A Facile Strategy for the Ion Current and Fluorescence Dual-Lock in Detection: Naphthalic Anhydride Azide (NAA)-Modified Biomimetic Nanochannel Sensor towards H2S
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hourglass-Shaped Alumina Nanochannels
2.2. Synthesis of the Probe Naphthalic Anhydride-Azide
2.3. Construction of Functionalized Nanochannels
2.4. Response of Functionalized Nanochannels to H2S
2.5. Analysis and Characterization
2.5.1. Ionic Current Measurement
2.5.2. Fluorescence Properties Measurement
2.5.3. Characterization
3. Results and Discussions
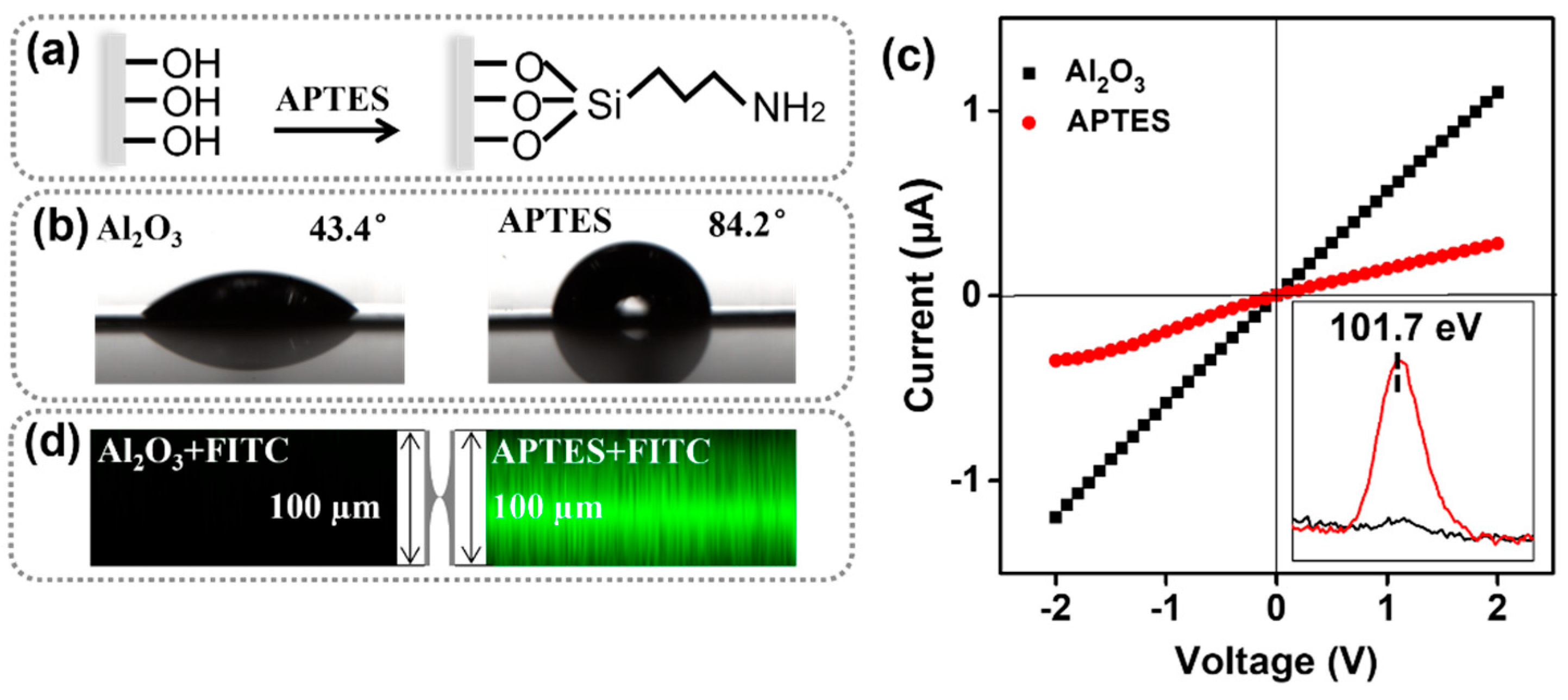
3.1. Construction and Modification of the Artificial Nanochannels
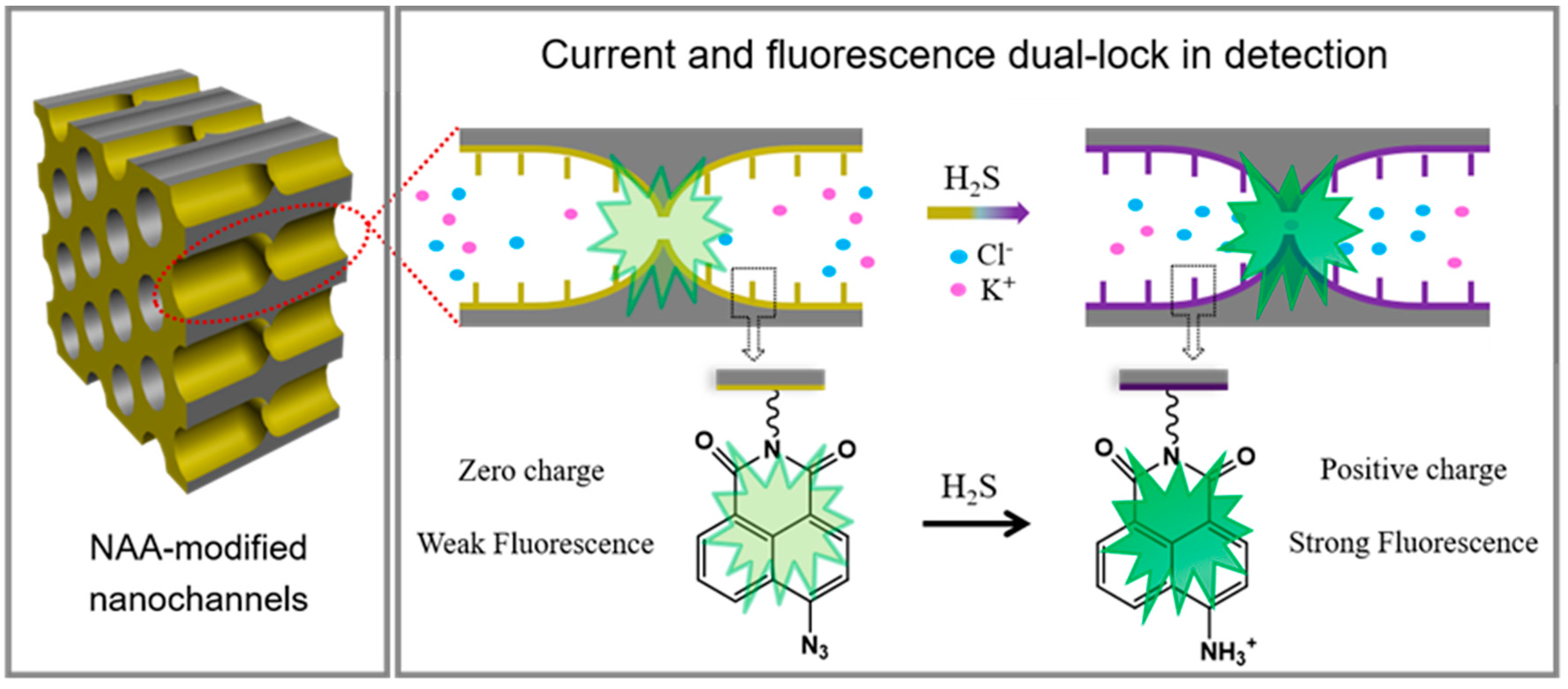
3.2. The Dual Lock-in Detection Mechanism
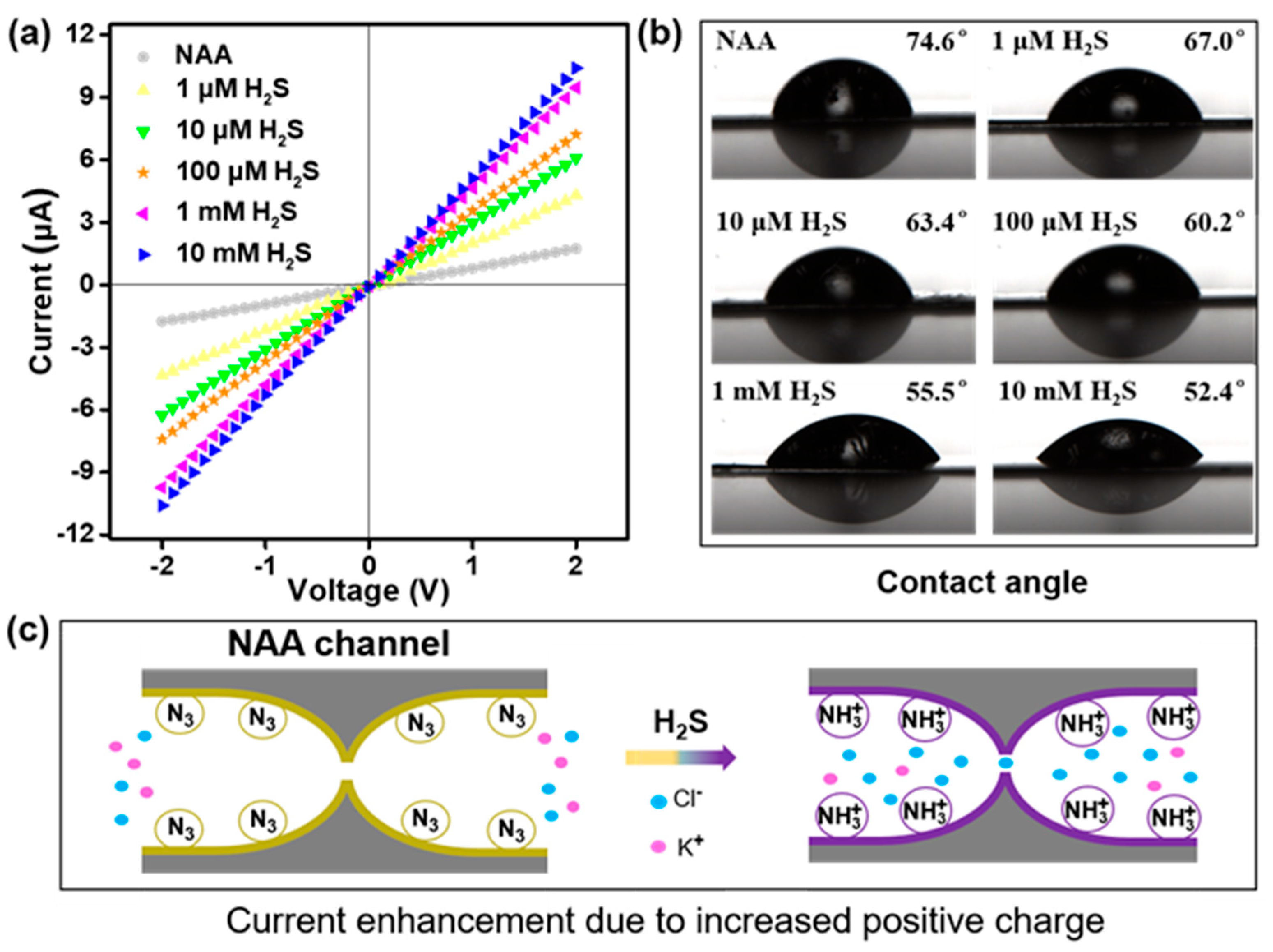
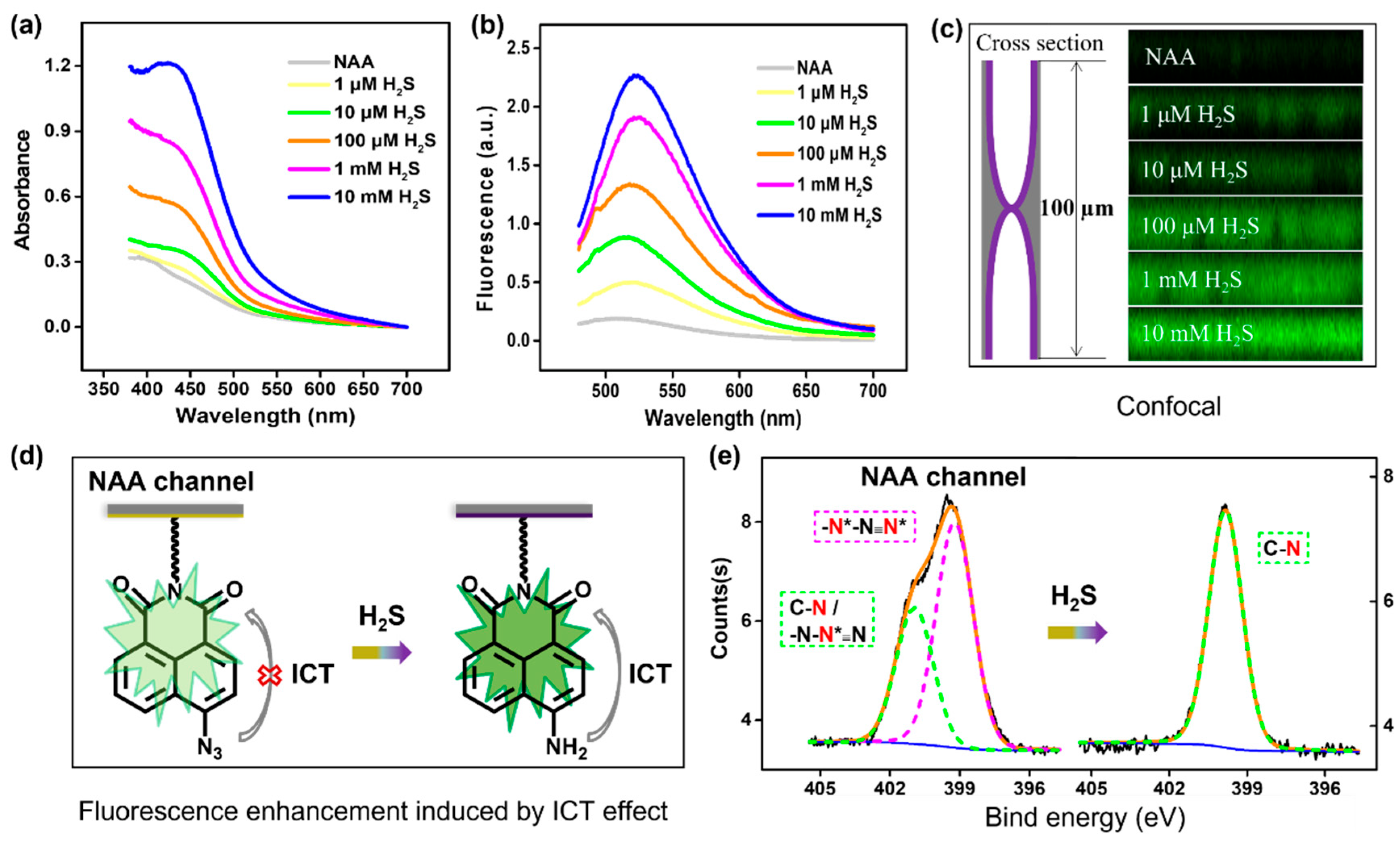
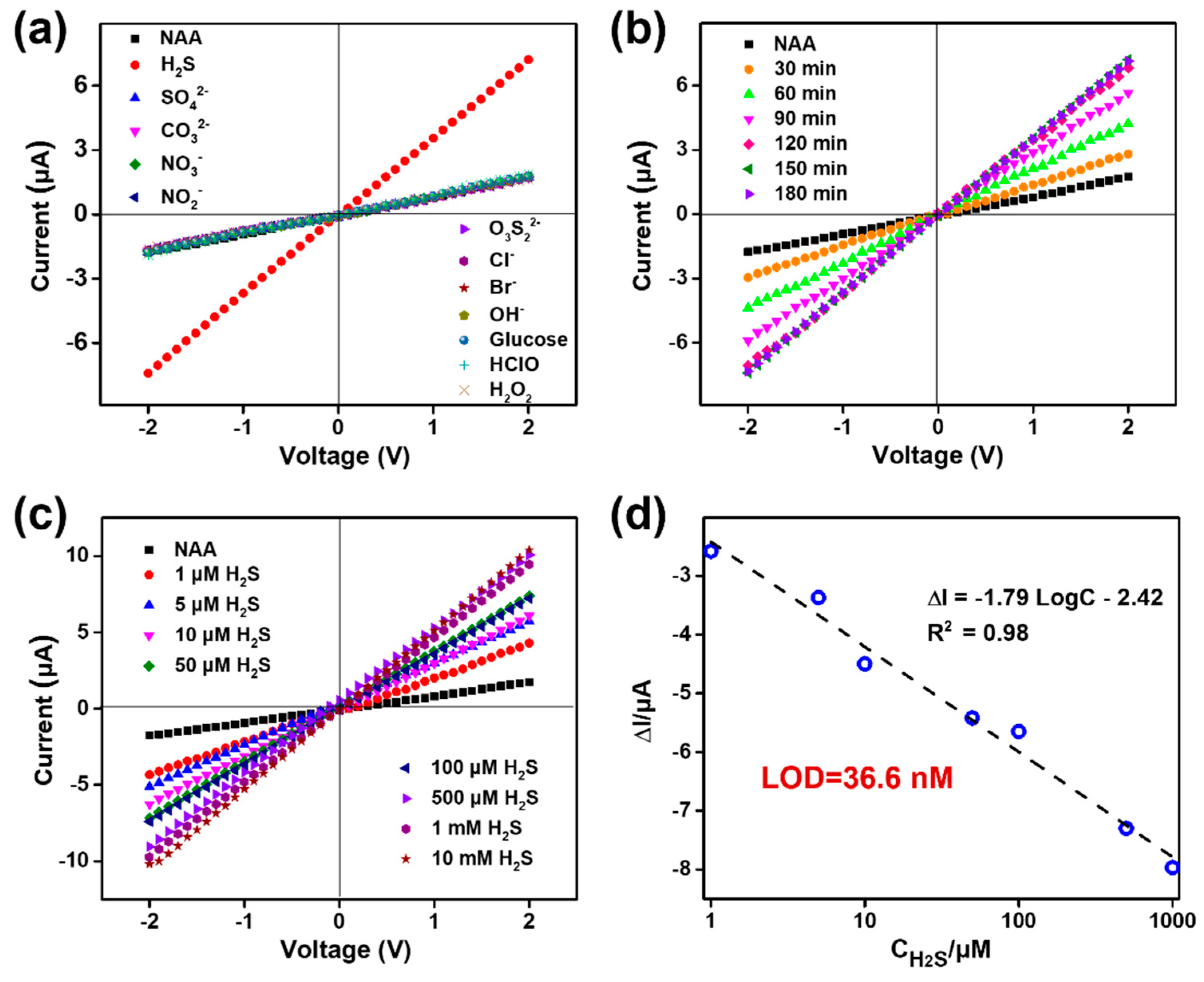
3.3. Selectivity, Reaction Time, and Sensitivity of the Nanosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prindle, A.; Liu, J.; Asally, M.; Ly, S.; Garcia-Ojalvo, J.; Suel, G.M. Ion channels enable electrical communication in bacterial communities. Nature 2015, 527, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, G.; Lin, M.; Kong, X.; Mi, L.; Liu, S.; Chen, G.; Tian, Y.; Jiang, L. Continuously Tunable Ion Rectification and Conductance in Submicrochannels Stemming from Thermoresponsive Polymer Self-Assembly. Angew. Chem. Int. Ed. Engl. 2019, 58, 12481–12485. [Google Scholar] [CrossRef]
- Xie, G.; Xiao, K.; Zhang, Z.; Kong, X.Y.; Liu, Q.; Li, P.; Wen, L.; Jiang, L. A Bioinspired Switchable and Tunable Carbonate-Activated Nanofluidic Diode Based on a Single Nanochannel. Angew. Chem. Int. Ed. Engl. 2015, 54, 13664–13668. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, K.; Wen, L.; Lu, H.; Liu, Y.; Kong, X.Y.; Xie, G.; Zhang, Z.; Bo, Z.; Jiang, L. Engineered Ionic Gates for Ion Conduction Based on Sodium and Potassium Activated Nanochannels. J. Am. Chem. Soc. 2015, 137, 11976–11983. [Google Scholar] [CrossRef]
- Zhao, X.P.; Wang, S.S.; Younis, M.R.; Xia, X.H.; Wang, C. Asymmetric Nanochannel-Ionchannel Hybrid for Ultrasensitive and Label-Free Detection of Copper Ions in Blood. Anal. Chem. 2018, 90, 896–902. [Google Scholar] [CrossRef]
- Shang, X.; Xie, G.; Kong, X.Y.; Zhang, Z.; Zhang, Y.; Tian, W.; Wen, L.; Jiang, L. An Artificial CO2 -Driven Ionic Gate Inspired by Olfactory Sensory Neurons in Mosquitoes. Adv. Mater. 2017, 29, 1603884. [Google Scholar] [CrossRef]
- Wu, K.; Kong, X.Y.; Xiao, K.; Wei, Y.; Zhu, C.; Zhou, R.; Si, M.; Wang, J.; Zhang, Y.; Wen, L. Engineered Smart Gating Nanochannels for High Performance in Formaldehyde Detection and Removal. Adv. Funct. Mater. 2019, 29, 1807953. [Google Scholar] [CrossRef]
- Zhao, X.-P.; Cao, J.; Nie, X.-G.; Wang, S.-S.; Wang, C.; Xia, X.-H. Label-free monitoring of the thrombin–aptamer recognition reaction using an array of nanochannels coupled with electrochemical detection. Electrochem. Commun. 2017, 81, 5–9. [Google Scholar] [CrossRef]
- Burns, J.R.; Seifert, A.; Fertig, N.; Howorka, S. A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane. Nat. Nanotechnol. 2016, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, Y.; Zhou, Y.; Xia, F.; Guo, W.; Jiang, L. Two-way nanopore sensing of sequence-specific oligonucleotides and small-molecule targets in complex matrices using integrated DNA supersandwich structures. Angew. Chem. Int. Ed. Engl. 2013, 52, 2007–2011. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, X.P.; Younis, M.R.; Li, Z.Q.; Xia, X.H.; Wang, C. Ultrasensitive Capture, Detection, and Release of Circulating Tumor Cells Using a Nanochannel-Ion Channel Hybrid Coupled with Electrochemical Detection Technique. Anal. Chem. 2017, 89, 10957–10964. [Google Scholar] [CrossRef]
- Freedman, K.J.; Otto, L.M.; Ivanov, A.P.; Barik, A.; Oh, S.H.; Edel, J.B. Nanopore sensing at ultra-low concentrations using single-molecule dielectrophoretic trapping. Nat. Commun. 2016, 7, 10217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.P.; Zhou, Y.; Zhang, Q.W.; Yang, D.R.; Wang, C.; Xia, X.H. Nanochannel-Ion Channel Hybrid Device for Ultrasensitive Monitoring of Biomolecular Recognition Events. Anal. Chem. 2019, 91, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, C.; Zhou, Y.; Jin, Y. Controllable Shrinking of Glass Capillary Nanopores Down to sub-10 nm by Wet-Chemical Silanization for Signal-Enhanced DNA Translocation. ACS Sens. 2017, 2, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Liu, L.; Wang, J.; Ma, K.; Lv, J. A Novel Biosensor Based on Molybdenum Disulfide (MoS2) Modified Porous Anodic Aluminum Oxide Nanochannels for Ultrasensitive microRNA-155 Detection. Small 2020, 16, e2001223. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, C.H.; Huang, S.Z.; Lei, W.; Ruan, Y.F.; Lu, H.J.; Zhao, W.; Xu, J.J.; Chen, H.Y. Ultrasmall Nanopipette: Toward Continuous Monitoring of Redox Metabolism at Subcellular Level. Angew. Chem. Int. Ed. Engl. 2018, 57, 13226–13230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, B.; Zhang, Z. Ionic Current Rectification Triggered Photoelectrochemical Chiral Sensing Platform for Recognition of Amino Acid Enantiomers on Self-Standing Nanochannel Arrays. Anal. Chem. 2020, 92, 8670–8674. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, W.; Gao, P.; Li, H.; Feng, G.; Zhao, Z.; Lou, X. Coordination of the electrical and optical signals revealing nanochannels with an ‘onion-like’ gating mechanism and its sensing application. NPG Asia Mater. 2016, 8, e234. [Google Scholar] [CrossRef] [Green Version]
- Donnarumma, E.; Trivedi, R.K.; Lefer, D.J. Protective Actions of H2S in Acute Myocardial Infarction and Heart Failure. Compr. Physiol. 2017, 7, 583–602. [Google Scholar]
- King, A.L.; Lefer, D.J. Cytoprotective actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp. Physiol. 2011, 96, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Lefer, D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Luan, L.; Guan, W.; Zhang, S.; Li, B.; Ji, W.; Fan, H. Hydrogen sulfide attenuates isoflurane-induced neuroapoptosis and cognitive impairment in the developing rat brain. BMC Anesthesiol. 2017, 17, 123. [Google Scholar] [CrossRef] [Green Version]
- Shefa, U.; Kim, M.S.; Jeong, N.Y.; Jung, J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxid. Med. Cell. Longev. 2018, 2018, 1873962. [Google Scholar] [CrossRef]
- Dallas, M.L.; Al-Owais, M.M.; Hettiarachchi, N.T.; Vandiver, M.S.; Jarosz-Griffiths, H.H.; Scragg, J.L.; Boyle, J.P.; Steele, D.; Peers, C. Hydrogen sulfide regulates hippocampal neuron excitability via S-sulfhydration of Kv2.1. Sci. Rep. 2021, 11, 8194. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, J.; Guo, W.; Zhu, Y.Z. Atherosclerosis and the Hydrogen Sulfide Signaling Pathway-Therapeutic Approaches to Disease Prevention. Cell. Physiol. Biochem. 2017, 42, 859–875. [Google Scholar] [CrossRef]
- Li, L.; Moore, P.K. An overview of the biological significance of endogenous gases: New roles for old molecules. Biochem. Soc. Trans. 2007, 35, 1138–1141. [Google Scholar] [CrossRef]
- Cha, J.-H.; Kim, D.-H.; Choi, S.-J.; Koo, W.-T.; Kim, I.-D. Sub-Parts-per-Million Hydrogen Sulfide Colorimetric Sensor: Lead Acetate Anchored Nanofibers toward Halitosis Diagnosis. Anal. Chem. 2018, 90, 8769–8775. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. AJP-Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A Source of Hydrogen Sulfide and a Mechanism of Its Release in the Brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Guo, Y.; Wang, S.; Chen, W.; Zhang, J.; Zheng, W.; Jiang, X. Nanocrystalline cellulose mediated seed-growth for ultra-robust colorimetric detection of hydrogen sulfide. Nanoscale 2017, 9, 9811–9817. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, X.; Tan, J.; Ding, Z.; Wang, W.; Yuan, D.; Li, G.; Zhang, H.; Zhang, X. Reductive cleavage of C=C bond as a new strategy for turn-on dual fluorescence in effective sensing of H2S. Chem. Sci. 2018, 9, 8369–8374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henthorn, H.A.; Pluth, M.D. Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef] [Green Version]
- Yassine, O.; Shekhah, O.; Assen, A.H.; Belmabkhout, Y.; Salama, K.N.; Eddaoudi, M. H2S Sensors: Fumarate-Based fcu-MOF Thin Film Grown on a Capacitive Interdigitated Electrode. Angew. Chem. Int. Ed. 2016, 55, 15879–15883. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, H.; Liu, Z.; Han, B.; Zhang, X. A Highly Efficient Chemical Sensor Material for H2S: α-Fe2O3 Nanotubes Fabricated Using Carbon Nanotube Templates. Adv. Mater. 2005, 17, 2993–2997. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.; Sun, Z.; Chen, S.; Gao, J.; Sun, Y.; Li, H. Switchable Nanochannel Biosensor for H2S Detection Based on an Azide Reduction Reaction Controlled BSA Aggregation. Anal. Chem. 2019, 91, 6149–6154. [Google Scholar] [CrossRef]
- Guo, J.; Yang, L.; Xu, H.; Zhao, C.; Dai, Z.; Gao, Z.; Song, Y. Biomineralization-Driven Ion Gate in TiO2 Nanochannel Arrays for Cell H2S Sensing. Anal. Chem. 2019, 91, 13746–13751. [Google Scholar] [CrossRef]
- Nesakumar, N.; Kesavan, S.; Li, C.-Z.; Alwarappan, S. Microfluidic Electrochemical Devices for Biosensing. J. Anal. Test. 2019, 3, 3–18. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, S.; Liu, Y.; Fan, X.; Zhang, M.; Zhai, J.; Jiang, L. Self-Assembled Porphyrin Nanofiber Membrane-Decorated Alumina Channels for Enhanced Photoelectric Response. ACS Nano 2018, 12, 11169–11177. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q.; Fan, X.; Zhang, M.; Zhai, J.; Jiang, L. An Effective Dark-Vis-UV Ternary Biomimetic Switching Based on N3/Spiropyran-Modified Nanochannels. Adv. Mater. 2018, 30, e1804862. [Google Scholar] [CrossRef]
- Xu, A.; Tang, Y.; Lin, W. Development of a mitochondrial-targeted two-photon fluorescence turn-on probe for formaldehyde and its bio-imaging applications in living cells and tissues. New J. Chem. 2018, 42, 8325–8329. [Google Scholar] [CrossRef]
- Montoya, L.A.; Pluth, M.D. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 4767–4769. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Yao, H.W.; Zhu, X.Y.; Guo, X.F.; Wang, H. A cell surface specific two-photon fluorescent probe for monitoring intercellular transmission of hydrogen sulfide. Anal. Chim. Acta 2017, 994, 1–9. [Google Scholar] [CrossRef]
- Zhao, X.P.; Liu, F.F.; Hu, W.C.; Younis, M.R.; Wang, C.; Xia, X.H. Biomimetic Nanochannel-Ionchannel Hybrid for Ultrasensitive and Label-Free Detection of MicroRNA in Cells. Anal. Chem. 2019, 91, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, H.; Zhou, G.; Ge, L.; Li, F. In situ growth of nano-gold on anodized aluminum oxide with tandem nanozyme activities towards sensitive electrochemical nanochannel sensing. Analyst 2020, 145, 6617–6624. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, I.; Zhang, D.; Zhang, X. A Facile Strategy for the Ion Current and Fluorescence Dual-Lock in Detection: Naphthalic Anhydride Azide (NAA)-Modified Biomimetic Nanochannel Sensor towards H2S. Chemosensors 2021, 9, 298. https://doi.org/10.3390/chemosensors9110298
Wu I, Zhang D, Zhang X. A Facile Strategy for the Ion Current and Fluorescence Dual-Lock in Detection: Naphthalic Anhydride Azide (NAA)-Modified Biomimetic Nanochannel Sensor towards H2S. Chemosensors. 2021; 9(11):298. https://doi.org/10.3390/chemosensors9110298
Chicago/Turabian StyleWu, I, Dan Zhang, and Xuanjun Zhang. 2021. "A Facile Strategy for the Ion Current and Fluorescence Dual-Lock in Detection: Naphthalic Anhydride Azide (NAA)-Modified Biomimetic Nanochannel Sensor towards H2S" Chemosensors 9, no. 11: 298. https://doi.org/10.3390/chemosensors9110298
APA StyleWu, I., Zhang, D., & Zhang, X. (2021). A Facile Strategy for the Ion Current and Fluorescence Dual-Lock in Detection: Naphthalic Anhydride Azide (NAA)-Modified Biomimetic Nanochannel Sensor towards H2S. Chemosensors, 9(11), 298. https://doi.org/10.3390/chemosensors9110298




