Advances in Colorimetric Assay Based on AuNPs Modified by Proteins and Nucleic Acid Aptamers
Abstract
:1. Introduction
2. Preparation and Properties of AuNPs
2.1. Preparation of AuNPs
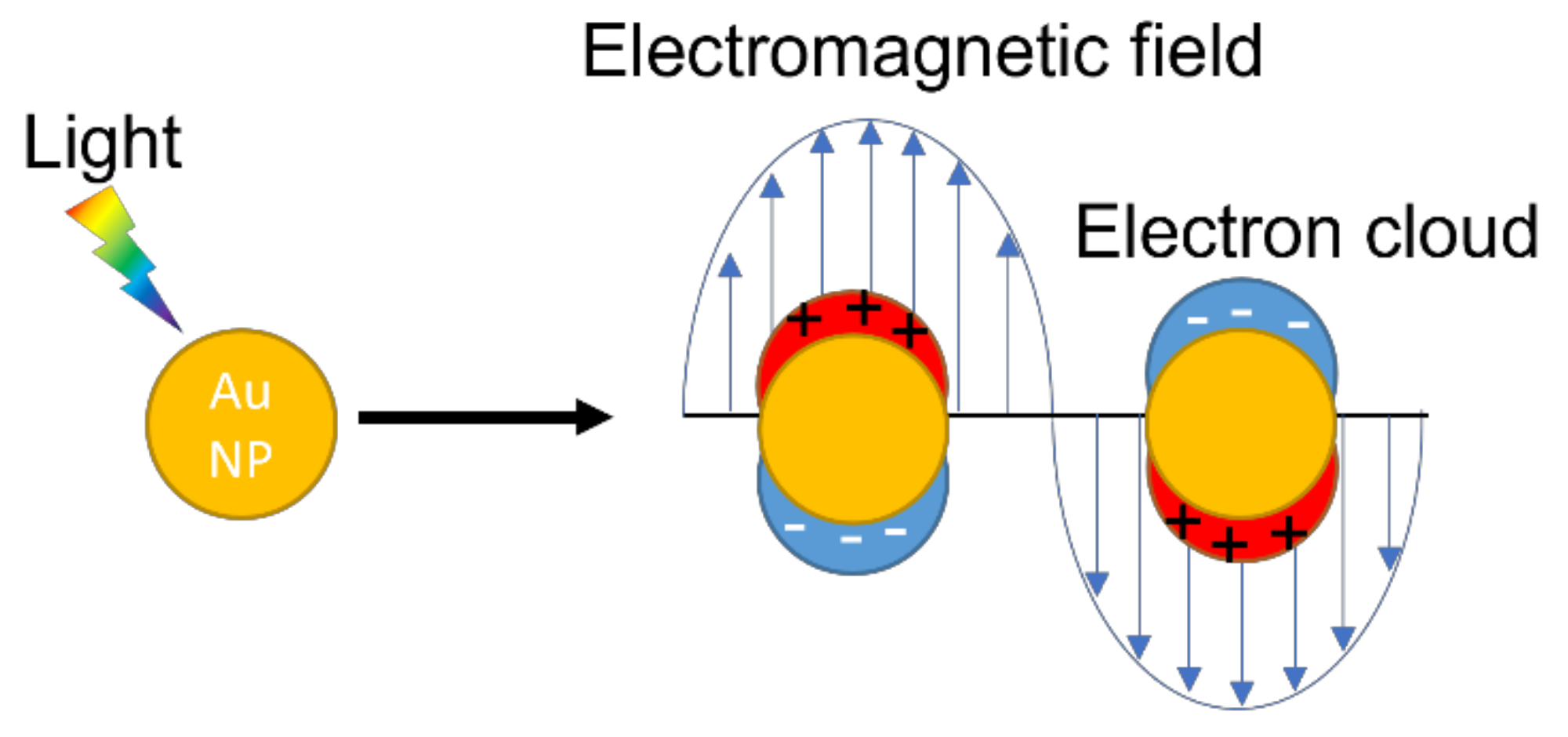
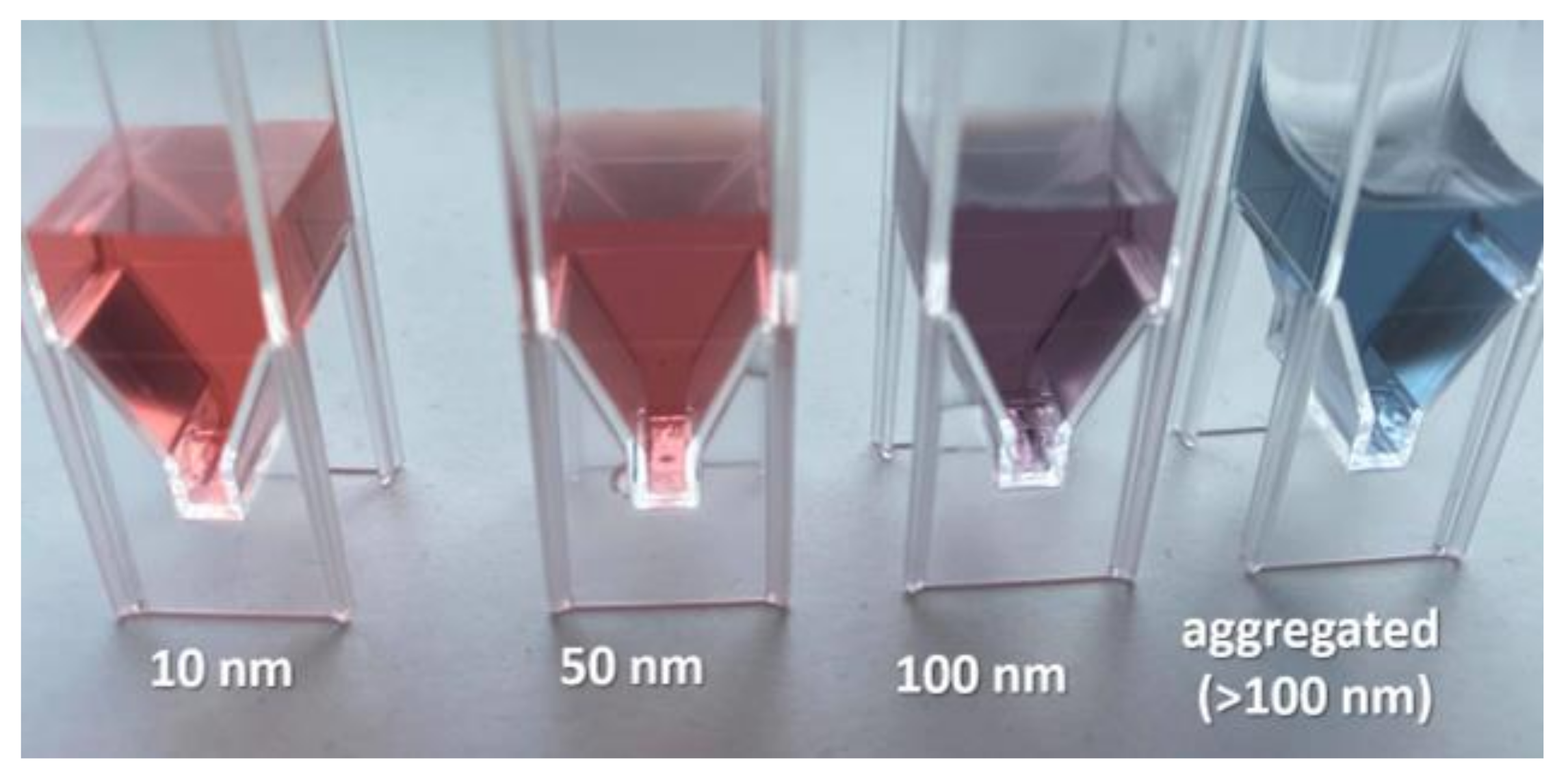
2.2. Properties of AuNPs
3. Methods of Modification of AuNPs by Nucleic Acid Aptamers and Proteins
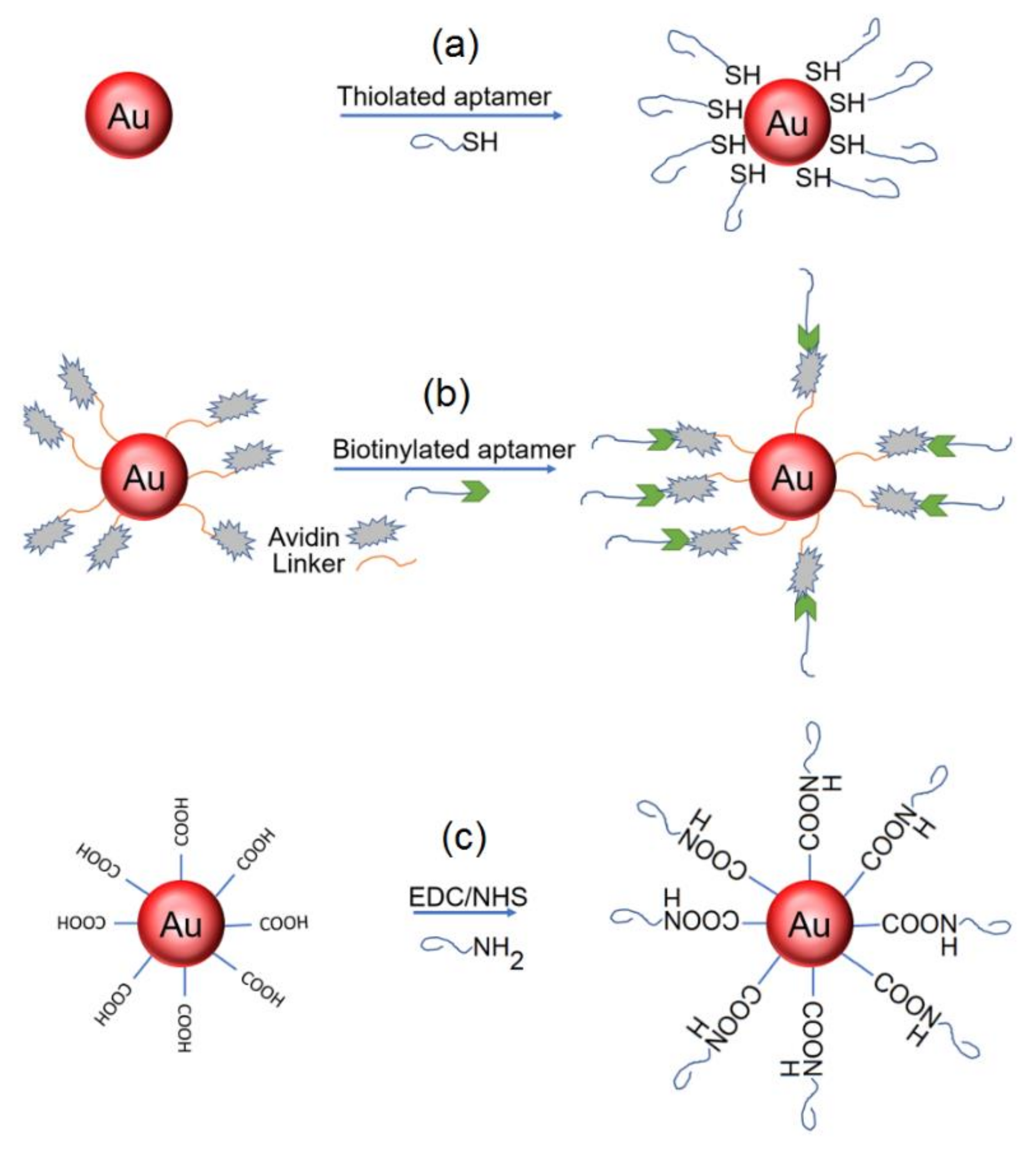
3.1. Modification of AuNPs by Nucleic Acid Aptamers
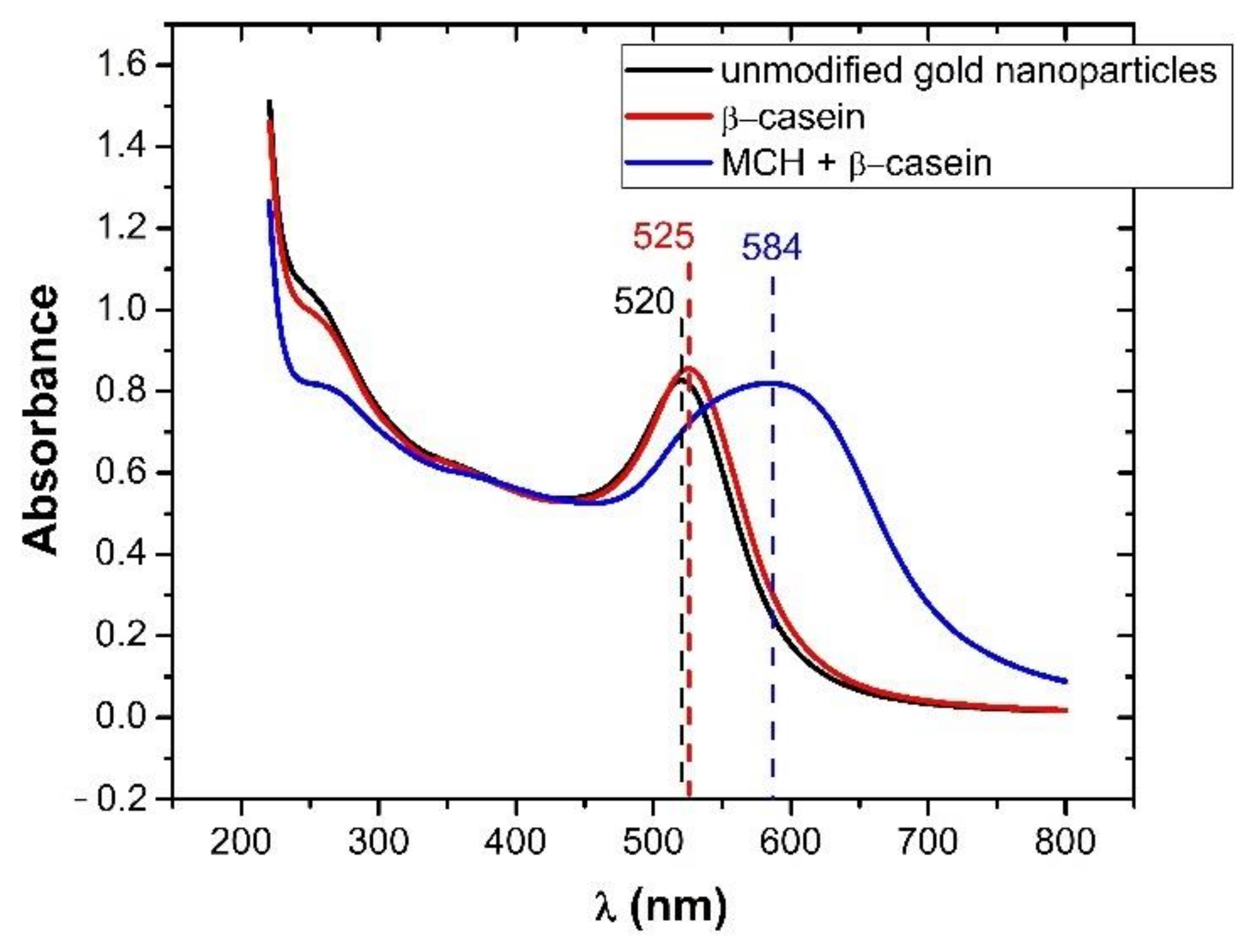
3.2. Modification of AuNPs by Proteins
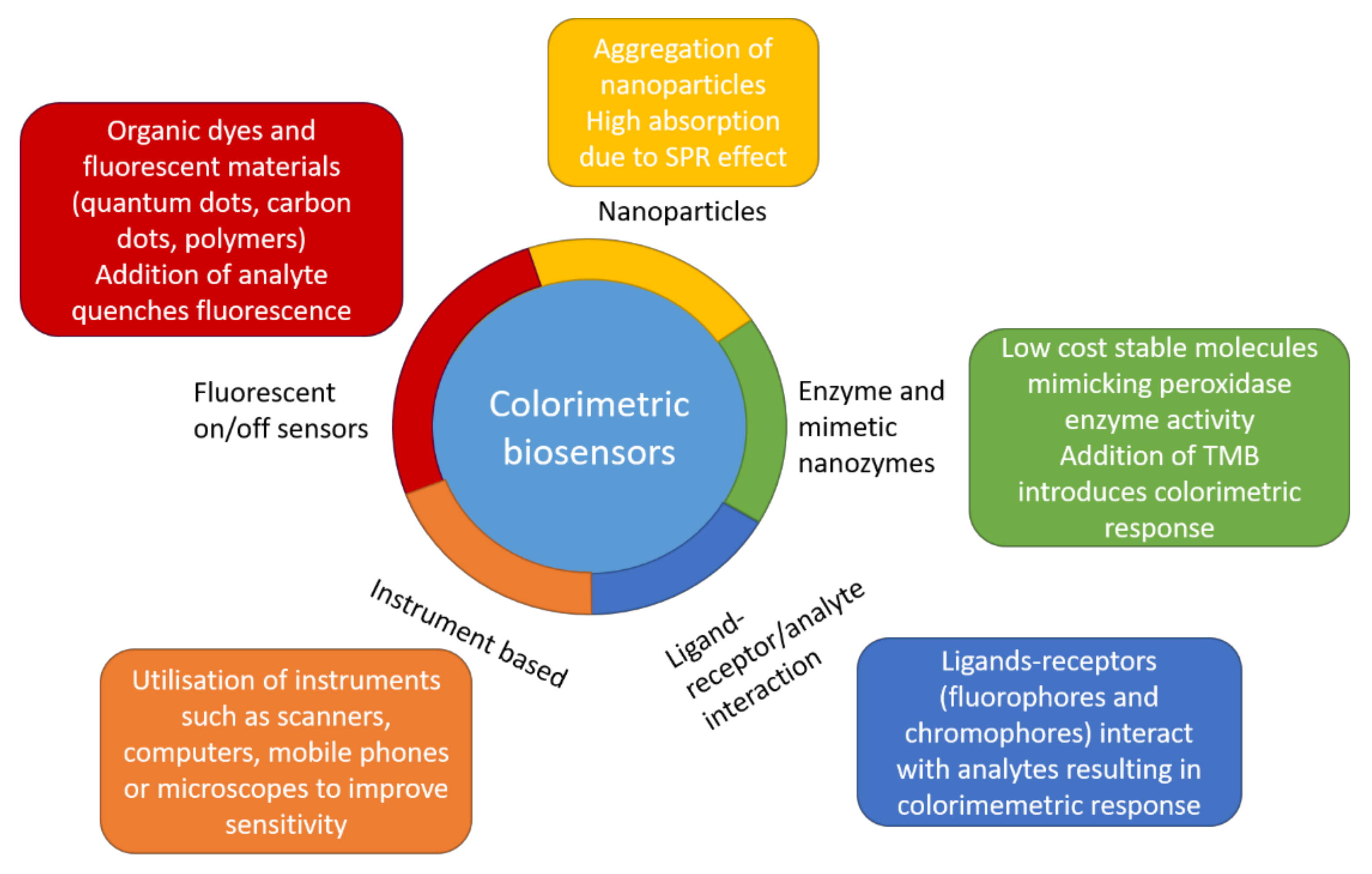
4. Colorimetric Assay Based on AuNPs Modified by Proteins and Peptides
5. Colorimetric Assay Based on AuNPs Modified by Nucleic Acid Aptamers
5.1. Detection of Small Molecules, Proteins, Cancer Markers and Cells by Colorimetry
5.2. Colorimetric Assay Based on AuNPs and DNA Aptamers for Antibiotic Detection
5.3. Colorimetric Assay Based on AuNPs and DNA Aptamers for Detection of Pathogenic Bacteria
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez-Santana, C.; Toscano-Garibay, J.D.; López-López, M.; Coria-Jiménez, V.R. Aptamers coupled to nanoparticles in the diagnosis and treatment of microbial infections. Enferm. Infecc. Microbiol. Clin. 2020, 38, 331–337. [Google Scholar] [CrossRef]
- Ni, X.; Xia, B.; Wang, L.; Ye, J.; Du, G.; Feng, H.; Zhou, X.; Zhang, T.; Wang, W. Fluorescent aptasensor for 17b-estradiol determination based on AuNPs quenching the fluorescence of Rhodamine B. Anal. Biochem. 2017, 523, 17–23. [Google Scholar] [CrossRef]
- António, M.; Ferreira, R.; Vitorino, R.; Daniel-da-Silva, A.L. A simple aptamer-based colorimetric assay for rapid detection of C-reactive protein using AuNPs. Talanta 2020, 214, 120868. [Google Scholar] [CrossRef]
- Majdi, H.; Salehi, R.; Pourhassan-Moghaddam, M.; Mahmoodi, S.; Poursalehi, Z.; Vasilescu, S. Antibody conjugated green synthesized chitosan-AuNPs for optical biosensing. Colloids Interface Sci. Commun. 2019, 33, 100207. [Google Scholar] [CrossRef]
- Lapenna, A.; Dell’Aglio, M.; Palazzo, G.; Mallardi, A. “Naked” AuNPs as colorimetric reporters for biogenic amine detection. Colloids Surf. A 2020, 600, 124903. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zhou, J.; Qi, Q.; Fu, L. A colorimetric and fluorescent gold nanoparticle-based dual-mode aptasensor for parvalbumin detection. Microchem. J. 2020, 159, 105413. [Google Scholar] [CrossRef]
- Shirani, M.; Kalantari, H.; Khodayar, M.J.; Kouchak, M.; Rahbar, N. A novel strategy for detection of small molecules based on aptamer/AuNPs/graphitic carbon nitride nanosheets as fluorescent biosensor. Talanta 2020, 219, 121235. [Google Scholar] [CrossRef]
- Akshaya, K.; Arthi, C.; Pavithra, A.J.; Poovizhi, P.; Shilpa Antinate, S.; Hikku, G.S.; Jeyasubramanian, K.; Murugesan, R. Bioconjugated AuNPs as an efficient colorimetric sensor for cancer diagnostics. Photodiagn. Photodyn Ther. 2020, 30, 101699. [Google Scholar] [CrossRef]
- Yang, T.; Luo, Z.; Tian, Y.; Qian, C.; Duan, Y. Design strategies of AuNPs-based nucleic acid colorimetric biosensors. Trends Anal. Chem. 2020, 124, 115795. [Google Scholar] [CrossRef]
- Holmes, H.N. Experiments in colloid chemistry. J. Chem. Educ. 1941, 18, 349. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Benkovičová, M.; Végsö, K.; Šiffalovič, P.; Jergel, M.; Majková, E.; Luby, Š.; Šatka, A. Preparation of sterically stabilized AuNPs for plasmonic applications. Chem. Pap. 2013, 67, 1225–1230. [Google Scholar] [CrossRef]
- Ivanov, M.R.; Bednar, H.R.; Haes, A.J. Investigations of the mechanism of gold nanoparticle stability and surface functionalization in capillary electrophoresis. ACS Nano 2009, 3, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Bali, K.; Dúzs, B.; Sáfrán, G.; Pécz, B.; Mészáros, R. Effect of added surfactant on poly(Ethylenimine)-assisted gold nanoparticle formation. Langmuir 2019, 35, 14007–14016. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of precision AuNPs using Turkevich method. KONA 2020, 37, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Bartosewicz, B.; Bujno, K.; Liszewska, M.; Budner, B.; Bazarnik, P.; Płociński, T.; Jankiewicz, B.J. Effect of citrate substitution by various α-hydroxycarboxylate anions on properties of AuNPs synthesized by Turkevich method. Colloids Surf. A 2018, 549, 25–33. [Google Scholar] [CrossRef]
- Kettemann, F.; Birnbaum, A.; Witte, S.; Wuithschick, M.; Pinna, N.; Kraehnert, R.; Rademann, K.; Polte, J. Missing piece of the mechanism of the Turkevich method: The critical role of citrate protonation. Chem. Mater. 2016, 28, 4072–4081. [Google Scholar] [CrossRef]
- Perezjuste, J.; Pastorizasantos, I.; Lizmarzan, L.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, J.; Ma, Z. Synthesis of gold nanostars with tunable morphology and their electrochemical application for hydrogen peroxide sensing. Electrochim. Acta 2013, 108, 435–440. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Chen, H.; Dai, S.; Liu, Y. Enhanced off-resonance optical nonlinearities of Au@CdS core-shell nanoparticles embedded in BaTiO3 thin films. Chem. Phys. Lett. 2003, 370, 1–6. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Studies on ligand exchange reaction of functionalized mercaptothiadiazole compounds onto citrate capped AuNPs. Mat. Chem. Phys. 2010, 122, 380–385. [Google Scholar] [CrossRef]
- Iqbal, M.; Usanase, G.; Oulmi, K.; Aberkane, F.; Bendaikha, T.; Fessi, H.; Zine, N.; Agusti, G.; Errachid, E.-S.; Elaissari, A. Preparation of AuNPs and determination of their particles size via different methods. Mat. Res. Bull. 2016, 79, 97–104. [Google Scholar] [CrossRef]
- Kesik, M.; Kanik, F.E.; Hızalan, G.; Kozanoglu, D.; Esenturk, E.N.; Timur, S.; Toppare, L. A Functional immobilization matrix based on a conducting polymer and functionalized AuNPs: Synthesis and its application as an amperometric glucose biosensor. Polymer 2013, 54, 4463–4471. [Google Scholar] [CrossRef]
- Aryal, S.; Remant, B.K.C.; Dharmaraj, N.; Bhattarai, N.; Kim, C.H.; Kim, H.Y. Spectroscopic identification of S-Au interaction in cysteine capped AuNPs. Spectrochim. Acta A 2006, 63, 160–163. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Han, L.; Zhao, Y.; Fan, A. Enhancement effect of p-iodophenol on gold nanoparticle-catalyzed chemiluminescence and its applications in detection of thiols and guanidine. Talanta 2018, 182, 523–528. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised AuNPs in a two-phase liquid–liquid system. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Briñas, R.P.; Maetani, M.; Barchi, J.J. A Survey of place-exchange reaction for the preparation of water-soluble AuNPs. J. Coll. Interface Sci. 2013, 392, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Yang, Y.S.; Ha, S.-C.; Cho, S.M.; Kim, Y.S.; Kim, H.Y.; Yang, H.; Kim, Y.T. Mixed-ligand nanoparticles of chlorobenzenemethanethiol and n-octanethiol as chemical sensors. Sens. Actuat. B 2005, 106, 189–198. [Google Scholar] [CrossRef]
- Dichello, G.A.; Fukuda, T.; Maekawa, T.; Whitby, R.L.D.; Mikhalovsky, S.V.; Alavijeh, M.; Pannala, A.S.; Sarker, D.K. Preparation of liposomes containing small AuNPs using electrostatic interactions. Eur. J. Pharm. Sci. 2017, 105, 55–63. [Google Scholar] [CrossRef]
- Razzaq, H.; Qureshi, R.; Cabo-Fernandez, L.; Schiffrin, D.J. Synthesis of Au clusters-redox centre hybrids by diazonium chemistry employing double layer charged AuNPs. J. Electroanal. Chem. 2018, 819, 9–15. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5−40 nm diameter AuNPs. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated synthesis of AuNPs. Colloids Surf. B. 2010, 80, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of AuNPs. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Gan, P.P.; Ng, S.H.; Huang, Y.; Li, S.F.Y. Green synthesis of AuNPs using palm oil mill effluent (POME): A low-cost and eco-friendly viable approach. Biores. Technol. 2012, 113, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Honey Mediated Green synthesis of AuNPs. Spectrochim. Acta A 2009, 73, 650–653. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Coriander leaf mediated biosynthesis of AuNPs. Mat. Lett. 2008, 62, 4588–4590. [Google Scholar] [CrossRef]
- Arunkumar, P.; Thanalakshmi, M.; Kumar, P.; Premkumar, K. Micrococcus luteus mediated dual mode synthesis of AuNPs: Involvement of extracellular α-amylase and cell wall teichuronic acid. Colloids Surf. B 2013, 103, 517–522. [Google Scholar] [CrossRef]
- Kumar, G.; Abd-Elfattah, A.; Soliman, H.; El-Matbouli, M. Establishment of medium for laboratory cultivation and maintenance of Fredericella sultana for in vivo experiments with Tetracapsuloides bryosalmonae (Myxozoa). J. Fish. Dis. 2013, 36, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasree, S.R.; Suman, T. Extracellular biosynthesis of AuNPs using a Gram negative bacterium Pseudomonas fluorescens. Asian Pac. J. Tropical Dis. 2012, 2, S796–S799. [Google Scholar] [CrossRef]
- Attia, Y.A.; Farag, Y.E.; Mohamed, Y.M.A.; Hussien, A.T.; Youssef, T. Photo-extracellular synthesis of AuNPs using baker’s yeast and their anticancer evaluation against Ehrlich ascites carcinoma Cells. New J. Chem. 2016, 40, 9395–9402. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathy, S.K.; Yun, S.-I. Fungus mediated synthesis of AuNPs and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process Biochem. 2012, 47, 701–711. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S. Biosynthesis of AuNPs: A green approach. J. Photochem. Photobiol. B 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Wu, G. Stabilized and size-tunable AuNPs formed in a quaternary ammonium-based room-temperature ionic liquid under γ-irradiation. Nanotechnology 2005, 16, 2360–2364. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-P.; Gopalan, A.I.; Santhosh, P.; Lee, S.H.; Nho, Y.C. Gamma radiation induced distribution of AuNPs into carbon nanotube-polyaniline composite. Compos. Sci. Technol. 2007, 67, 811–816. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]
- Riabinina, D.; Zhang, J.; Chaker, M.; Margot, J.; Ma, D. Size control of AuNPs synthesized by laser ablation in liquid media. ISRN Nanotechnol. 2012, 2012, 297863. [Google Scholar] [CrossRef] [Green Version]
- Corbierre, M.K.; Beerens, J.; Lennox, R.B. AuNPs generated by electron beam lithography of gold(I)−thiolate thin films. Chem. Mater. 2005, 17, 5774–5779. [Google Scholar] [CrossRef]
- Ogundare, O.D.; Adeoye, M.O.; Adetunji, A.R.; Adewoye, O.O. Production and characterization of AuNPs from itagunmodi gold deposit. In Proceedings of the 1st African International Conference/Workshop on Applications of Nanotechnology to Energy, Health and Environment, UNN, Nsukka, Nigeria, 23–29 March 2014; pp. 117–122. [Google Scholar]
- Jauregui-Gomez, D.; Bermejo-Gallardo, O.M.; Moreno-Medrano, E.D.; Perez-Garcia, M.G.; Ceja, I.; Soto, V.; Carvajal-Ramos, F.; Gutierrez-Becerra, A. Freeze-drying storage method based on pectin for AuNPs. Nanomat. Nanotechnol. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. AuNPs: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Pereira, E.; Baptista, P.; Gomes, I.; Figueiredo, S.; Soares, L.; Franco, R. AuNPs as (bio)chemical sensors. In Comprehensive Analytical Chemistry. AuNPs in Analytical Chemistry, 1st ed.; Valcárcel, M., López-Lorente, A.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 529–567. ISBN 978-0-444-63285-2. [Google Scholar]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in AuNPs: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with AuNPs: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef] [Green Version]
- He, Y.Q.; Liu, S.P.; Kong, L.; Liu, Z.F. A study on the sizes and concentrations of AuNPs by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim. Acta A 2005, 61, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Li, X. Optimal size of AuNPs for surface-enhanced Raman spectroscopy under different conditions. J. Nanomat. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Piovarci, I.; Hianik, T.; Ivanov, I.N. Detection of chymotrypsin by optical and acoustic methods. Biosensors 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Nanomaterials exhibiting enzyme-like properties (nanozymes): Current advances and future perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. AuNPs of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of AuNPs through the blood brain barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katas, H.; Lim, C.S.; Nor Azlan, A.Y.H.; Buang, F.; Mh Busra, M.F. Antibacterial activity of biosynthesized AuNPs using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef]
- Urmann, K.; Modrejewski, J.; Scheper, T.; Walter, J.-G. Aptamer-modified nanomaterials: Principles and applications. BioNanoMaterials 2017, 18, 20160012. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Liu, H.; Zhang, X.; Tan, W. Aptamer-conjugated AuNPs for bioanalysis. Nanomedicine 2013, 8, 983–993. [Google Scholar] [CrossRef]
- Chung, C.H.; Kim, J.H.; Jung, J.; Chung, B.H. Nuclease-resistant DNA aptamer on AuNPs for the simultaneous detection of Pb2+ and Hg2+ in human serum. Biosens. Bioelectron. 2013, 41, 827–832. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.; Xu, Q.; Wang, J.; Wang, L.; Lou, X. Zeta-potential data reliability of gold nanoparticle biomolecular conjugates and its application in sensitive quantification of surface absorbed protein. Colloids Surf. B. 2016, 148, 541–548. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Y.; Li, X.; Zhou, T.; Ma, H.; Qiang, H.; Liu, Y. Colorimetric detection of potassium ions using aptamer-functionalized AuNPs. Anal. Chim. Acta 2013, 787, 189–192. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Ceña, V.; Majoral, J.-P. Dendrimer–and polymeric nanoparticle–aptamer bioconjugates as nonviral delivery systems: A new approach in medicine. Drug Discov. Today 2020, 25, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Ismail, S.I. Aptamers chemistry: Chemical modifications and conjugation strategies. Molecules 2020, 25, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iosin, M.; Toderas, F.; Baldeck, P.L.; Astilean, S. Study of protein–gold nanoparticle conjugates by fluorescence and surface-enhanced Raman scattering. J. Mol. Struct. 2009, 924–926, 196–200. [Google Scholar] [CrossRef]
- Chirra, H.D.; Sexton, T.; Biswal, D.; Hersh, L.B.; Hilt, J.Z. Catalase-coupled AuNPs: Comparison between the carbodiimide and biotin–streptavidin methods. Acta Biomater. 2011, 7, 2865–2872. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: AuNPs for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Selegård, R.; Aili, D.; Liedberg, B. Peptide functionalized AuNPs for colorimetric detection of matrilysin (MMP-7) activity. Nanoscale 2013, 5, 8973. [Google Scholar] [CrossRef]
- Laromaine, A.; Koh, L.; Murugesan, M.; Ulijn, R.V.; Stevens, M.M. Protease-triggered dispersion of nanoparticle assemblies. J. Am. Chem. Soc. 2007, 129, 4156–4157. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, L.; Wang, X.; Xiang, Y.; Tong, A. A new colorimetric strategy for monitoring caspase 3 activity by HRP-mimicking DNAzyme–peptide conjugates. Analyst 2014, 139, 1178–1183. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, K.H.; Park, Y.H.; Ko, S.; Kim, Y.-P. Colorimetric assay of matrix metalloproteinase activity based on metal-induced self-assembly of carboxy AuNPs. Biosens. Bioelectron. 2013, 41, 833–839. [Google Scholar] [CrossRef]
- Choi, Y.; Ho, N.-H.; Tung, C.-H. Sensing phosphatase activity by using AuNPs. Angew. Chem. Int. Ed. 2007, 46, 707–709. [Google Scholar] [CrossRef]
- Serizawa, T.; Hirai, Y.; Aizawa, M. Detection of enzyme activities based on the synthesis of AuNPs in HEPES buffer. Mol. BioSyst. 2010, 6, 1565. [Google Scholar] [CrossRef]
- Lévy, R. Peptide-capped AuNPs: Towards artificial proteins. ChemBioChem 2006, 7, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Andresen, H.; Ghadiali, J.E.; Stevens, M.M. Kinase-actuated immunoaggregation of peptide-conjugated AuNPs. Small 2010, 6, 1509–1513. [Google Scholar] [CrossRef]
- Oishi, J.; Asami, Y.; Mori, T.; Kang, J.-H.; Tanabe, M.; Niidome, T.; Katayama, Y. Measurement of homogeneous kinase activity for cell lysates based on the aggregation of AuNPs. ChemBioChem 2007, 8, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Guarise, C.; Pasquato, L.; De Filippis, V.; Scrimin, P. AuNPs-based protease assay. Proc. Natl. Acad. Sci. USA 2006, 103, 3978–3982. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Ge, D.; Yang, K.-L. Colorimetric protease assay by using AuNPs and oligopeptides. Sens. Actuators B 2014, 201, 234–239. [Google Scholar] [CrossRef]
- Chandrawati, R.; Stevens, M.M. Controlled assembly of peptide-functionalized AuNPs for label-free detection of blood coagulation Factor XIII activity. Chem. Commun. 2014, 50, 5431. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Xie, Y.; Zhang, H.; Wang, P.; Cheung, H.-Y.; Yang, M.; Sun, H. A general colorimetric method for detecting protease activity based on peptide-induced gold nanoparticle aggregation. RSC Adv. 2014, 4, 6560–6563. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Li, J.-C.; Chen, S.-H.; Liu, T.-Y.; Kuo, C.-H.; Huang, W.-T.; Lin, C.-S. An optical biosensing platform for proteinase activity using AuNPs. Biomaterials 2010, 31, 6087–6095. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, P.; Wang, Y.; Zhang, J.; Aili, D.; Liedberg, B. Biofunctionalized AuNPs for colorimetric sensing of botulinum neurotoxin A light chain. Anal. Chem. 2014, 86, 2345–2352. [Google Scholar] [CrossRef]
- Liu, P.; Han, L.; Wang, F.; Petrenko, V.A.; Liu, A. Gold nanoprobe functionalized with specific fusion protein selection from phage display and its application in rapid, selective and sensitive colorimetric biosensing of Staphylococcus aureus. Biosens. Bioelectron. 2016, 82, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Piovarci, I.; Melikishvili, S.; Tatarko, M.; Hianik, T.; Thompson, M. Detection of sub-nanomolar concentration of trypsin by thickness-shear mode acoustic biosensor and spectrophotometry. Biosensors 2021, 11, 117. [Google Scholar] [CrossRef]
- Sajjanar, B.; Kakodia, B.; Bisht, D.; Saxena, S.; Singh, A.K.; Joshi, V.; Tiwari, A.K.; Kumar, S. Peptide-activated AuNPs for selective visual sensing of virus. J. Nanopart. Res. 2015, 17, 234. [Google Scholar] [CrossRef]
- Shinde, S.; Kim, D.-Y.; Saratale, R.; Syed, A.; Ameen, F.; Ghodake, G. A spectral probe for detection of aluminum (III) ions using surface functionalized AuNPs. Nanomaterials 2017, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chansuvarn, W.; Imyim, A. Visual and colorimetric detection of mercury(II) ion using AuNPs stabilized with a dithia-diaza ligand. Microchim. Acta 2012, 176, 57–64. [Google Scholar] [CrossRef]
- Wei, H.; Li, B.; Li, J.; Dong, S.; Wang, E. DNAzyme-based colorimetric sensing of lead (Pb2+) using unmodified gold nanoparticle probes. Nanotechnology 2008, 19, 095501. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Wang, Q.; Luo, Y.; Gao, Y. Detection of Ag+ ions via an anti-aggregation mechanism using unmodified AuNPs in the presence of thiamazole. Talanta 2021, 222, 121506. [Google Scholar] [CrossRef]
- Karami, C.; Taher, M.A. Colorimetric sensor of cobalt ions in aqueous solution using AuNPs modified with glycyrrhizic acid. Plasmonics 2018, 13, 1315–1323. [Google Scholar] [CrossRef]
- Chen, N.; Chen, J.; Yang, J.-H.; Bai, L.-Y.; Zhang, Y.-P. Colorimetric detection of cadmium ions using DL-mercaptosuccinic acid-modified AuNPs. J. Nanosci. Nanotechnol. 2016, 16, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, C.; Lu, L.; Yang, F.; Yang, X. A label-free colorimetric sensor for sulfate based on the inhibition of peroxidase-like activity of cysteamine-modified AuNPs. Sens. Actuators B 2015, 215, 437–444. [Google Scholar] [CrossRef]
- McVey, C.; Logan, N.; Thanh, N.T.K.; Elliott, C.; Cao, C. Unusual switchable peroxidase-mimicking nanozyme for the determination of proteolytic biomarker. Nano Res. 2019, 12, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ji, J.; Wang, D.; Gou, S.; Xue, Z.; Zhao, L.; Feng, S. Highly sensitive and selective colorimetric sensing of histidine by NAC functionalized AuNPs in aqueous medium with real sample application. Microchem. J. 2021, 160, 105661. [Google Scholar] [CrossRef]
- Aili, D.; Selegård, R.; Baltzer, L.; Enander, K.; Liedberg, B. Colorimetric protein sensing by controlled assembly of AuNPs functionalized with synthetic receptors. Small 2009, 5, 2445–2452. [Google Scholar] [CrossRef]
- Andresen, H.; Mager, M.; Grießner, M.; Charchar, P.; Todorova, N.; Bell, N.; Theocharidis, G.; Bertazzo, S.; Yarovsky, I.; Stevens, M.M. Single-step homogeneous immunoassays utilizing epitope-tagged AuNPs: On the mechanism, feasibility, and limitations. Chem. Mater. 2014, 26, 4696–4704. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress on the construction of nanozymes-based biosensors and their applications to food safety assay. Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advance in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Stobiecka, M.; Coopersmith, K.; Hepel, M. Resonance elastic light scattering (RELS) spectroscopy of fast non-Langmuirian ligand-exchange in glutathione-induced gold nanoparticle assembly. J. Colloids Interface Sci. 2010, 350, 168–177. [Google Scholar] [CrossRef]
- Stobiecka, M.; Jakiela, S.; Chalupa, A.; Bednarczyk, P.; Dworakowska, B. Mitochondria-based biosensors with piezometric and RELS transduction for potassium uptake and release investigations. Biosens. Bioelectron. 2017, 88, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M. Novel plasmonic field-enhanced nanoassay for trace detection of proteins. Biosens. Bioelectron. 2014, 55, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Chalupa, A. Modulation of plasmon-enhanced resonance energy transfer to gold nanoparticles by protein survivin channeled-shell gating. J. Phys. Chem. B 2015, 119, 13227–13235. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2019, 229, 115463. [Google Scholar] [CrossRef]
- Grel, H.; Ratajczak, K.; Jakiela, S.; Stobiecka, M. Gated resonance energy transfer (gRET) controlled by programmed death protein Ligand 1. Nanomaterials 2020, 10, 1592. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kim, B.C. A colorimetric assay for detection of 6-OH-BDE-47 using 6-OH-BDE-47-specific aptamers and AuNPs. Sens. Actuators B 2017, 248, 298–304. [Google Scholar] [CrossRef]
- Smith, J.E.; Griffin, D.K.; Leny, J.K.; Hagen, J.A.; Chávez, J.L.; Kelley-Loughnane, N. Colorimetric detection with aptamer-gold nanoparticle conjugates coupled to an android-based color analysis application for use in the field. Talanta 2014, 121, 247–255. [Google Scholar] [CrossRef] [PubMed]
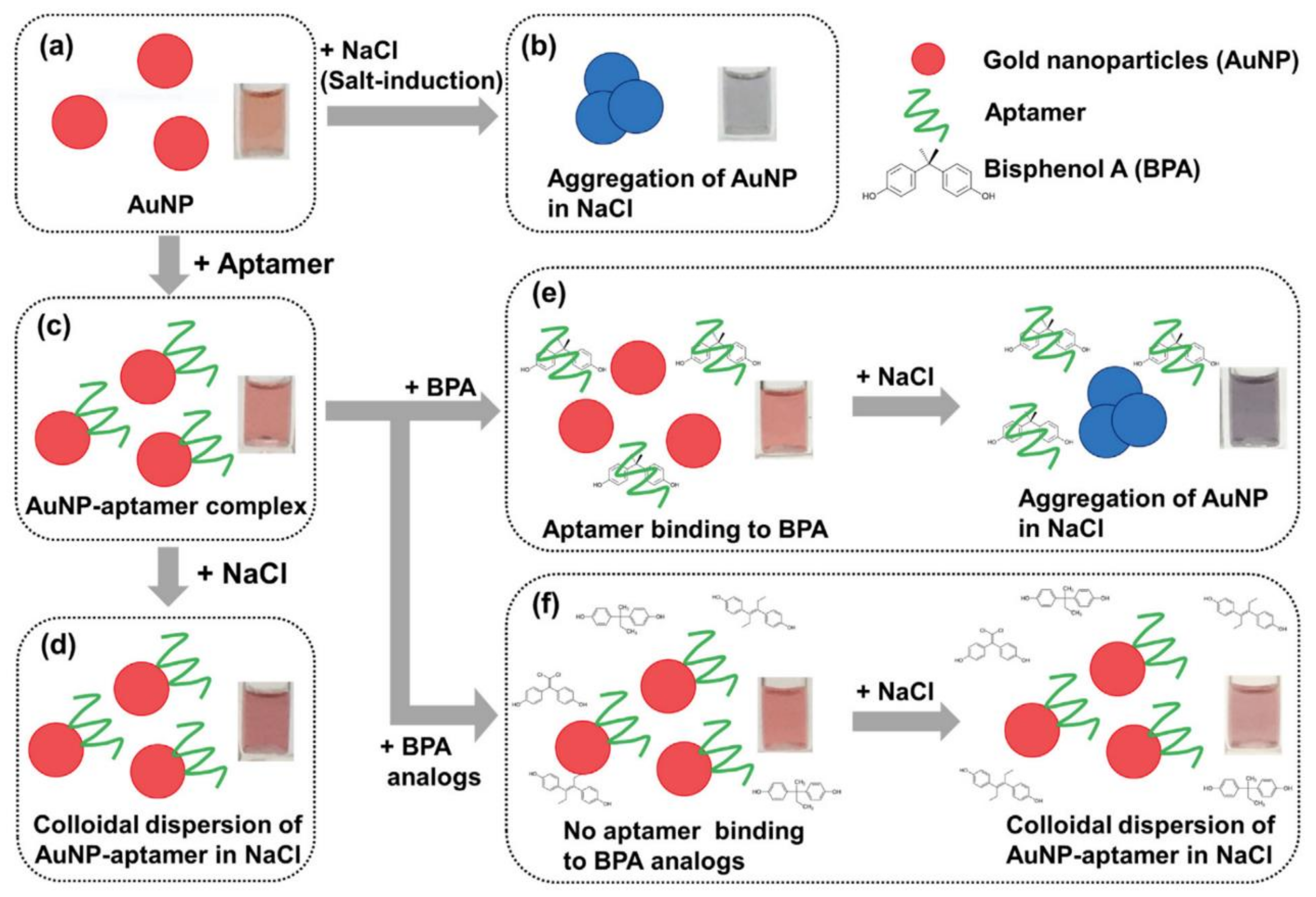
- Lee, E.-H.; Lee, S.K.; Kim, M.J.; Lee, S.-W. Simple and rapid detection of bisphenol A using a gold nanoparticle-based colorimetric aptasensor. Food Chem. 2019, 287, 205–213. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Ye, J.; Xu, L.; Xu, H.; Zhan, S.; Xia, B.; Wang, L. Colorimetric detection of bisphenol A based on unmodified aptamer and cationic polymer aggregated AuNPs. Anal. Biochem. 2016, 499, 51–56. [Google Scholar] [CrossRef]
- Peng, Y.; Li, L.; Mu, X.; Guo, L. Aptamer-gold nanoparticle-based colorimetric assay for the sensitive detection of thrombin. Sens. Actuators B 2013, 177, 818–825. [Google Scholar] [CrossRef]
- Shayesteh, O.H.; Ghavami, R. A novel label-free colorimetric aptasensor for sensitive determination of PSA biomarker using AuNPs and a cationic polymer in human serum. Spectrochim. Acta 2020, 226, 117644. [Google Scholar] [CrossRef]
- Borghei, Y.-S.; Hosseini, M.; Dadmehr, M.; Hosseinkhani, S.; Ganjali, M.R.; Sheikhnejad, R. Visual detection of cancer cells by colorimetric aptasensor based on aggregation of AuNPs induced by DNA hybridization. Anal. Chim. Acta 2016, 904, 92–97. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in AuNPs-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Huang, P.; Wu, F.-Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020, 304, 125377. [Google Scholar] [CrossRef]
- Javidi, M.; Housaindokht, M.R.; Verdian, A.; Razavizadeh, B.M. Detection of chloramphenicol using a novel apta-sensing platform based on aptamer terminal-lock in milk samples. Anal. Chim. Acta 2018, 1039, 116–123. [Google Scholar] [CrossRef]
- Birader, K.; Kumar, P.; Tammineni, Y.; Alice, J.B.; Reddy, S.; Suman, P. Colorimetric aptasensor for on-site detection of oxytetracycline antibiotic in milk. Food Chem. 2021, 356, 129659. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, X.; Lai, Y. Colorimetric aptasensor for sensitive detection of kanamycin based on target-triggered catalytic hairpin assembly amplification and DNA-gold nanoparticle probes. Microchem. J. 2021, 162, 105858. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Jia, J.; Xiang, Y. Colorimetric aptasensors for determination of tobramycin in milk and chicken eggs based on DNA and AuNPs. Food Chem. 2018, 249, 98–103. [Google Scholar] [CrossRef]
- Majdinasab, A.; Mishra, K.R.; Tang, X.; Marty, J.L. Detection of antibiotics in food: New achievements in the development of biosensors. Trends Anal. Chem. 2020, 127, 115883. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Lai, G.; Liu, S.; Li, B.; Yu, A. Sensitive and rapid aptasensing of chloramphenicol by colorimetric signal transduction with a DNAzyme-functionalized gold nanoprobe. Food Chem. 2019, 270, 287–292. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J.; Li, Y.; Gao, H.; Guo, J.; Shen, F.; Sun, C. A novel colorimetric aptasensor using cysteamine-stabilized AuNPs as probe for rapid and specific detection of tetracycline in raw milk. Food Contr. 2015, 54, 7–15. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Li, B.; Du, B. Aptamer biorecognition-triggered hairpin switch and nicking enzyme assisted signal amplification for ultrasensitive colorimetric bioassay of kanamycin in milk. Food Chem. 2021, 339, 128059. [Google Scholar] [CrossRef]
- Luan, Q.; Miao, Y.; Gan, N.; Cao, Y.; Li, T.; Chen, Y. A POCT colorimetric aptasensor for streptomycin detection using porous silica beads- enzyme linked polymer aptamer probes and exonuclease-assisted target recycling for signal amplification. Sens. Actuators B 2017, 251, 349–358. [Google Scholar] [CrossRef]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar Chhillar, A.; Singh Rana, J. Detection of pathogenic bacteria with special emphasis to biosensors integrated with AuNPs. Sens. Int. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Subjakova, V.; Oravczova, V.; Tatarko, M.; Hianik, T. Advances in electrochemical aptasensors and immunosensors for detection of bacterial pathogens in food. Electrochim. Acta 2021, 389, 138724. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef] [PubMed]
- Trunzo, E.N.; Hong, K.L. Recent progress in the identification of aptamers against bacteria origins and their diagnostic applications. Int. J. Molec. Sci. 2020, 21, 5074. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wu, S.; Duan, N.; Ma, X.; Xia, Y.; Chen, J.; Ding, Z.; Wang, Z. A sensitive gold nanoparticle-based colorimetric aptasensor for Staphylococcus aureus. Talanta 2014, 127, 163–168. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Q.; Wu, J.; Dai, Z.; Wang, Y. Naked-eyes detection of Shigella flexneri in food samples based on a novel gold nanoparticle-based colorimetric aptasensor. Food Control 2019, 98, 333–341. [Google Scholar] [CrossRef]
- Du, J.; Singh, H.; Dong, W.-j.; Bai, Y.-h.; Yi, T.-H. Colorimetric detection of Listeria monocytogenes using one-pot biosynthesized flower-shaped AuNPs. Sens. Actuators B 2018, 265, 285–292. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Li, J.; Wu, J. A trigger-based aggregation of aptamer-functionalized AuNPs for colorimetry: An example on detection of Escherichia coli O157:H7. Sens. Actuators B 2021, 339, 129865. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, S.; Song, X.; Xu, K.; Wang, J.; Li, J.; Zhao, C.; Jin, M. One-step colorimetric detection of Staphylococcus aureus based on target-induced shielding against the peroxidase mimicking activity of aptamer-functionalized gold-coated iron oxide nanocomposites. Talanta 2021, 232, 122448. [Google Scholar] [CrossRef]
- Ozalp, V.C.; Bayramoglu, G.; Erdem, Z.; Arica, M.Y. Pathogen detection in complex samples by quartz crystal microbalance sensor coupled to aptamer functionalized core–shell type magnetic separation. Anal. Chim. Acta 2015, 853, 533–540. [Google Scholar] [CrossRef]
- Tatarko, M.; Spagnolo, S.; Oravczova, V.; Süle, J.; Hun, M.; Hucker, A.; Hianik, T. Changes of viscoelastic properties of aptamer sensing layers following interaction with Listeria innocua. Sensors 2021, 21, 5585. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, F.; Wang, R.; Li, Y. Whole-bacterium SELEX of DNA aptamers for rapid detection of E. coli O157:H7 using a QCM sensor. J. Biotechnol. 2018, 266, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, J.; Pang, B.; Wang, X.; Shi, Y.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of AuNPs. Microchim. Acta 2020, 187, 504. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Z.; Song, Y.; Yang, X.; Chen, S.; Fu, S.; Qin, X.; Zhang, W.; Man, C.; Jiang, Y. A novel smartphone-based colorimetric aptasensor for on-site detection of Escherichia coli O157:H7 in milk. J. Dairy Sci. 2021, 104, 8506–8516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Yao, S.; Shang, M.; Zhao, C.; Li, J.; Wang, J. A multicolor sensing system for simultaneous detection of four foodborne pathogenic bacteria based on Fe3O4/MnO2 nanocomposites and the etching of gold nanorods. Food Chem. Toxicol. 2021, 14, 112035. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A.; Kumar, S.; Pinnaka, A.K.; Singhal, N.K. Naked eye colorimetric detection of Escherichia coli using aptamer conjugated graphene oxide enclosed AuNPs. Sens. Actuators B 2021, 329, 129100. [Google Scholar] [CrossRef]
- Duan, N.; Yang, W.; Wu, S.; Zou, Y.; Wang, Z. A Visual and sensitive detection of Escherichia coli based on aptamer and peroxidase-like mimics of copper-metal organic framework nanoparticles. Food Anal. Meth. 2020, 13, 1433–14411. [Google Scholar] [CrossRef]
- Dehghani, Z.; Hosseini, M.; Mohammadnejad, J.; Bakhshi, B.; Rezayan, A.H. Colorimetric aptasensor for Campylobacter jejuni cells by exploiting the peroxidase like activity of Au@Pd nanoparticles. Microchim. Acta 2018, 185, 448. [Google Scholar] [CrossRef]
- Chen, W.; Teng, J.; Yao, L.; Xu, J.; Liu, G. Selection of specific DNA aptamers for hetero-sandwich-based colorimetric determination of Campylobacter jejuni in food. J. Agricult. Food Chem. 2020, 68, 8455–8461. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-S.; Chon, J.-W.; Kim, D.-H.; Hyeon, J.-Y.; Seo, K.-H. New colorimetric aptasensor for rapid on-site detection of Campylobacter jejuni and Campylobacter coli in chicken carcass samples. Anal. Chim. Acta 2018, 1029, 78–85. [Google Scholar] [CrossRef]
- Shin, W.R.; Sekhon, S.S.; Rhee, S.K.; Ko, J.H.; Ahn, J.Y.; Min, J.; Kim, Y.H. Aptamer-based paper strip sensor for detecting Vibrio fischeri. ACS Comb. Sci. 2018, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hong, Y.; Xiao, Z.; Zhou, Y.; Jiang, Y.; Huang, M.; Xu, X.; Zhou, G. Colorimetric determination of: Salmonella typhimurium based on aptamer recognition. Anal. Meth. 2016, 8, 6560–6565. [Google Scholar] [CrossRef]
- Man, Y.; Ban, M.; Li, A.; Jin, X.; Du, Y.; Pan, L. A microfluidic colorimetric biosensor for in-field detection of Salmonella in fresh-cut vegetables using thiolated polystyrene microspheres, hose-based microvalve and smartphone imaging APP. Food Chem. 2021, 354, 129578. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2018, 218, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zeng, J.; Wang, J.; Wang, X.; Wang, Y.; Gan, N. Dual-mode aptasensor for simultaneous detection of multiple food-borne pathogenic bacteria based on colorimetry and microfluidic chip using stir bar sorptive extraction. Microchim. Acta 2021, 188, 244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Hu, H.; Huang, Y.; Yang, X.; Wang, Q.; Chen, X. Improving the detection limit of Salmonella colorimetry using long ssDNA of asymmetric-PCR and non-functionalized AuNPs. Anal. Biochem. 2021, 626, 114229. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.; He, L.; Tan, L.; Jia, Z.; Gan, N. The universal dual-mode aptasensor for simultaneous determination of different bacteria based on naked eyes and microfluidic-chip together with magnetic DNA encoded probes. Talanta 2021, 225, 122062. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, R.; Jia, L. Enhancement of the peroxidase-like activity of aptamers modified gold nanoclusters by bacteria for colorimetric detection of Salmonella typhimurium. Talanta 2021, 221, 121476. [Google Scholar] [CrossRef]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Y.; Jiang, F.; Zhou, J.; Li, Y.; Liang, C.; Dang, L.; Lu, A.; Zhang, G. Development of Cell-SELEX Technology and its application in cancer diagnosis and therapy. Int. J. Mol. Sci. 2016, 17, 2079. [Google Scholar] [CrossRef] [Green Version]

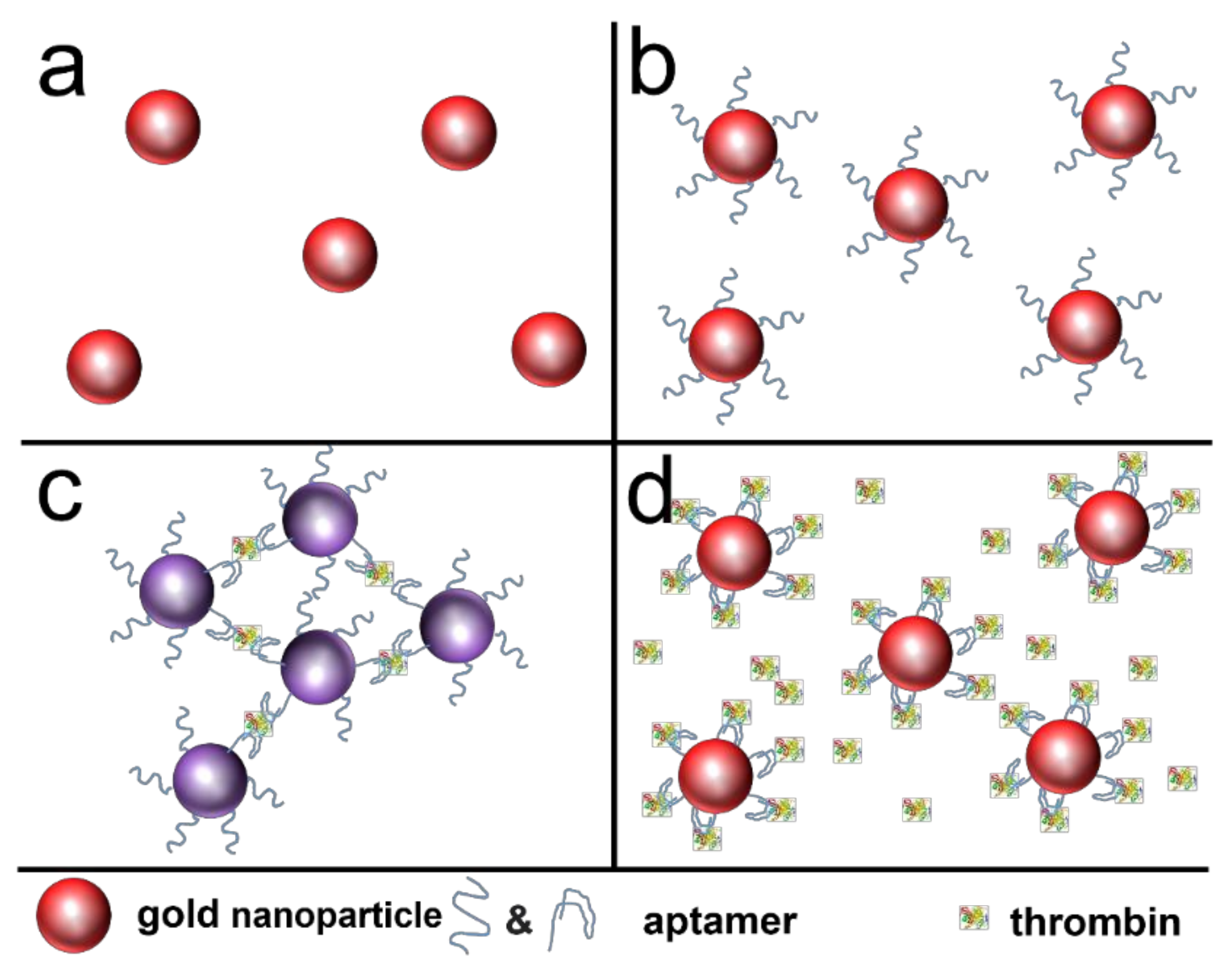

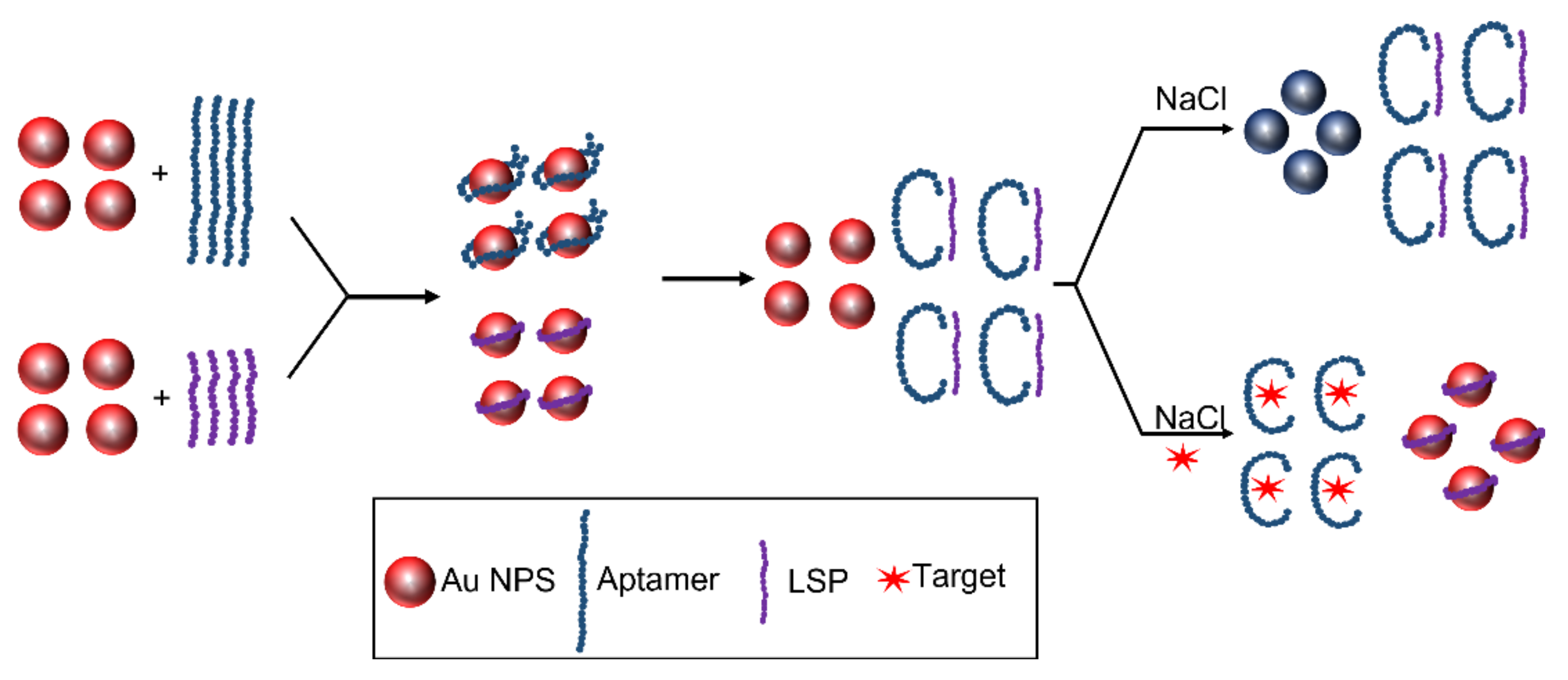

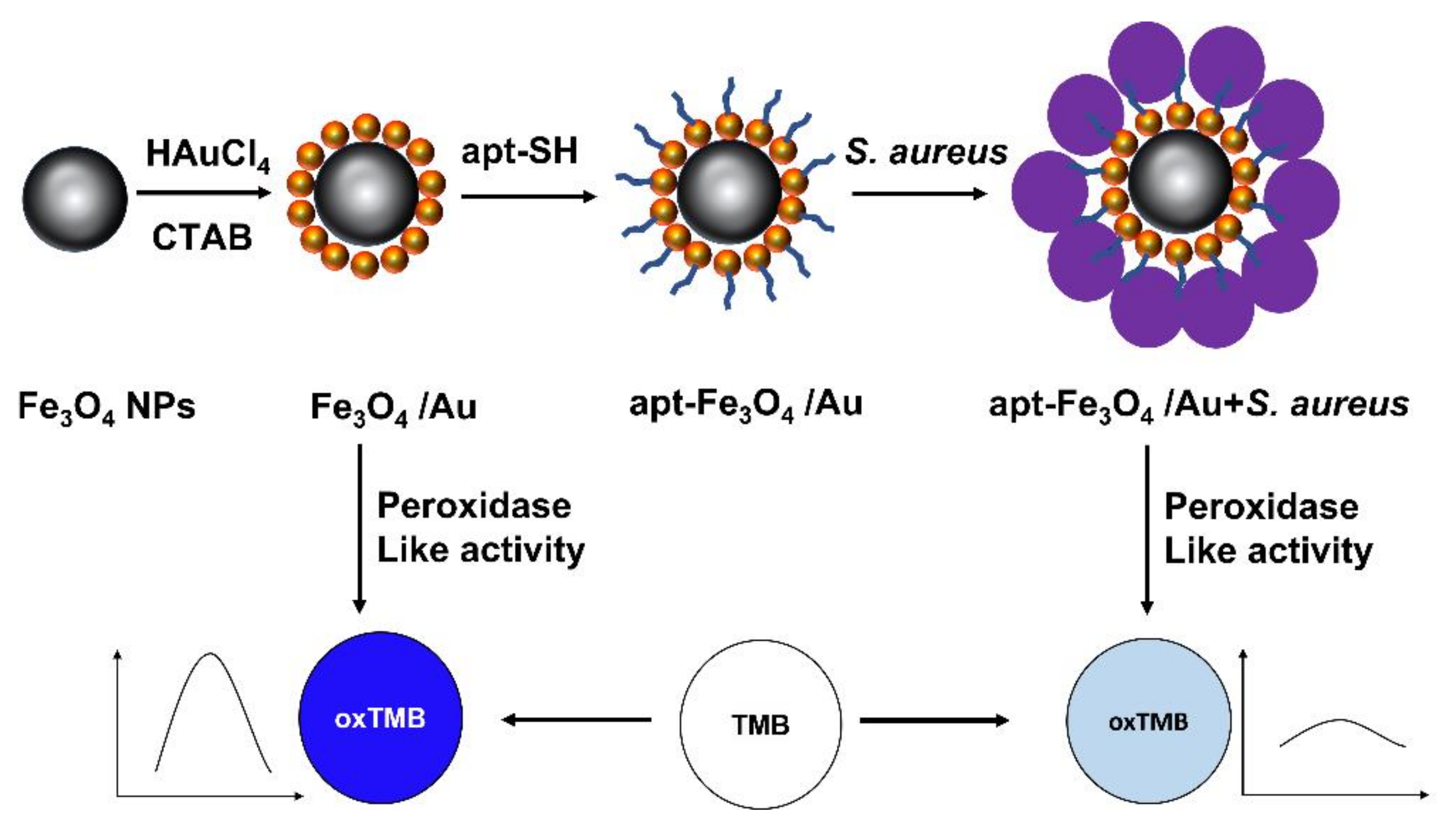

| Method | Reduction Agent | Stabilization Agent | Size [nm] | References |
|---|---|---|---|---|
| Bottom to top (from HAuCl4) | ||||
| Turkevich method | Sodium citrate | Citrate ions | 5–40 | [11] |
| Modified Turkevich method | Sodium borohydrate | Citrate ions | 7–30 | [24,25,26,27,28] |
| Burst-Schiffrin | Sodium borohydrate | Thiols | 2–30 | [30,31,32,33] |
| ”Green” methods | Plant extracts Bacteria Fungi Yeast | Various chemicals present in plants and microorganism | 2.4–200 | [35,36,37,38,39,40,41,42,43,44,45] |
| Radiolytic method | Gamma radiation | Ionic liquid | 10–50 | [46] |
| Top to bottom (from bulk gold) | ||||
| Laser ablation | N/A | Surfactants | 3–13 | [49] |
| Lithography | N/A | Thiols | 2–4.5 | [50] |
| Milling method | N/A | Thiols | 24–36 | [51] |
| Parameters | TSM Biosensor | AuNPs Assay |
|---|---|---|
| Detection time | 30 min | 30 min |
| KM | 0.92 ± 0.44 nM | 0.56 ± 0.10 nM |
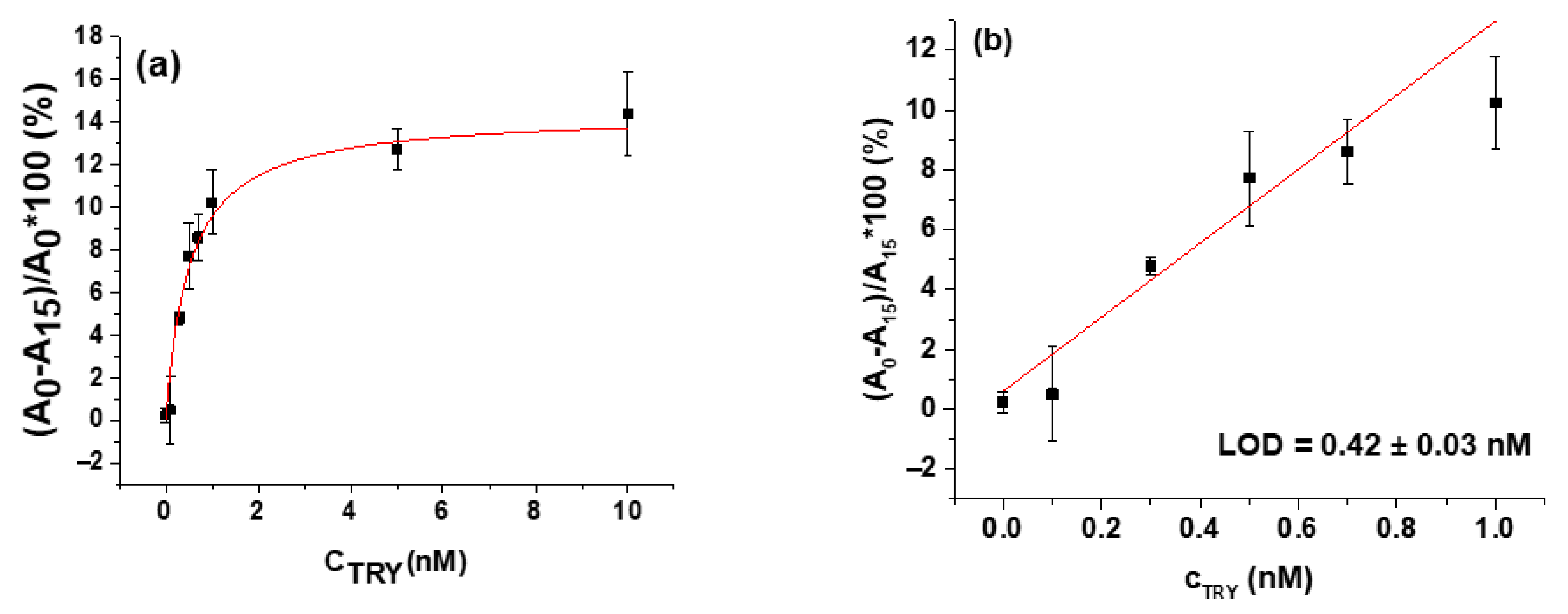
| Limit of detection | 0.48 ± 0.08 nM | 0.42 ± 0.03 nM |
| Signal detection | Acoustic wave at surface | UV-vis absorbance in a volume |
| Protease | Modification of AuNPs | LOD | Detection Time | References |
|---|---|---|---|---|
| MMP-7 | Peptide | 5 nM | 30 min | [74] |
| MMP-7 | carboxy-PEG-thiol | 10 nM | 2 h | [77] |
| Thermolysin | peptide | 90 zg/mL | 2 h | [75] |
| Phosphatase | peptide | 34 nM | 4 min | [78] |
| Kinase | peptide | 1.5 nM | 2 h | [81] |
| Kinase | peptide | 0.125 mg/mL | 60 min | [82] |
| Thrombin | peptide | 5 nM | 60 min | [83] |
| Lethal factor of Bacillus anthracis | peptide | 25 nM | 60 min | [83] |
| Blood coagulation factor XIII | peptide | 0.01 U/mL | 2 h | [85] |
| MMP-2 | peptide | 5 nM | 10 min | [86] |
| MMP-2 | gelatin | 20 ng/mL | 30 min | [86] |
| Botulinum neurotoxin | peptide | 0.1 nM | 4 h | [88] |
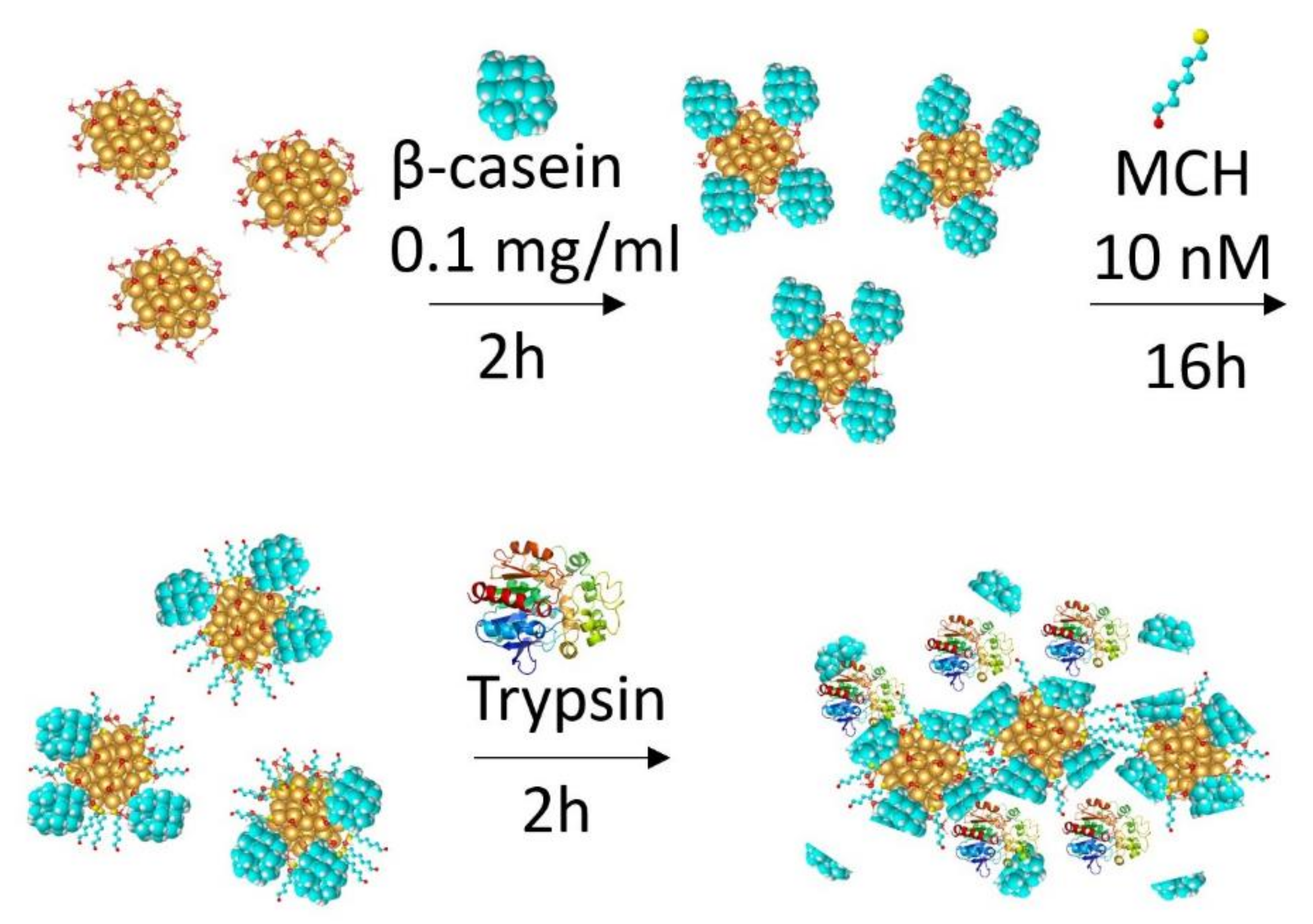
| Chymotrypsin | β-casein | 0.15 ± 0.01 nM | 30 min | [59] |
| Trypsin | peptide | 0.5 nM | 30 min | [84] |
| Trypsin | gelatin | 1.25 × 10−2 U | 10 min | [87] |
| Trypsin | β-casein | 0.42 ± 0.03 nM | 30 min | [90] |
| Antibiotic | Detection Strategy | Permissible Contamination (ng/mL) | Linear Range (ng/mL) | LOD (ng/mL) | Detection Time (min) | Sample/ Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Chloramphenicol | AuNPs aggregation | 0.3 | 16.16–581.63 | 2.26 | 10 | Milk, chicken /96.1–109.98 | [119] |
| Chloramphenicol | AuNPs aggregation | 0.3 | 0.03–3.23 | 0.01 | 5 | Milk/NA | [120] |
| Chloramphenicol | AuNPs/hemin/G-quadruplex/DNAzyme/cDNA | 0.3 | 0.001–100 | 0.13 × 10−3 | 60 | Milk, honey, river water/96.0–106.0 | [125] |
| Tetracycline | AuNPs aggregation | 100 | 22.22–1333.31 | 14.62 | 10 | Milk, chicken /1.06–5.57 | [119] |
| Tetracycline | Cysteamine-stabilized AuNPs | 100 | 200–2000 | 39 | 14 | Milk/91.28–100.87 | [126] |
| Oxytetracycline | AuNPs aggregation | 100 | NA | 5 | 10 | Milk/NA | [121] |
| Kanamycin | AuNPs aggregation | 145 | 0.002–2.42 | 0.005 | 60 | Milk, honey, river water/96.0–106.0 | [122] |
| Kanamycin | Platinum NPs, nicking enzyme | 145 | 5 × 10−4-200 | 2 × 10−4 | 60 | Cow and goat milk/95.3-104.2 | [127] |
| Tobramycin | AuNPs aggregation | 200 | 18.70–93.50 | 10.89 | 20 | Milk, chicken egg /93.1–105.1 | [123] |
| Streptomycin | Porous SiO2 beads/enzyme linked aptamer/exonuclease-assisted target recycling | 200 | 0.003–20 | 0.001 | N/A | Milk/90–112 | [128] |
| Bacteria | Detection Strategy | Permissible Contami-Nation (CFU/mL) | Linear Range (CFU/mL) | LOD (CFU/mL) | Detection Time (min) | Sample/ Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | AuNPs aggregation | 100 | 10–106 | 9 | <240 | Milk/NA | [134] |
| Staphylococcus aureus | Au-coated iron oxide/nanozyme | 100 | 10–106 | 10 | 12 | Milk, urine/86.5–122.3 | [138] |
| Staphylococcus aureus | Immunomagnetic separation/AuNPs/peroxidase catalysis | 100 | 10–106 | 10 | 65 | Pork, milk/88.2–119.8 | [142] |
| Shigella flexneri | AuNPs aggregation | NA | 102–106 | 80 | 20 | Spiked salmon/ 88.51–110.20 | [135] |
| Listeria monocytogeneses | Flower-shaped AuNPs aggregation | 10–100 | NA | 100.4 ng (genomic DNA) | 20 | NA | [136] |
| E. coli O157:H7 | A trigger-based AuNPs aggregation using HCl-NaOH-MgCl2 | 20–100 | 1.2 × 102–9 × 103 | 40.46–147.6 | 60 | Spiked tap water/ 90.2–120 | [137] |
| E. coli O157:H7 | AuNPs/MWCNT/carbonyl iron powder | 20–100 | NA | 5.24 × 102 | 60 | Milk/NA | [143] |
| E. coli O157:H7 | Fe3O4/MnO2/Au nanorods/catalysis | 20–100 | NA | 1.3 | 40 | NA | [144] |
| E. coli O157:H7 | AuNPs-coated by graphene oxide | 20–100 | 10–109 | 10 | 60 | Coconut water, litchi juice/ NA | [145] |
| E. coli O157:H7 | Cu-MOF/catalysis of colorless peroxidase substrate | 20–100 | 16~1.6×106 | 2 | NA | Milk/96.0–102.6 | [146] |
| Campylobacter jejuni | Peroxidase activity of Au@Pd NPs/TMB | 500< | 102–106 | 100 | NA | Milk/98.0–113.0 | [147] |
| Campylobacter jejuni | Hetero-sandwich platform-Antibody/HRP-modified aptamer | 500< | 17–1.7×106 | 10 | NA | Milk/NA | [148] |
| Campylobacter jejuni | AuNPs aggregation induced by MgCl2 | 500< | 105–108 | 7.2 × 105 | 30 | Chicken/NA | [149] |
| Vibrio fischeri | Aptamer sandwich assay | Non pathogenic | 4 × 10–4 × 105 | 103~104 | 10 | Buffer | [150] |
| Salmonella typhimurium | AuNPs, catalase mediated | Absence in 25 g of food | 10–106 | 10 | NA | Chicken/92.0–109.0 | [151] |
| Salmonella typhimurium | Microfluidic, polystyrene and AuNPs aggregation | Absence in 25 g of food | 60–60 × 105 | 60 | NA | Salad/91.68–113.76 | [152] |
| Salmonella typhimurium | Separation of aptamer-modified magnetic NPs. | Absence in 25 g of food | 15–1.5 × 106 | 15 | NA | Milk/96.7–99.8 | [153] |
| Salmonella typhimurium | Colorimetry/microfluidic chip/SBSE/(TMB)-H2O2 | Absence in 25 g of food | N/A | 100 | NA | NA | [154] |
| Salmonella typhimurium | AuNPs aggregation/long ssDNA | Absence in 25 g of food | 2.04 × 102–2.04 × 106 | 2.56 | NA | Lettuce/91.84–110.20 | [155] |
| Salmonella typhimurium | Colorimetry/microfluidic chip/MDE/hemin | Absence in 25 g of food | 102–108 | 100 | 3 | Milk, water/ 91.7–103.4 | [156] |
| Salmonella typhimurium | aptamers@BSA-AuNCs/TMB | Absence in 25 g of food- | 10–106 | 1 | NA | Eggs/92.4–110 | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melikishvili, S.; Piovarci, I.; Hianik, T. Advances in Colorimetric Assay Based on AuNPs Modified by Proteins and Nucleic Acid Aptamers. Chemosensors 2021, 9, 281. https://doi.org/10.3390/chemosensors9100281
Melikishvili S, Piovarci I, Hianik T. Advances in Colorimetric Assay Based on AuNPs Modified by Proteins and Nucleic Acid Aptamers. Chemosensors. 2021; 9(10):281. https://doi.org/10.3390/chemosensors9100281
Chicago/Turabian StyleMelikishvili, Sopio, Ivan Piovarci, and Tibor Hianik. 2021. "Advances in Colorimetric Assay Based on AuNPs Modified by Proteins and Nucleic Acid Aptamers" Chemosensors 9, no. 10: 281. https://doi.org/10.3390/chemosensors9100281
APA StyleMelikishvili, S., Piovarci, I., & Hianik, T. (2021). Advances in Colorimetric Assay Based on AuNPs Modified by Proteins and Nucleic Acid Aptamers. Chemosensors, 9(10), 281. https://doi.org/10.3390/chemosensors9100281







