Fabrication of Zinc Oxide Nanoparticles Deposited on (3-Aminopropyl) Triethoxysilane-Treated Silicon Substrates by an Optimized Voltage-Controlled Electrophoretic Deposition and Their Application as Fluorescence-Based Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Material
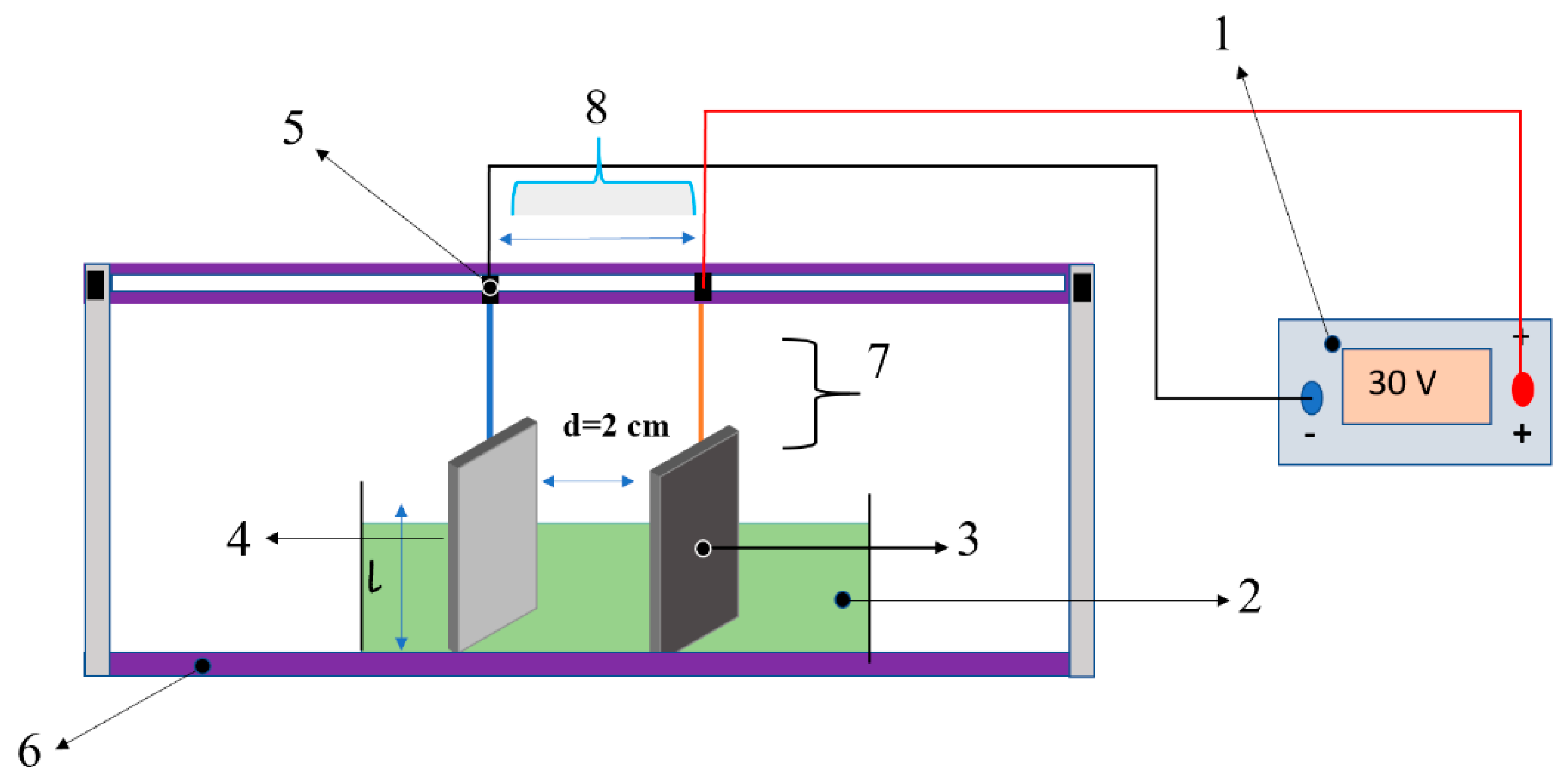
2.2. EPD Deposition Setup
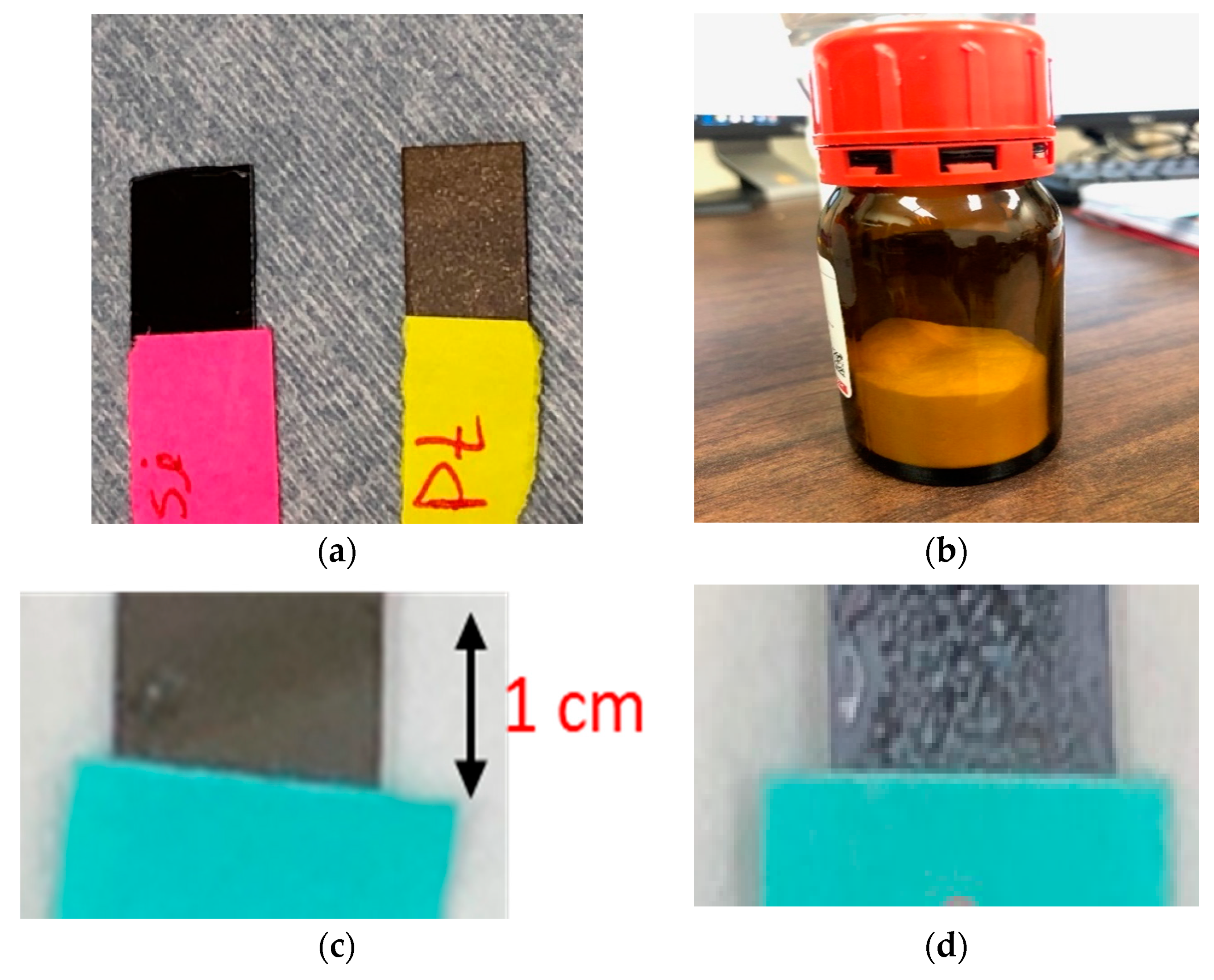
2.3. Electrodes Preparation
2.4. 3-Aminopropyl-Triethoxysilane (APTES) Surface Functionalization Treatment of ZnO Substrates
2.5. Microfabrication Processes
2.6. Synthesizing EPD ZnO Solution
2.7. ZnO Nanostructure Characterization
3. Results
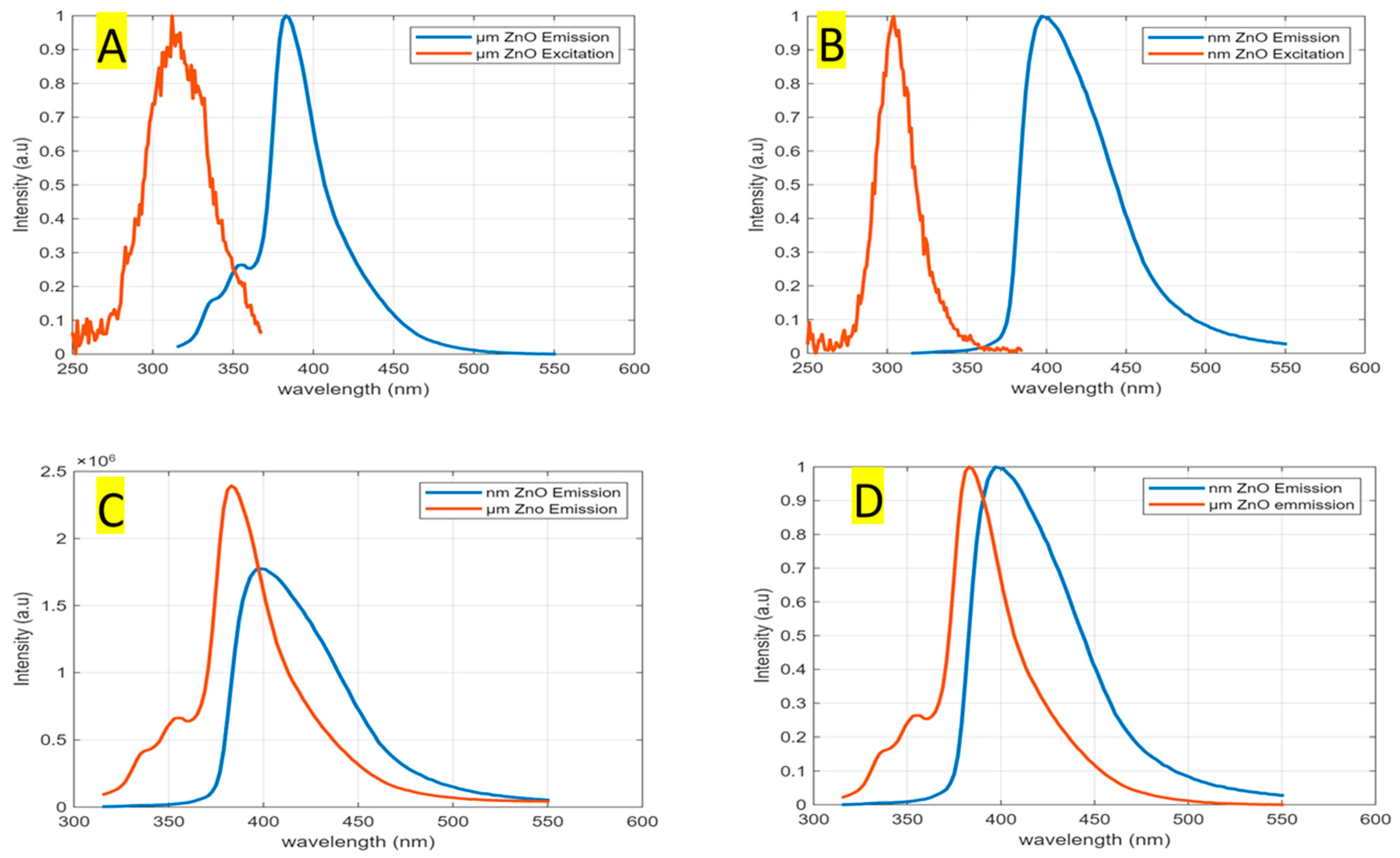
3.1. Fluorescence Spectra for Sensing ZnO Microparticles and Nanoparticles in DI Water
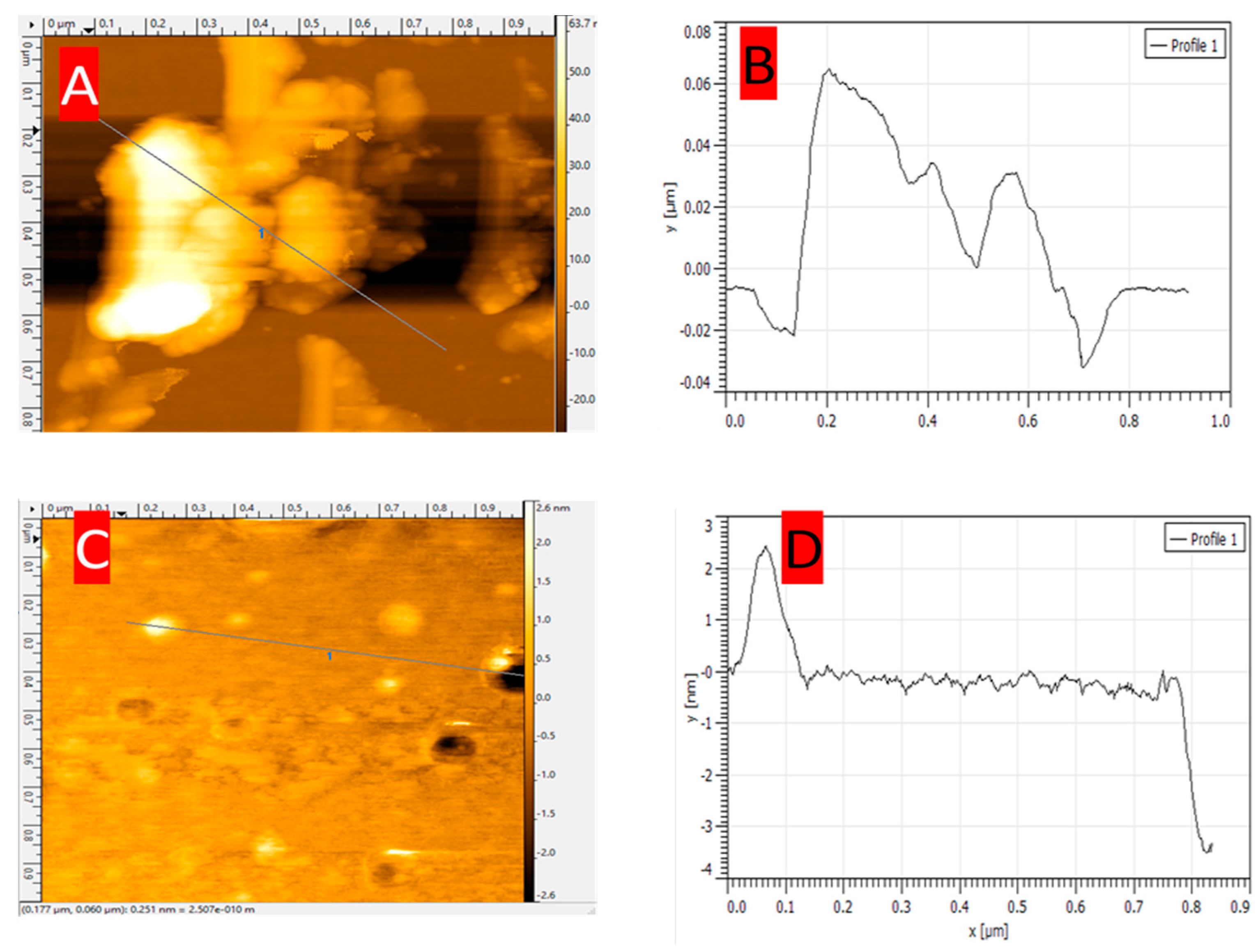
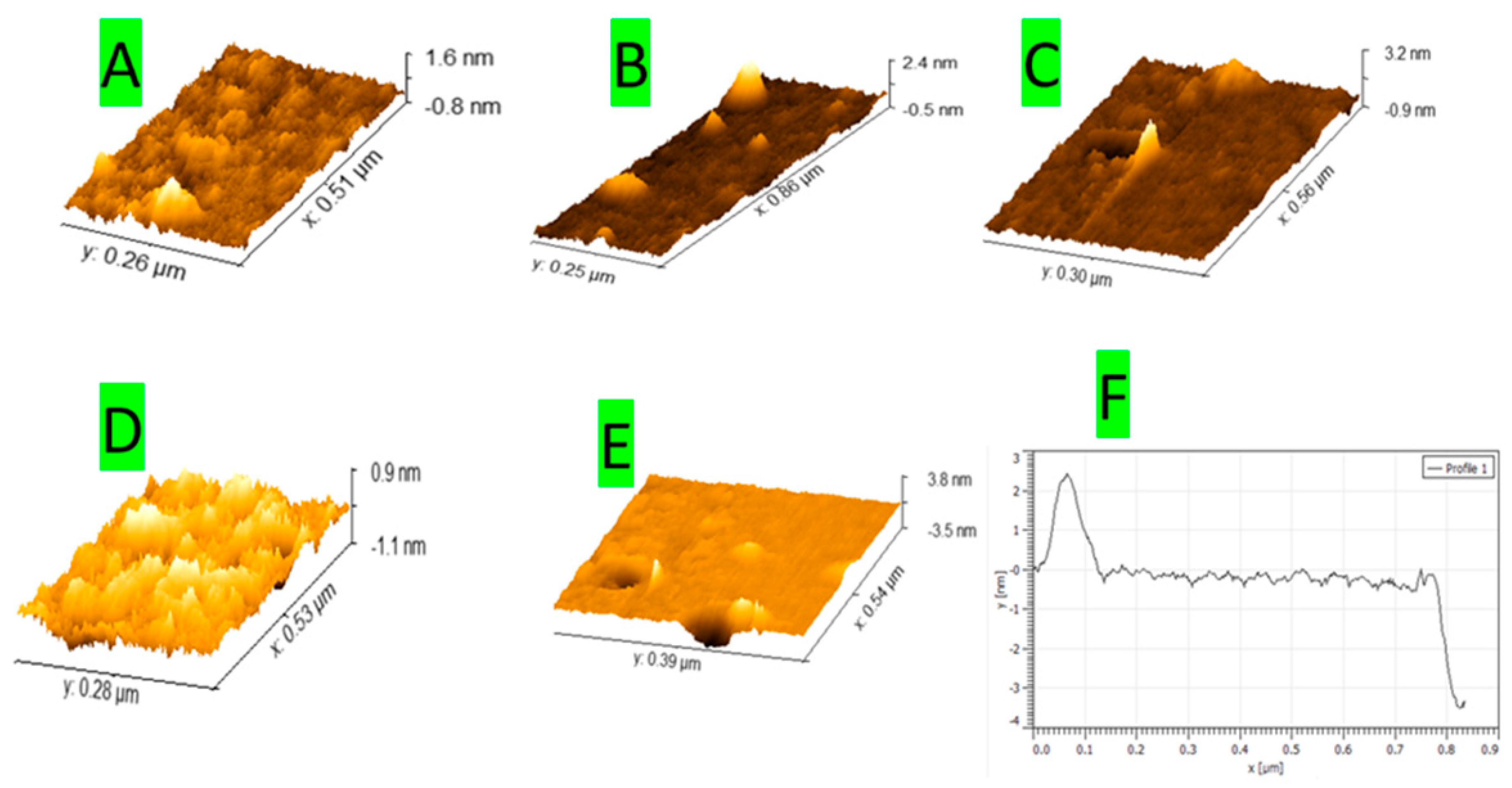
3.2. Atomic Force Microscopy Charachtrization of EPD ZnO on p-Type Si
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, S.; Umar, A.; Bhasin, K.K.; Baskoutas, S. Chemical Sensing Applications of Zno Nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, Properties and Applications of Zno Nanomaterials with Oxygen Vacancies: A Review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Jin, J.; Wang, H.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Wang, X. Application of Graphene Oxides and Graphene Oxide-Based Nanomaterials in Radionuclide Removal from Aqueous Solutions. Sci. Bull. 2016, 61, 1583–1593. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.P.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-Nanomaterial-Based Flexible Batteries for Wearable Electronics. Adv. Mater. 2019, 31, 1800716. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue Luminescence of Zno Nanoparticles Based on Non-Equilibrium Processes: Defect Origins and Emission Controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Tachikawa, S.; Noguchi, A.; Tsuge, T.; Hara, M.; Odawara, O.; Wada, H. Optical Properties of Zno Nanoparticles Capped with Polymers. Materials 2011, 4, 1132–1143. [Google Scholar] [CrossRef] [Green Version]
- Dagdeviren, C.; Hwang, S.-W.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, Biocompatible Electronics and Energy Harvesters Based on Zno. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef]
- Franco, A., Jr.; Pessoni, H. Effect of Gd Doping on the Structural, Optical Band-Gap, Dielectric and Magnetic Properties of Zno Nanoparticles. Phys. B Condens. Matter 2017, 506, 145–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.-H.; Zheng, J.-C.; Zhu, Z.-Z. Direct to Indirect Band Gap Transition in Ultrathin Zno Nanowires under Uniaxial Compression. Appl. Phys. Lett. 2009, 94, 113114. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.W.; Wei, A.; Lei, Y.; Cai, X.; Li, C.M.; Dong, Z.L. Zinc Oxide Nanocomb Biosensor for Glucose Detection. Appl. Phys. Lett. 2006, 88, 233106. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent Advances in Zno Nanostructures and Thin Films for Biosensor Applications. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Sun, X.W.; Wang, J.; Lei, Y.; Cai, X.; Li, C.M.; Dong, Z.; Huang, W. Enzymatic Glucose Biosensor Based on Zno Nanorod Array Grown by Hydrothermal Decomposition. Appl. Phys. Lett. 2006, 89, 123902. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elkady, M.; Farghali, A.; Salem, A.M.; El-Hamid, A.A. Fabrication of Novel Magnetic Zinc Oxide Cellulose Acetate Hybrid Nano-Fiber to Be Utilized for Phenol Decontamination. J. Taiwan Inst. Chem. Eng. 2017, 78, 307–316. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, B. A Mechanochemical Synthesis of Nanostructured Zinc Oxide Via Acetate Route for Lpg Sensing. J. Exp. Nanosci. 2014, 9, 501–511. [Google Scholar] [CrossRef]
- Tonto, P.; Mekasuwandumrong, O.; Phatanasri, S.; Pavarajarn, V.; Praserthdam, P. Preparation of Zno Nanorod by Solvothermal Reaction of Zinc Acetate in Various Alcohols. Ceram. Int. 2008, 34, 57–62. [Google Scholar] [CrossRef]
- Ohyama, M.; Kouzuka, H.; Yoko, T. Sol-Gel Preparation of Zno Films with Extremely Preferred Orientation Along (002) Plane from Zinc Acetate Solution. Thin Solid Film. 1997, 306, 78–85. [Google Scholar] [CrossRef]
- Sakohara, S.; Ishida, M.; Anderson, M.A. Visible Luminescence and Surface Properties of Nanosized Zno Colloids Prepared by Hydrolyzing Zinc Acetate. J. Phys. Chem. B 1998, 102, 10169–10175. [Google Scholar] [CrossRef]
- Belhaj, M.; Dridi, C.; Elhouichet, H.; Valmalette, J.C. Study of Zno Nanoparticles Based Hybrid Nanocomposites for Optoelectronic Applications. J. Appl. Phys. 2016, 119, 095501. [Google Scholar] [CrossRef]
- Lai, L.-T.; Chang, S.-J.; Yang, C.-C.; Young, S.-J. Uv-Enhanced 2-D Nanostructured Zno Field Emitter with Adsorbed Pt Nanoparticles. IEEE Electron Device Lett. 2018, 39, 1932–1935. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, G.; Xu, M.; Su, Y.; Tai, H.; Du, H.; Jiang, Y. Visible Light-Assisted Room Temperature Gas Sensing with Zno-Ag Heterostructure Nanoparticles. Sens. Actuators B Chem. 2018, 259, 269–281. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, S.-J.; Chen, W.-S.; Hsueh, T.-J. Transparent Zno-Nanowire-Based Device for Uv Light Detection and Ethanol Gas Sensing on C-Si Solar Cell. RSC Adv. 2016, 6, 11146–11150. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-Temperature Gas Sensing of Zno-Based Gas Sensor: A Review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Comini, E.; Hartiti, B.; Moumen, A.; Arachchige, H.M.M.; Fadili, S.; Thevenin, P.; Kamal, A. Effect of Vanadium Doping on Zno Sensing Properties Synthesized by Spray Pyrolysis. Mater. Des. 2018, 139, 56–64. [Google Scholar]
- Fan, Z.; Wang, D.; Chang, P.-C.; Tseng, W.-Y.; Lu, J.G. Zno Nanowire Field-Effect Transistor and Oxygen Sensing Property. Appl. Phys. Lett. 2004, 85, 5923–5925. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chang, Y.; Long, Y. Influence of Sn Doping on Zno Sensing Properties for Ethanol and Acetone. Mater. Sci. Eng. C 2012, 32, 817–821. [Google Scholar] [CrossRef]
- Miura, N.; Akisada, K.; Wang, J.; Zhuiykov, S.; Ono, T. Mixed-Potential-Type No X Sensor Based on Ysz and Zinc Oxide Sensing Electrode. Ionics 2004, 10, 1–9. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.; Chen, Y.; Wang, T.-H.; He, X.; Li, J.; Lin, C. Fabrication and Ethanol Sensing Characteristics of Zno Nanowire Gas Sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; She, G.-W.; Wang, H.; Ou, X.-M.; Zhang, X.-H.; Lee, C.-S.; Lee, S.-T. Zno Nanotube Arrays as Biosensors for Glucose. J. Phys. Chem. C 2009, 113, 20169–20172. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, W.; Zhang, X.; Wang, B.; Jiang, H. Zno-Based Amperometric Enzyme Biosensors. Sensors 2010, 10, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arya, S.K.; Pandey, P.; Malhotra, B.; Saha, S.; Sreenivas, K.; Gupta, V. Cholesterol Biosensor Based on Rf Sputtered Zinc Oxide Nanoporous Thin Film. Appl. Phys. Lett. 2007, 91, 063901. [Google Scholar] [CrossRef]
- Lim, S.; Kwon, S.; Kim, H. Zno Thin Films Prepared by Atomic Layer Deposition and Rf Sputtering as an Active Layer for Thin Film Transistor. Thin Solid Film. 2008, 516, 1523–1528. [Google Scholar] [CrossRef]
- Kwon, S.; Bang, S.; Lee, S.; Jeon, S.; Jeong, W.; Kim, H.; Gong, S.C.; Chang, H.J.; Park, H.-H.; Jeon, H. Characteristics of the Zno Thin Film Transistor by Atomic Layer Deposition at Various Temperatures. Semicond. Sci. Technol. 2009, 24, 035015. [Google Scholar] [CrossRef]
- Lim, S.; Kwon, S.-J.; Kim, H.; Park, J.-S. High Performance Thin Film Transistor with Low Temperature Atomic Layer Deposition Nitrogen-Doped Zno. Appl. Phys. Lett. 2007, 91, 183517. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, J.; Cagin, E.; Chen, D.; Phillips, J. Zno Thin-Film Transistors with Polycrystalline (Ba, Sr) Ti O 3 Gate Insulators. Appl. Phys. Lett. 2006, 88, 212903. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-C.; Peng, G.-T.; Liu, T.-F. Optoelectronic Properties and Anisotropic Stress of Mo: Zno Thin Films Deposited on Flexible Substrates by Radio Frequency Magnetron Sputtering. Appl. Opt. 2020, 59, 1454–1460. [Google Scholar] [CrossRef]
- Kaur, G.; Mitra, A.; Yadav, K. Pulsed Laser Deposited Al-Doped Zno Thin Films for Optical Applications. Prog. Nat. Sci. Mater. Int. 2015, 25, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.; Harvey, J.; Leaf, J.; Fry, C.; Wolden, C. Transport Phenomena in High Performance Nanocrystalline Zno: Ga Films Deposited by Plasma-Enhanced Chemical Vapor Deposition. Thin Solid Film. 2005, 473, 35–40. [Google Scholar] [CrossRef]
- Tan, S.T.; Chen, B.; Sun, X.; Fan, W.; Kwok, H.S.; Zhang, X.; Chua, S. Blueshift of Optical Band Gap in Zno Thin Films Grown by Metal-Organic Chemical-Vapor Deposition. J. Appl. Phys. 2005, 98, 013505. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Bhatti, K.; Qindeel, R.; Alonizan, N.; Althobaiti, H.S. Characterizations of Multilayer Zno Thin Films Deposited by Sol-Gel Spin Coating Technique. Results Phys. 2017, 7, 651–655. [Google Scholar] [CrossRef]
- Coke, M. Electronic Properties of Zinc Oxide and Related Materials Grown by Molecular Beam Epitaxy; UCL (University College London): London, UK, 2017. [Google Scholar]
- Banerjee, P.; Lee, W.-J.; Bae, K.-R.; Lee, S.B.; Rubloff, G.W. Structural, Electrical, and Optical Properties of Atomic Layer Deposition Al-Doped Zno Films. J. Appl. Phys. 2010, 108, 043504. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Hecht, D.S.; Gruner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Kanakamedala, K.; DeSoto, J.; Sarkar, A.; Race, T.D. Study of Electrospray Assisted Electrophoretic Deposition of Carbon Nanotubes on Insulator Substrates. Electron. Mater. Lett. 2015, 11, 949–956. [Google Scholar] [CrossRef]
- Maulik, S.; Sarkar, A.; Basu, S.; Daniels-Race, T. Voltage-Controlled Spray Deposition of Multiwalled Carbon Nanotubes on Semiconducting and Insulating Substrates. J. Electron. Mater. 2018, 47, 4604–4609. [Google Scholar] [CrossRef]
- Sarkar, A.; Daniels-Race, T. Electrophoretic Deposition of Carbon Nanotubes on 3-Amino-Propyl-Triethoxysilane (Aptes) Surface Functionalized Silicon Substrates. Nanomaterials 2013, 3, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeur, B. Molecular Fluorescence. Digit. Encycl. Appl. Phys. 2003, 477–531. [Google Scholar] [CrossRef]
- Cosby, A.G.; Quevedo, G.; Boros, E. A High-Throughput Method to Measure Relative Quantum Yield of Lanthanide Complexes for Bioimaging. Inorg. Chem. 2019, 58, 10611–10615. [Google Scholar] [CrossRef]
- Levitus, M. Tutorial: Measurement of Fluorescence Spectra and Determination of Relative Fluorescence Quantum Yields of Transparent Samples. Methods Appl. Fluoresc. 2020, 8, 033001. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence Quantum Yields and Their Relation to Lifetimes of Rhodamine 6g and Fluorescein in Nine Solvents: Improved Absolute Standards for Quantum Yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Serpone, N. Relative Photonic Efficiencies and Quantum Yields in Heterogeneous Photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 104, 1–12. [Google Scholar] [CrossRef]
- Ware, W.R.; Rothman, W. Relative Fluorescence Quantum Yields Using an Integrating Sphere. The Quantum Yield of 9, 10-Diphenylanthracene in Cyclohexane. Chem. Phys. Lett. 1976, 39, 449–453. [Google Scholar]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative Fluorescence Quantum Yields Using a Computer-Controlled Luminescence Spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and Absolute Determination of Fluorescence Quantum Yields of Transparent Samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Comparison of Methods and Achievable Uncertainties for the Relative and Absolute Measurement of Photoluminescence Quantum Yields. Anal. Chem. 2011, 83, 3431–3439. [Google Scholar] [CrossRef]
- Meijer, M.S.; Rojas-Gutierrez, P.A.; Busko, D.; Howard, I.A.; Frenzel, F.; Würth, C.; Resch-Genger, U.; Richards, B.S.; Turshatov, A.; Capobianco, J.A. Absolute Upconversion Quantum Yields of Blue-Emitting Liyf 4: Yb 3+, Tm 3+ Upconverting Nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 22556–22562. [Google Scholar] [CrossRef] [Green Version]
- Porres, L.; Holland, A.; Pålsson, L.-O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Kaiser, M.; Wilhelm, S.; Grauel, B.; Hirsch, T.; Resch-Genger, U. Excitation Power Dependent Population Pathways and Absolute Quantum Yields of Upconversion Nanoparticles in Different Solvents. Nanoscale 2017, 9, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, U.J.; Murphy, K.R.; Stedmon, C.A. Fluorescence Quantum Yields of Natural Organic Matter and Organic Compounds: Implications for the Fluorescence-Based Interpretation of Organic Matter Composition. Front. Mar. Sci. 2015, 2, 98. [Google Scholar] [CrossRef]
- McCartney, M.; Whitaker, A.; Wood, A. George Gabriel Stokes: Life, Science and Faith; Oxford University Press: New York, NC, USA, 2019. [Google Scholar]
- Li, S.; Sun, Z.; Li, R.; Dong, M.; Zhang, L.; Qi, W.; Zhang, X.; Wang, H. Zno Nanocomposites Modified by Hydrophobic and Hydrophilic Silanes with Dramatically Enhanced Tunable Fluorescence and Aqueous Ultrastability toward Biological Imaging Applications. Sci. Rep. 2015, 5, 8475. [Google Scholar] [CrossRef] [Green Version]
- Guo-Bin, Z.; Chao-Shu, S.; Zheng-Fu, H.; Jun-Yan, S.; Zhu-Xi, F.; Kirm, M.; Zimmerer, G. Photoluminescent Properties of Zno Films Deposited on Si Substrates. Chin. Phys. Lett. 2001, 18, 441. [Google Scholar] [CrossRef]
- Ohshima, T.; Thareja, R.; Yamagata, Y.; Ikegami, T.; Ebihara, K.; Narayan, J. Laser-Ablated Plasma for Deposition of Zno Thin Films on Various Substrates. Sci. Technol. Adv. Mater. 2001, 2, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wei, S.-H.; Zunger, A. Intrinsic N-Type Versus P-Type Doping Asymmetry and the Defect Physics of Zno. Phys. Rev. B 2001, 63, 075205. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.L.; Kashif, M.; Hashim, U.; Liu, W.-W. Effect of Different Solvents on the Structural and Optical Properties of Zinc Oxide Thin Films for Optoelectronic Applications. Ceram. Int. 2014, 40, 753–761. [Google Scholar] [CrossRef]
- Sengupta, J.; Sahoo, R.; Mukherjee, C. Effect of Annealing on the Structural, Topographical and Optical Properties of Sol–Gel Derived Zno and Azo Thin Films. Mater. Lett. 2012, 83, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Besra, L.; Liu, M. A Review on Fundamentals and Applications of Electrophoretic Deposition (Epd). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar]
- Kim, Y.Y.; Kong, B.H.; Cho, H.K. Vertically Arrayed Ga-Doped Zno Nanorods Grown by Magnetron Sputtering: The Effect of Ga Contents and Microstructural Evaluation. J. Cryst. Growth 2011, 330, 17–21. [Google Scholar] [CrossRef]
- Lee, S.M.; Ikeda, S.; Yagi, T.; Harada, T.; Ennaoui, A.; Matsumura, M. Fabrication of Cuins 2 Films from Electrodeposited Cu/in Bilayers: Effects of Preheat Treatment on Their Structural, Photoelectrochemical and Solar Cell Properties. Phys. Chem. Chem. Phys. 2011, 13, 6662–6669. [Google Scholar] [CrossRef]


| Zinc Weight Percentage to EPD Solution (%) | C2H6O2 Weight Percentage to EPD Solution (%) | DI Water Weight Percentage to EPD Solution (%) | Average Particle Diameter (nm) |
|---|---|---|---|
| 1 (0.22 g) | 19 | 80 | 50 |
| 5 (1.1 g) | 15 | 80 | 50 |
| 10 (2.2 g) | 10 | 80 | 50 |
| 10 (2.2 g) | 10 | 80 | 100 |
| Emission | Excitation | Stoke Shift | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Point of Study | Peak Wavelength (nm) | Energy (eV) | FWHM (nm) | FQY | Peak Wavelength (nm) | Energy (eV) | FWHM (nm) | Energy (eV) | |
| Particles Sizes | |||||||||
| ZnO nanoparticles | 399 | 3.10 | 60 | 0.52 | 304 | 4.08 | 27 | 0.971 | |
| ZnO microparticles | 383 | 3.24 | 32 | 0.35 | 312 | 3.98 | 43 | 0.74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazzazi, F.; Young, A.; O’Loughlin, C.; Daniels-Race, T. Fabrication of Zinc Oxide Nanoparticles Deposited on (3-Aminopropyl) Triethoxysilane-Treated Silicon Substrates by an Optimized Voltage-Controlled Electrophoretic Deposition and Their Application as Fluorescence-Based Sensors. Chemosensors 2021, 9, 5. https://doi.org/10.3390/chemosensors9010005
Hazzazi F, Young A, O’Loughlin C, Daniels-Race T. Fabrication of Zinc Oxide Nanoparticles Deposited on (3-Aminopropyl) Triethoxysilane-Treated Silicon Substrates by an Optimized Voltage-Controlled Electrophoretic Deposition and Their Application as Fluorescence-Based Sensors. Chemosensors. 2021; 9(1):5. https://doi.org/10.3390/chemosensors9010005
Chicago/Turabian StyleHazzazi, Fawwaz, Alex Young, Christopher O’Loughlin, and Theda Daniels-Race. 2021. "Fabrication of Zinc Oxide Nanoparticles Deposited on (3-Aminopropyl) Triethoxysilane-Treated Silicon Substrates by an Optimized Voltage-Controlled Electrophoretic Deposition and Their Application as Fluorescence-Based Sensors" Chemosensors 9, no. 1: 5. https://doi.org/10.3390/chemosensors9010005
APA StyleHazzazi, F., Young, A., O’Loughlin, C., & Daniels-Race, T. (2021). Fabrication of Zinc Oxide Nanoparticles Deposited on (3-Aminopropyl) Triethoxysilane-Treated Silicon Substrates by an Optimized Voltage-Controlled Electrophoretic Deposition and Their Application as Fluorescence-Based Sensors. Chemosensors, 9(1), 5. https://doi.org/10.3390/chemosensors9010005






