Scholar] [CrossRef]
- Cheng, K.L. Explanation of misleading Nernst slope by Boltzmann equation. Microchem. J. 1998, 59, 457–461. [Google Scholar] [CrossRef]
- Ghorbani-Bidkorbeh, F.; Shahrokhian, S.; Mohammadi, A.; Dinarvand, R. Simultaneous voltammetric determination of tramadol and acetaminophen using carbon nanoparticles modified glassy carbon electrode. Electrochim. Acta 2010, 55, 2752–2759. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Manjunatha, R.; Nagaraju, D.H.; Suresh, G.S.; Melo, J.S.; D’Souza, S.F.; Venkatesha, T.V. Electrochemical detection of acetaminophen on the functionalized MWCNTs modified electrode using layer-by-layer technique. Electrochim. Acta 2011, 56, 6619–6627. [Google Scholar] [CrossRef]
- Matsuda, H.; Ayabe, Y. The theory of the cathode-ray polarography of Randles-Sevcik. Z. Elektrochem. Angew. Phys. Chem. 1955, 59, 494–503. [Google Scholar]
- Ribeiro, A.C.F.; Barros, M.C.F.; Veríssimo, L.M.P.; Santos, C.I.A.V.; Cabral, A.M.T.D.P.V.; Gaspar, G.D.; Esteso, M.A. Diffusion coefficients of paracetamol in aqueous solutions. J. Chem. Thermodyn. 2012, 54, 97–99. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Howlader, M.M.R.; Hu, N.-X.; Deen, M.J. Electrochemical sensing of acetaminophen using multi-walled carbon nanotube and β-cyclodextrin. Sens. Actuators B 2018, 254, 896–909. [Google Scholar] [CrossRef]
- Arancibia, V.; Penagos-Llanos, J.; Nagles, E.; García-Beltrán, O.; Hurtado, J.J. Development of a microcomposite with single-walled carbon nanotubes and Nd2O3 for determination of paracetamol in pharmaceutical dosage by adsorptive voltammetry. J. Pharm. Anal. 2019, 9, 62–69. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G. Enhanced voltammetric detection of paracetamol by using carbon nanotube modified electrode as an electrochemical sensor. J. Electrochem. Sci. Eng. 2019, 10, 29–40. [Google Scholar] [CrossRef]
- Mulyasuryani, A.; Tjahjanto, R.T.; Andawiyah, R.a. Simultaneous voltammetric detection of acetaminophen and caffeine base on cassava starch—Fe3O4 nanoparticles modified glassy carbon electrode. Chemosensors 2019, 7, 49. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, Y.; Fan, J.; Liu, H.; Shi, Q.; Liu, W.; Peng, Q.; Zang, G. Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen. Molecules 2019, 24, 2320. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.-P.; Zhang, L.-N.; Zhang, Y.-C.; Shen, L. Phosphorus-doped graphene-based electrochemical sensor for sensitive detection of acetaminophen. Anal. Chim. Acta 2018, 1036, 26–32. [Google Scholar] [CrossRef]



| Electrode | Rct (Ω) | Electroactive Surface Area (cm2) |
|---|---|---|
| Bare PGE | 2.67 ± 0.08 × 104 | 0.0224 ± 0.0003 |
| PVP/PGE | 2.31 ± 1.3 × 104 | 0.0385 ± 0.0013 |
| SWCNT/PGE | 117 ± 9.5 | 0.1114 ± 0.0039 |
| Mixed PVP-SWCNT/PGE | 1.72 ± 0.12 × 104 | 0.0367 ± 0.0006 |
| PVP/SWCNT/PGE | 82 ± 6.3 | 0.1126 ± 0.0006 |
| Modified Electrodes | Method | LOD (μM) | Linear Range (μM) | Ref. |
|---|---|---|---|---|
| Nafion/TiO2-graphene/GCE | DPV | 0.21 | 1–100 | [16] |
| Hexacyanoferate(III) intercalated Ni−Al layered double hydroxide/GCE | CA | 0.80 | 3–1500 | [17] |
| Chitosan/CPE | SWV | 0.51 | 0.8–20, 40–100 | [18] |
| MWCNT/β-cyclodextrin/GCE | DPV | 0.0115 | 0.05–300 | [55] |
| SWCNT/Nd2O3/CPE | SWV | 0.05 | 0.10–9.5 | [56] |
| Poly(methyl orange) CNT-paste electrode | DPV | 0.38 | 2–50 | [57] |
| Cassava starch-Fe3O4/GCE | DPV | 16 | 50–2000 | [58] |
| Nafion/Cu-nanowires/GO/GCE | DPV | 0.04 | 1–100 | [59] |
| Phosphorus-doped graphene/GCE | DPV | 0.36 | 1.5–120 | [60] |
| PGE/SWCNT/PVP/PGE | SWV | 0.38 | 1–500 | This work |
| Sample | Prepared Concentration (μM) | Added 1 (μM) | Found (μM) | Recovery (%) | RSD 2 (%) |
|---|---|---|---|---|---|
| Paracetamol syrup | 49.62 | - | 50.14 | 101.59 | 1.45 |
| (50 mg/mL) | 49.62 | 15 | 63.45 | 98.19 | 2.02 |
| 49.62 | 30 | 81.02 | 101.76 | 1.53 | |
| Paracetamol tablet | 50.62 | - | 51.73 | 102.19 | 2.94 |
| (250 mg/tablet) | 50.62 | 15 | 67.24 | 102.47 | 1.83 |
| 50.62 | 30 | 81.23 | 100.76 | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinyou, P.; Blay, V.; Chansaenpak, K.; Lisnund, S. Paracetamol Sensing with a Pencil Lead Electrode Modified with Carbon Nanotubes and Polyvinylpyrrolidone. Chemosensors 2020, 8, 133. https://doi.org/10.3390/chemosensors8040133
Pinyou P, Blay V, Chansaenpak K, Lisnund S. Paracetamol Sensing with a Pencil Lead Electrode Modified with Carbon Nanotubes and Polyvinylpyrrolidone. Chemosensors. 2020; 8(4):133. https://doi.org/10.3390/chemosensors8040133
Chicago/Turabian StylePinyou, Piyanut, Vincent Blay, Kantapat Chansaenpak, and Sireerat Lisnund. 2020. "Paracetamol Sensing with a Pencil Lead Electrode Modified with Carbon Nanotubes and Polyvinylpyrrolidone" Chemosensors 8, no. 4: 133. https://doi.org/10.3390/chemosensors8040133
APA StylePinyou, P., Blay, V., Chansaenpak, K., & Lisnund, S. (2020). Paracetamol Sensing with a Pencil Lead Electrode Modified with Carbon Nanotubes and Polyvinylpyrrolidone. Chemosensors, 8(4), 133. https://doi.org/10.3390/chemosensors8040133







