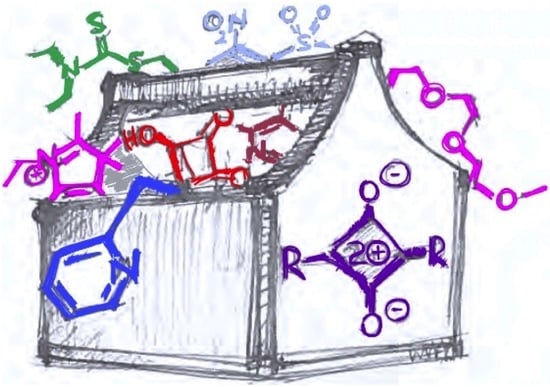
Squaraine-Based Optical Sensors: Designer Toolbox for Exploring Ionic and Molecular Recognitions
Abstract
:1. Introduction: A Case for Squaraine Scaffold
2. Synthesis of Squaraine Dyes: General Considerations
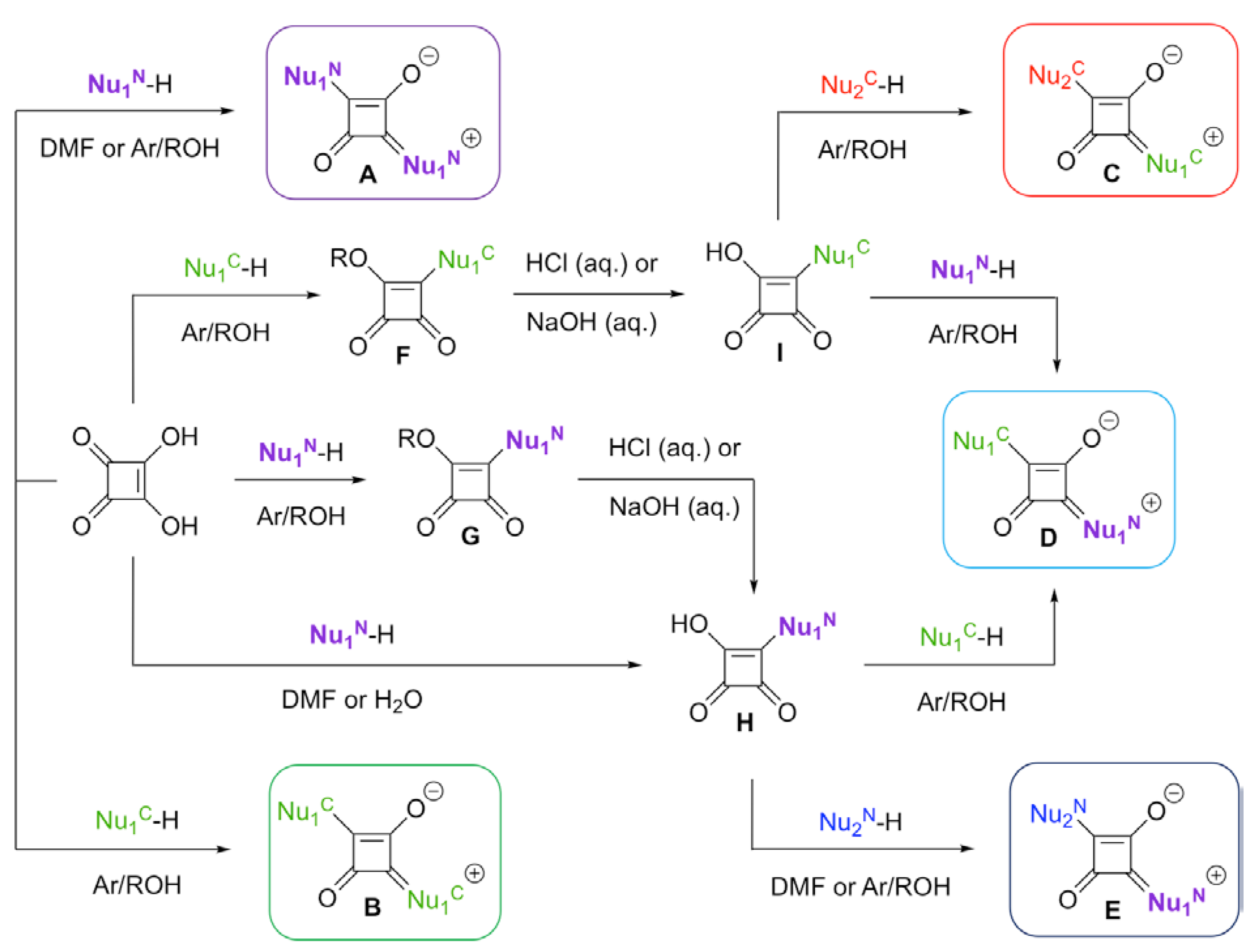
2.1. Squaric Acid-Based Syntheses
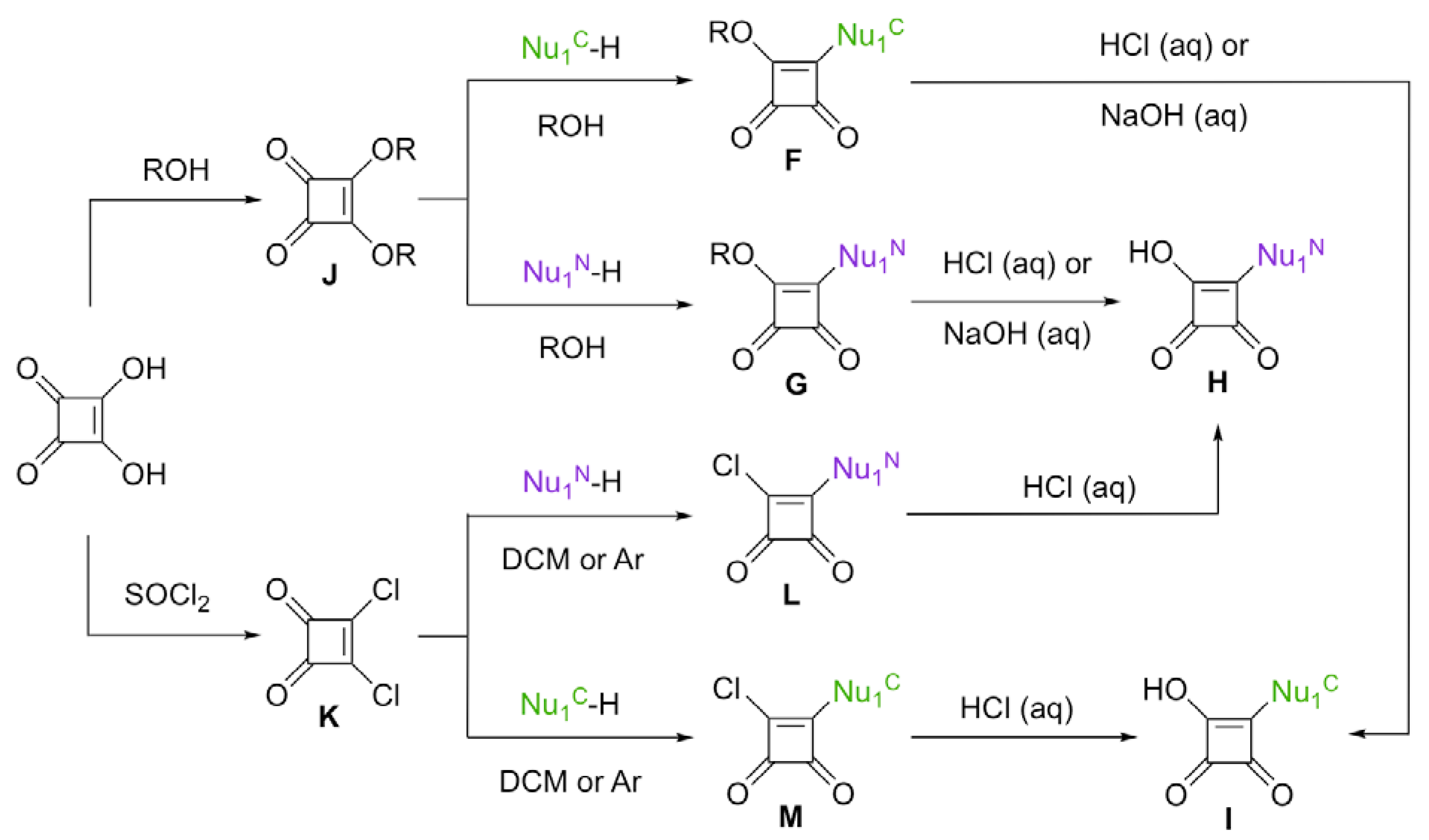
2.2. Squaric Acid Ester and Squaryl Chloride-Based Approaches
3. Chelating Group Approach for Sensor Designs
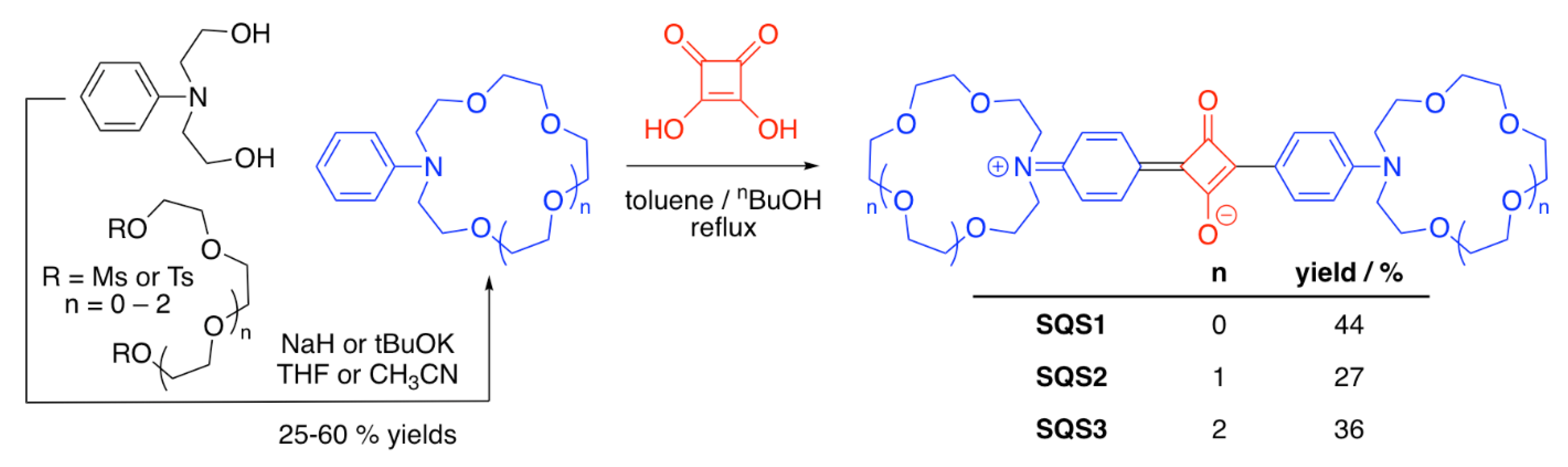
3.1. Crown Ether-Containing Squaraine Sensors
3.2. Podand-Containing Squaraine Sensors
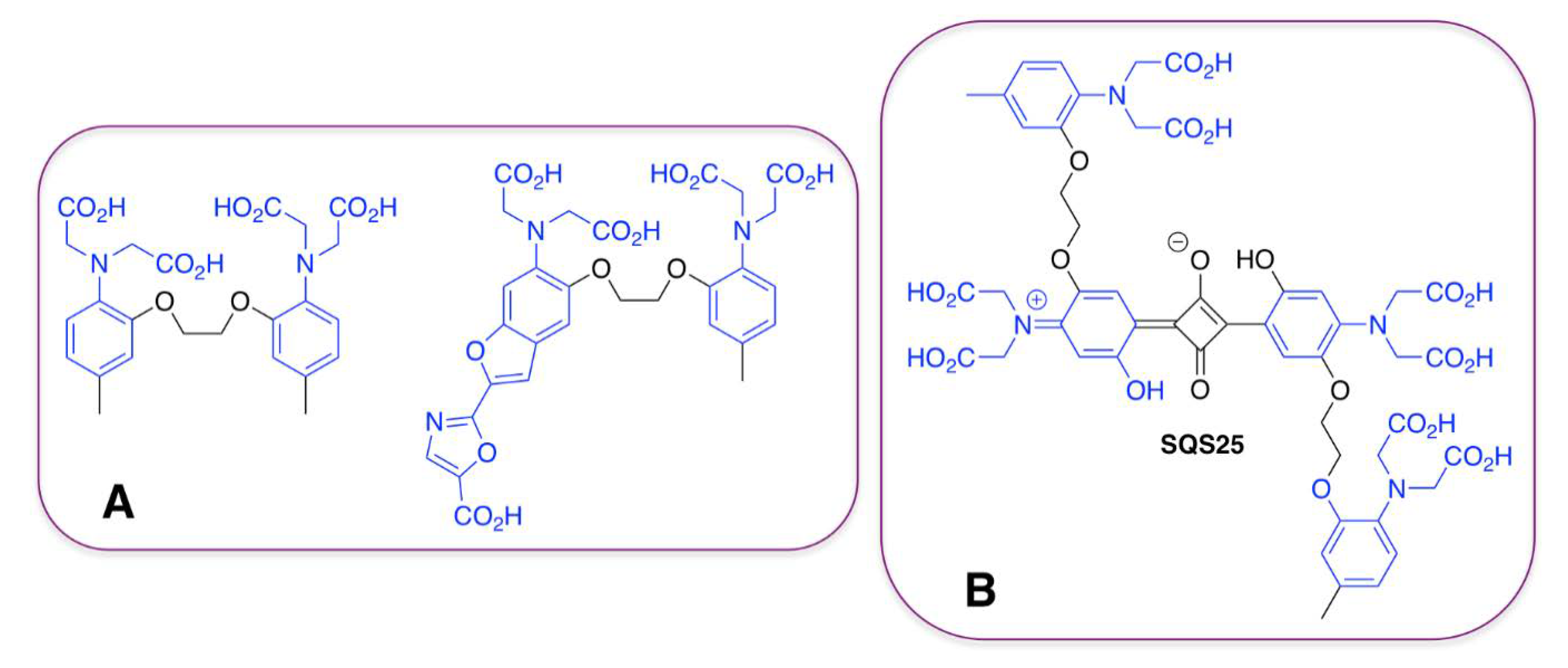
3.3. Tetracarboxylate Binding Motif
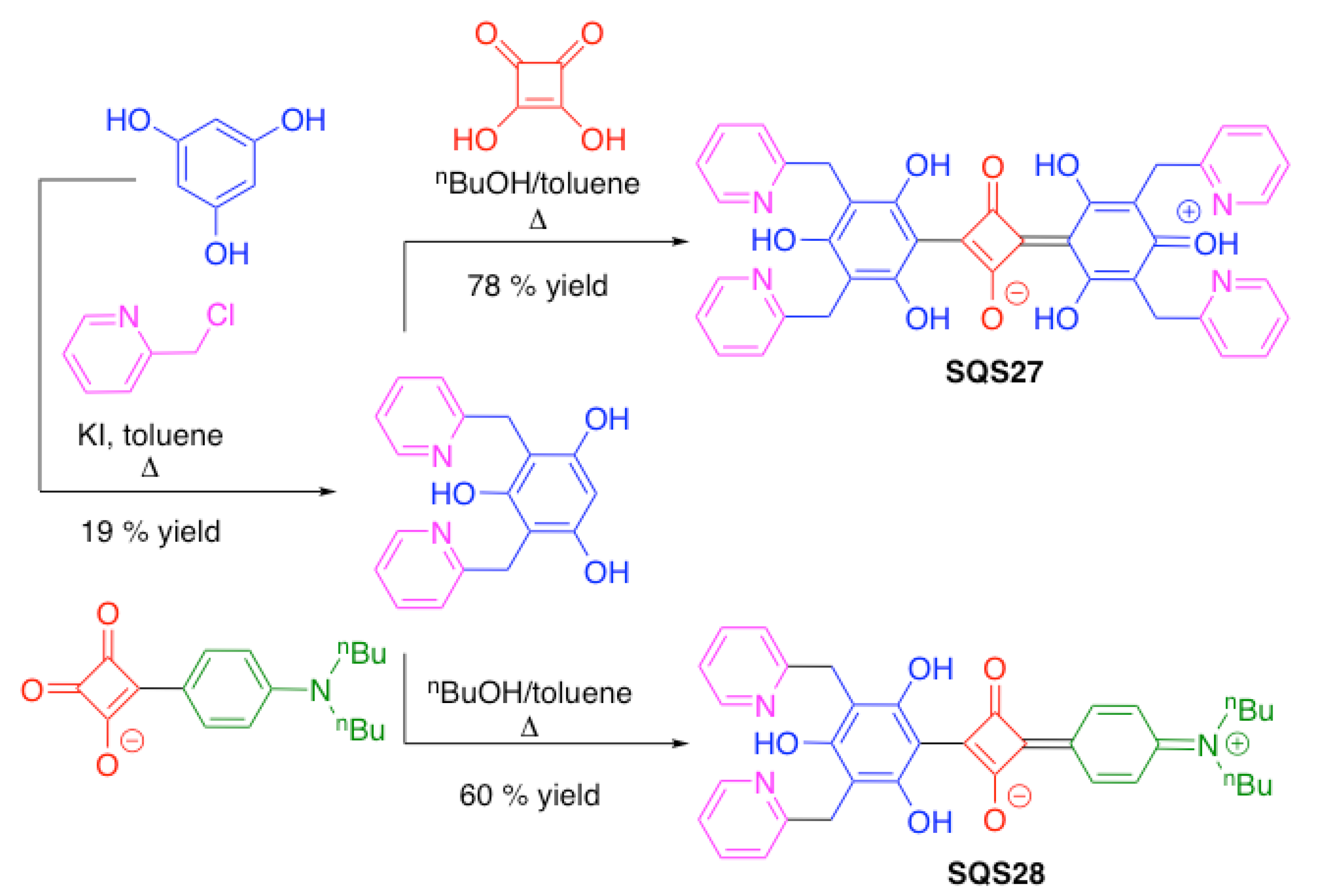
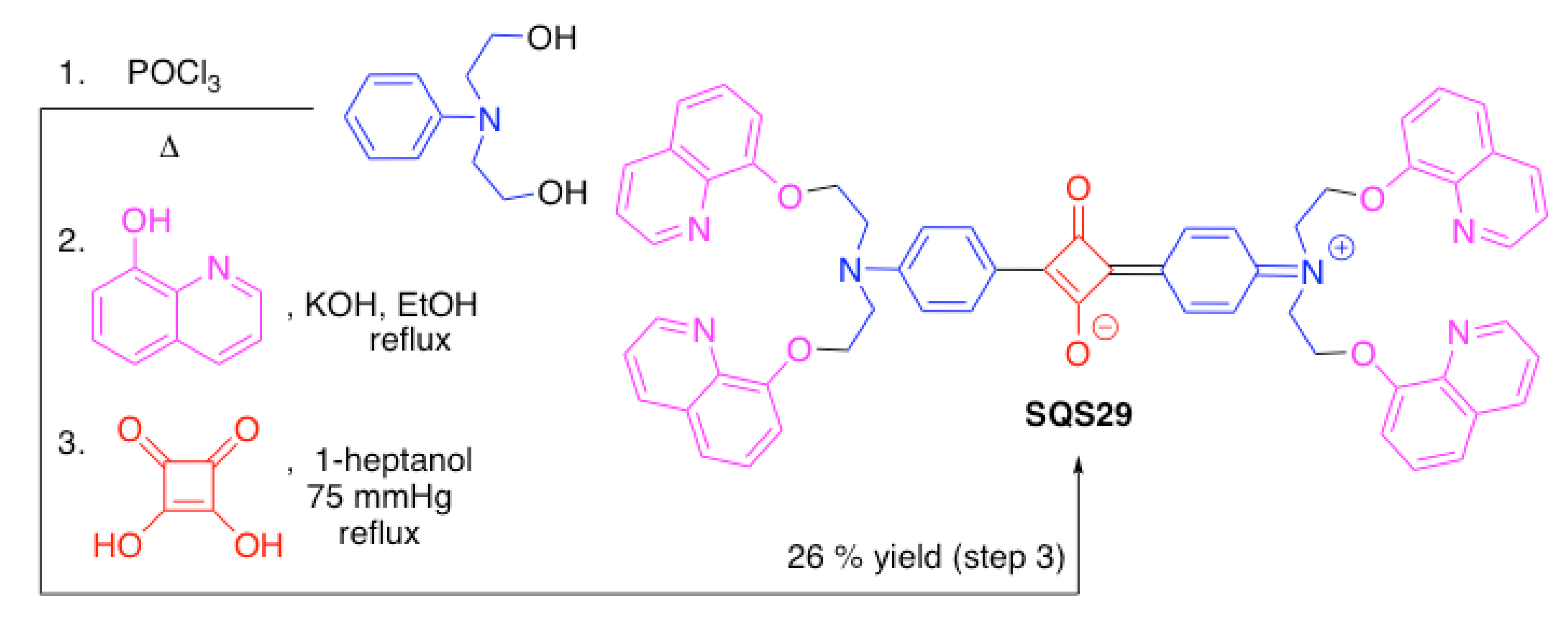
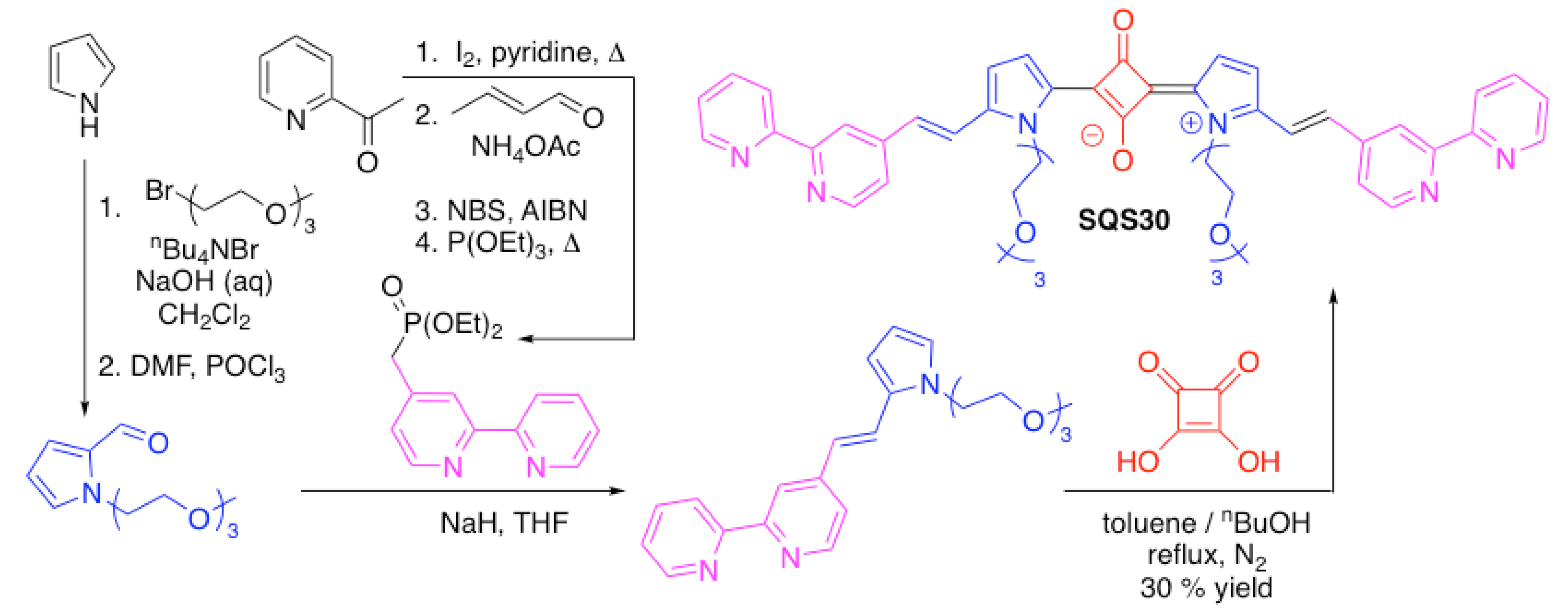
3.4. Nitrogen-Containing Heterocycles as Binding Motifs
4. Squaraine Scaffolds with Acidic NH
5. Electrophilicity of Squaraine Ring’s Carbon as Key Element in “Turn-Off” and “Turn-On” Sensing Paradigms
5.1. Nucleophilic Addition as a Tool for Controlling Energy Transfer
5.2. Nucleophile-Induced Skeletal Rearrangement
6. Chemo-Uncaging Strategies for Sensing
7. Squaraine–Metal Scaffold for Sensing
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.; Jo, Y.J.; Sun, X.; Qiao, W.; Ok, J.; Kim, T.-I.; Li, Z. Squaraine dyes for photovoltaic and biomedical applications. Adv. Funct. Mater. 2021, 31, 2008201. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine dyes: Molecular design for different applications and remaining challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.J.; Wallace, K.J. Squaraine dyes in molecular recognition and self-assembly. Chem. Commun. 2009, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kwon, B.; Liu, W.; Anslyn, E.V.; Wang, P.; Kim, J.S. Chromogenic/fluorogenic ensemble chemosensing systems. Chem. Rev. 2015, 115, 7893–7943. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platform. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Khopkar, S.; Shankarling, G. Synthesis, photophysical properties and applications of NIR absorbing unsymmetrical squaraines: A review. Dyes Pigments 2019, 170, 107645. [Google Scholar] [CrossRef]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Saini, R.; Kumar, A.; Luxami, V.; Kaur, N.; Singh, P.; Kumar, S. Chemodosimeters: An approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols. Coord. Chem. Rev. 2012, 256, 1992–2028. [Google Scholar] [CrossRef]
- Mukkanti, A.; Periasamy, M. Methods of synthesis of cyclobutenediones. ARKIVOC 2005, 11, 48–77. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Wang, H. Squaraine dyes: The hierarchical synthesis and its application in optical detection. J. Photochem. Photobiol. C 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Hu, L.; Yan, Z.; Xu, H. Advances in synthesis and application of near-infrared absorbing squaraine dyes. RSC Adv. 2013, 3, 7667–7676. [Google Scholar] [CrossRef]
- Sprenger, H.-E.; Ziegenbein, W. Cyclobutenediylium dyes. Angew. Chem. Int. Ed. 1968, 7, 530–535. [Google Scholar] [CrossRef]
- Mayerhöffer, U.; Fimmel, B.; Würthner, F. Bright near-infrared fluorophores based on squaraines by unexpected halogen effects. Angew. Chem. Int. Ed. 2012, 51, 164–167. [Google Scholar] [CrossRef]
- Wendling, L.A.; Koster, S.; Murray, J.E.; West, R. Syntheses and properties of 1,2- and 1,3-diquinocyclobutanediones. J. Org. Chem. 1997, 42, 1126–1130. [Google Scholar] [CrossRef]
- Law, K.Y.; Bailey, F.C. Squaraine chemistry. Synthesis, characterization, and optical properties of a class of novel unsymmetrical squaraines: [4-(dimethylamino)phenyl](4′-methoxymethyl)squaraine and it derivatives. J. Org. Chem. 1992, 57, 3278–3286. [Google Scholar] [CrossRef]
- Law, K.Y.; Bailey, F.C. Squaraine chemistry. A new approach to symmetrical and unsymmetrical protoconductive squaraines. Characterization and solid state properties of these materials. Can. J. Chem. 1993, 71, 494–505. [Google Scholar] [CrossRef]
- Hamilton, D.G.; Lynch, D.E.; Smith, G. Synthesis and solid-state structure of the unsymmetrical squaraine dye 2-[4-(dibutylamino)-2-hydroxyphenyl]-4-[4-(dibutylamino)phenyl]-cyclobutenebis(ylium)-1,3-diolate. Aust. J. Chem. 1996, 49, 1339–1343. [Google Scholar] [CrossRef]
- Piggot, P.M.T.; Hall, L.A.; White, A.J.P.; Williams, D.J. Attempted syntheses of lanthanide(III) complexes of the anisole and anilinosquarate ligands. Inorg. Chem. 2003, 42, 8344–8352. [Google Scholar] [CrossRef]
- de Oliveira, V.E.; de Carvalho, G.S.; Yoshida, M.I.; Donnici, C.L.; Speziali, N.L.; Diniz, R.; de Oliveira, L.F.C. Bis(dicyanomethylene)squarate squaraines in their 1,2- and 1,3-forms: Synthesis, crystal structure and spectroscopic study of compounds containing alkali metals and tetrabutylammonium ions. J. Mol. Struct. 2009, 936, 239–249. [Google Scholar] [CrossRef]
- Neuse, E.W.; Green, B.R. Dianilino derivatives of squaric acid. J. Org. Chem. 1974, 39, 3881–3887. [Google Scholar] [CrossRef]
- Khopkar, S.; Deshpande, S.; Shankarling, G. Greener protocol for the synthesis of NIR fluorescent indolenine-based symmetrical squaraine colorants. ACS Sustain. Chem. Eng. 2018, 6, 10798–10805. [Google Scholar] [CrossRef]
- Zappimbulso, N.; Capozzi, M.A.M.; Porcheddu, A.; Punzi, A. Solvent-free reactions for the synthesis of indolenine-based squaraines and croconaines: Comparison of thermal heating, mechanochemical milling, and IR irradiation. ChemSusChem 2021, 14, 1363–1369. [Google Scholar] [CrossRef]
- Gauger, J.; Manecke, G. Kondensationsproducte der quadratsäure mit primären unde sekundären aminen, I. Chem. Ber. 1970, 103, 2696–2706. [Google Scholar] [CrossRef]
- Keil, D.; Hartmann, H. Synthesis and characterization of a new class of unsymmetric squaraine dyes. Dyes Pigments 2001, 49, 161–179. [Google Scholar] [CrossRef]
- Buchynskyy, A.; Kempin, U.; Vogel, S.; Hennig, L.; Findeisen, M.; Müller, D.; Giesa, S.; Knoll, H.; Welzel, P. Synthesis of fluorescent derivatives of the antibiotic moenomycin A. Eur. J. Org. Chem. 2002, 2002, 1149–1162. [Google Scholar] [CrossRef]
- Kim, S.; Han, S. High performance squarylium dyes for high-tech use. Color. Technol. 2001, 117, 61–67. [Google Scholar] [CrossRef]
- Xie, J.; Comeau, A.B.; Seto, T. Squaric acids: A new motif for designing inhibitors of protein tyrosine phosphatates. Org. Lett. 2004, 6, 83–86. [Google Scholar] [CrossRef]
- López, C.; Vega, M.; Sanna, E.; Rotger, C.; Costa, A. Efficient microwave-assisted preparation of squaric acid monoamides in water. RSC Adv. 2013, 3, 7249–7253. [Google Scholar] [CrossRef]
- Jyothish, K.; Arun, K.T.; Ramaiah, D. Synthesis of novel quinaldine-based squaraine dyes: Effect of substituents and role of electronic factors. Org. Lett. 2004, 6, 3965–3968. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jyothish, K.; Ramaiah, D. Dual-mode semisquaraine-based sensor for selective detection of Hg2+ in a micellar medium. Org. Lett. 2007, 9, 121–125. [Google Scholar] [CrossRef]
- Jyothish, K.; Avirah, R.R.; Ramaiah, D. Synthesis of new cholesterol- and sugar-anchored squaraine dyes: Further evidence of how electronic factors influence dye formation. Org. Lett. 2006, 8, 111–114. [Google Scholar] [CrossRef]
- Mahs, G.; Hegenberg, P. Synthesis and derivatives of squaric acid. Angew. Chem. Int. Ed. 1966, 5, 888–893. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Ried, W. The preparative chemistry of cylobutenediones. III. Synthesis of squaric acid, benzocyclobutenedione and their derivatives. Synthesis 1978, 869–880. [Google Scholar] [CrossRef]
- Schmidt, A.H. Reactions of squaric acid and its derivatives. Synthesis 1980, 961–994. [Google Scholar] [CrossRef]
- Röesch, A.T.; Zhu, Q.; Robben, J.; Tassinari, F.; Meskers, S.C.J.; Naaman, R.; Palmans, A.R.A.; Meijer, E.W. Helicity control in the aggregation of achiral squaraine dyes in solution and thin films. Chem. Eur. J. 2021, 27, 298–306. [Google Scholar] [CrossRef]
- Lim, N.C.; Morton, M.D.; Jenkins, H.A.; Brückner, C. Squaric acid N-hydroxylamides: Synthesis, structure, and properties of vinylogous hydroxamic acid analogues. J. Org. Chem. 2003, 68, 9233–9241. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.M.; Roon, R.J.; Koerner, J.F.; Taylor, N.J.; Honek, J.F. A 3-amino-4-hydroxy-3-cyclobutene-1,2-dione-containing glutamate analog exhibiting high affinity to excitatory amino acid receptors. J. Med. Chem. 1995, 38, 4433–4438. [Google Scholar] [CrossRef] [PubMed]
- Ivanovsky, S.A.; Dorogov, M.V.; Kravchenko, D.V.; Ivachtchenko, A.V. Synthesis of the substituted 3-cyclobutene-1,2-diones. Synth. Commun. 2007, 37, 2527–2542. [Google Scholar] [CrossRef]
- Lunelli, B. New, optimized preparation of 1,2-dichlorocyclobuten-3,4-dione (C4O2Cl2) from squaric acid and oxalyl chloride. Tetrahedron Lett. 2007, 48, 3595–3597. [Google Scholar] [CrossRef]
- Garbay, G.; Tailliez, T.; Pavlopoulou, E.; Oriou, J.; Bezirdjolou, M.; Hadziioannou, G.; Cloutet, E.; Brochon, C. Triaryl-1,4-diamine-based polysquaraines: Effect of co-solvent and monomer insertion on optoelectronic properties. Polymer Chem. 2018, 9, 1288–1292. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Plaul, W.; Aimene, A.; Hotz, M.; Hoch, M. Oxocarbons and related compounds, VIII. Squaric amide chlorides: Simple methods of their preparation and cyclization of specially substituted representatives to annulated cyclobutenediones. Liebigs Ann. Chem. 1985, 1021–1035. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Yang, D.; Zhao, S.; Zhang, L.; Yang, L.; Wu, J.; Huang, Y.; Xu, Z.; Lu, Z. An azulene-containing low bandgap small molecule for organic photovoltaics with high open circuit voltage. Chem. Eur. J. 2016, 22, 14527–14530. [Google Scholar] [CrossRef]
- Hsueh, S.-Y.; Lai, C.-C.; Chiu, S.-H. Squaraine-based [2]rotaxanes that function as visibly active molecular switches. Chem. Eur. J. 2010, 16, 2997–3000. [Google Scholar] [CrossRef] [Green Version]
- Yagi, S.; Fujie, Y.; Hyodo, Y.; Nakazumi, H. Synthesis, structure, and complexation properties with transition metal cations of a novel methane-bridged bisquarylium dye. Dyes Pigments 2002, 52, 245–252. [Google Scholar] [CrossRef]
- Cole, E.L.; Arunkumar, E.; Xiao, S.; Smith, B.A.; Smith, B.D. Water-soluble, deep-red fluorescent squaraine rotaxanes. Org. Biomol. Chem. 2012, 10, 5769–5773. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.H.; Thiel, S.H.; Gaschler, O. Oxocarbons and related compounds. Part 24. Chlorosquarylation of indoles. J. Chem. Soc. Perkin Trans. 1996, 1, 495–496. [Google Scholar] [CrossRef]
- Dehmlow, E.V. Preparation of 3-alkoxy-4-alkyl-3-cyclobutene-1,2-dione. Chem. Ber. 1980, 113, 1–8. [Google Scholar] [CrossRef]
- Campbell, E.F.; Park, A.K.; Kinney, W.A.; Fengl, R.W.; Liebeskind, L.S. Synthesis of 3-hydroxy-3-cyclobutene-1,2-dione based amino acids. J. Org. Chem. 1995, 60, 1470–1472. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, Q.; Jiang, G.; He, L.; Zhang, B.; Wang, X. The synthesis of 1O2 photosensitization of halogenated asymmetric aniline-based squaraines. New. J. Chem. 2011, 35, 1128–1132. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef]
- Krakowiak, K.E.; Bradshaw, J.S.; Zamecka-Krakowiak, D.J. Synthesis of aza-crown ethers. Chem. Rev. 1989, 89, 929–972. [Google Scholar] [CrossRef]
- Das, S.; Thomas, G.; Thomas, K.J.; George, M.V.; Bedja, I.; Kamat, P.V. Crown ether derivatives of squaraine: New near-infrared-absorbing, redox-active fluorophores for alkali metal recognition. Anal. Proc. 1995, 32, 213–215. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Marcos, M.D.; Pardo, T.; Sancenón, F.; Martínez-Máñez, R.; Rurack, K. Anilinopyridine-metal complexes for the selective chromogenic sensing of cyanide anion. J. Coord. Chem. 2018, 71, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.-X.; Qin, S.-Y.; Zhou, Z.-Y.; Yam, V.W.-W. Synthesis, structure, and ion-binding studies of cobalt(II) complexes with aza-crown substituted salicylaldimine Schiff base ligand. Inorg. Chim. Acta 2003, 346, 49–56. [Google Scholar] [CrossRef]
- Deveci, P.; Taner, B.; Ustundag, Z.; Ozcan, E.; Solak, A.O.; Kilic, Z. Synthesis, enhanced spectroscopic characterization and electrochemical grafting of N-(4-aminophenyl)aza-18-crown-6: Application of DEPT, HETCOR, HMBC-NMR and x-ray photoelectron spectroscopy. J. Mol. Struct. 2010, 982, 162–168. [Google Scholar] [CrossRef]
- Das, S.; Thomas, K.G.; Kamat, P.V.; George, M.V. Photochemistry of squaraine dyes. 8. Photophysical properties of crown ether squaraine fluoroionophores and their metal ion complexes. J. Phys. Chem. 1994, 98, 9291–9296. [Google Scholar] [CrossRef]
- Ros-Lis, J.V.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Spieles, M.; Rurack, K. Squaraines as reporter units: Insights into their photophysics, protonation, and metal-ion coordination behavior. Chem. Eur. J. 2008, 14, 10101–10114. [Google Scholar] [CrossRef]
- Ogus, U.; Akkaya, E.U. One-pot synthesis of a red-fluorescent chemosensor from an azacrown, phloroglucinol and squaric acid: A simple in-solution construction of a functional molecular device. Tetrahedron Lett. 1997, 38, 4509–4512. [Google Scholar] [CrossRef]
- Oguz, U.; Akkaya, E.U. One-pot synthesis of squaraine fluoroionophores. J. Org. Chem. 1998, 63, 6059–6060. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, S.-K.; Park, S.-H.; Yoon, C.-M.; Keum, S.-R. Novel fluorescent chemosensor for Li+ based on a squarylium dye carrying a monoazacrown moiety. Dyes Pigments 1999, 43, 21–25. [Google Scholar] [CrossRef]
- Oguz, U.; Akkaya, E.U. A squraine-based sodium selective fluorescent chemosensor. Tetrahedron Lett. 1998, 39, 5857–5860. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Nie, L.; Xie, C.; Yan, Z. Colorimetric detection of trace Hg2+ with near-infrared absorbing squaraine functionalized by dibenzo-18-crown-6 and its mechanism. Spectrochim. Acta A 2013, 104, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, S.; Kutuk, I.; Ozdemir, T. A near IR di-styryl BODIPY-based ratiometric fluorescent chemosensor for Hg(II). Tetrahedron Lett. 2010, 51, 892–894. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yim, D.; Jang, W.-D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef]
- Ros-Lis, J.V.; Martínez-Máñez, R.; Rurack, K.; Sancenón, F.; Soto, J.; Spieles, M. Highly selective chromogenic signaling of Hg2+ in aqueous media at nanomolar levels employing a squaraine-based reporter. Inorg. Chem. 2004, 43, 5183–5185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheg, Q.; Wang, G.; Fu, N. Ultrasensitive detection of lead (II) based on the disaggregation of a polyether bridged squaraine fluorescent probe. Sens. Actuators B 2016, 237, 802–809. [Google Scholar] [CrossRef]
- Manna, U.; Das, G. Positional isomeric effect of acyclic hosts on supramolecular recognition of anionic guests. Coord. Chem. Rev. 2021, 440, 213931. [Google Scholar] [CrossRef]
- Steed, J.W. A modular approach to anion binding podands: Adaptability in properties. Chem. Commun. 2006, 2637–3649. [Google Scholar] [CrossRef]
- Jeunesse, C.; Armspach, D.; Matt, D. Playing with podands based on cone-shaped cavities. How can a cavity influence the properties of an appended metal centre? Chem. Commun. 2005, 5603–5614. [Google Scholar] [CrossRef]
- Ajayaghosh, A.; Arunkumar, E.; Daub, J. A highly specific Ca2+-ion sensor: Signaling by exciton interaction in a rigid-flexible-rigid bichromophoric “H” foldamer. Angew. Chem. Int. Ed. 2002, 41, 1766–1769. [Google Scholar] [CrossRef]
- Arunkumar, E.; Chithra, P.; Ajayaghosh, A. A controlled supramolecular approach toward cation-specific chemosensors: Alkaline earth metal ion-driven exciton signaling in squaraine tethered podands. J. Am. Chem. Soc. 2004, 126, 6590–6598. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Arunkumar, E. 1H NMR spectral evidence for a specific host-guest complexation induced charge localization in squaraine dyes. Org. Lett. 2005, 7, 3135–3138. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, Y.; Wang, G.; Chen, Y.; Lin, X.; Fu, N. A coordination driven deaggregation approach toward Hg2+-specific chemosensors based on thioether linked squaraine-aniline dyads. Sens. Actuators B 2014, 198, 201–209. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Guo, L.; Fu, N.; Dong, H.; Yan, Y. A squaraine-based colorimetric and “turn on” fluorescent sensor for selective detection of Hg2+ in an aqueous medium. Org. Lett. 2011, 13, 1162–1165. [Google Scholar] [CrossRef]
- Chen, C.; Dong, H.; Chen, Y.; Guo, L.; Wang, Z.; Sun, J.-J.; Fu, N. Dual-mode unsymmetrical squaraine-based sensors for selective detection of Hg2+ in aqueous media. Org. Biomol. Chem. 2011, 9, 8195–8201. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Chen, Y.; Fan, J.; Wang, G.; Lin, S. A bifunctional “turn on” fluorescent probe for trace level Hg2+ and EDTA in aqueous solution via chelator promoted cation induced deaggregation signaling. Sens. Actuators B 2014, 203, 435–443. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Chen, H.; Tang, Z.; Wang, G.; Fu, N. As EDTA promoted coordination induced disaggregation for specific Hg2+ detection. Dyes Pigments 2015, 113, 181–188. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Zhu, H.-J.; Xu, W.-J.; Wang, G.-M.; Fu, N.-Y. A squaraine based fluorescent probe for mercury ion via coordination induced deaggregation signaling. Chin. Chem. Lett. 2014, 25, 1291–1295. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, Q.; Zhang, B.; Wang, X. A new squaraine and Hg2+-based chemosensor with tunable measuring range for thiol-containing amino acids. New J. Chem. 2011, 35, 45–48. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, Q.; Lei, W.; Wang, J.; Zhang, B.; Wang, X. Supramolecular assembly of a new squaraine and β-cyclodextrin for detection of thiol-containing amino acids in water. Supramol. Chem. 2011, 23, 657–662. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Lin, Q.; Fu, N. A fluorescent probe for the dual-channel detection of Hg2+/Ag+ and its Hg2+-based complex for detection of mercapto biomolecules with a tunable measuring range. Sens. Actuators B 2012, 173, 874–881. [Google Scholar] [CrossRef]
- Tsien, R.Y. New Calcium indicators and buffers with high selectivity against magnesium and protons: Design, synthesis, and properties of prototype structures. Biochemistry 1980, 19, 2396–2404. [Google Scholar] [CrossRef]
- Crynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new general of Ca2+ indicators with greatly improved fluorescent properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Roopa, R.; Kumar, N.; Kumar, M.; Bhalla, V. Design and applications of small molecular probes for calcium detection. Chem. Asian J. 2019, 14, 4493–4505. [Google Scholar] [CrossRef]
- Akkaya, E.U.; Turkyilmaz, S. A squaraine-based near IR fluorescent chemosensor for calcium. Tetrahedron Lett. 1997, 38, 4513–4516. [Google Scholar] [CrossRef]
- Elgemeie, G.H.; Azzam, R.A.; Osman, R.R. Recent advances in synthesis, metal complexes and biological evaluation of 2-aryl, 2-pyridyl and 2-pyrimidylbenzothiazoles as potential chemotherapeutics. Inorg. Chim. Acta 2020, 502, 119302. [Google Scholar] [CrossRef]
- Karasik, A.A.; Musina, E.I.; Strelnik, I.D.; Dayanova, I.R.; Elistratova, J.G.; Mustafina, A.R.; Sinyashin, O.G. Luminescent complexes on a scaffold of P2N2-ligands: Design of materials for analytical and biomedical applications. Pure Appl. Chem. 2019, 91, 839–849. [Google Scholar] [CrossRef]
- Chi, Y.; Tong, B.; Chou, P.-T. Metal complexes with pyridyl azolates: Design, preparation and applications. Coord. Chem. Rev. 2014, 281, 1–25. [Google Scholar] [CrossRef]
- Hancock, R.D. The pyridyl group in ligand design for selective metal ion complexation and sensing. Chem. Soc. Rev. 2013, 42, 1500–1524. [Google Scholar] [CrossRef]
- Kumar, S.; Siddhant, S.; Kumar, A.; Kumar, P. Recognition, mechanistic investigation and applications for the detection of biorelevant Cu2+/Fe2+/Fe3+ ions by ruthenium(II)-polypyridyl based fluorescent sensors. Dalton Trans. 2021, 50, 2705–2721. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Hu, Y.; Yoon, J. Fluorescent chemosensors for various analytes including reactive oxygen species, biothiols, metal ions, and toxic gases. ACS Omega 2018, 3, 13731–13751. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chatterjee, N.; Bharadwaj, P.K. Selective sensing first-row transition metal ions through fluorescence enhancement. RSC Adv. 2014, 4, 26585–26620. [Google Scholar] [CrossRef]
- Esteves, C.V.; Costa, J.; Bernard, H.; Tripier, R.; Delgado, R. A squaraine-based dipicolylamine derivative acting as a turn-on mercury(II) fluorescent probe in water. New J. Chem. 2020, 44, 6589–6600. [Google Scholar] [CrossRef]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fu, A.; You, J.; Gao, G.; Lan, J.; Chen, L. Squaraine-based colorimetric and fluorescent sensors for Cu2+-specific detection and fluorescence imaging in living cells. Tetrahedron 2010, 66, 3695–3701. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, Y.; Fan, J.; Wang, R.; Fu, N. A squaraine and Hg2+-based colorimetric and “turn on” fluorescent probe for cysteine. Talanta 2013, 114, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, Q.; Wu, J.; Fu, N. Design and synthesis of a squaraine based near-infrared fluorescent probe for the ratiometric detection of Zn2+ ions. Dyes Pigments 2013, 99, 699–704. [Google Scholar] [CrossRef]
- Xia, G.; Liu, Y.; Ye, B.; Sun, J.; Wang, H. A squaraine-based colorimetric and F− dependent chemosensor for recyclable CO2 gas detection: Highly sensitive off-on-off response. Chem. Commun. 2015, 51, 13802–13805. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ye, B.; Xia, G.; Zhao, X.; Wang, H. A colorimetric and fluorescent chemosensor for the highly sensitive detection of CO2 gas: Experiment and DFT calculation. Sens. Actuators B 2016, 233, 76–82. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, X.; Yoon, J. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Nag, S.; Banerjee, P. Coumarin functionalized molecular scaffolds for the effectual detection of hazardous fluoride and cyanide. Dalton Trans. 2021, 50, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Hewage, H.S.; Anslyn, E.V. Pattern-based recognition of thiols and metals using a single squaraine indicator. J. Am. Chem. Soc. 2009, 131, 13099–13106. [Google Scholar] [CrossRef]
- Sklavounos, A.A.; Pefkianakis, E.K.; Toubanaki, D.T.; Vougiokalakis, G.C.; Calokerinos, A.C. A squaraine derivative for cost-effective, quick, and highly sensitive determination of mercury and thiols and pH sensing. ChemPusChem 2016, 81, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Ros-Lis, J.V.; Garcia, B.; Jimenez, D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Gonzalvo, F.; Valldecabres, M.C. Squaraines as fluoro-chromogenic probes for thiol-containing compounds and their application to the detection of biorelevant thiols. J. Am. Chem. Soc. 2004, 126, 4064–4065. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Lin, T.T.; Wu, J.; Xu, J. Triphenylethylene- and tetraphenylethylene-functionalized 1,3-bis(pyrrol-2-yl)squaraine dyes: Synthesis, aggregation-caused quenching to aggregation-induced emission, and thiol detection. ACS Omega 2018, 3, 16424–16435. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Zhang, J.; Liu, K.; Ding, L.; Peng, H.; Belfield, K.D.; Fang, Y. Squaraine-hydrazine adducts for fast and colorimetric detection of aldehydes in aqueous media. Sens. Actuators B 2019, 292, 88–93. [Google Scholar] [CrossRef]
- Ros-Lis, J.V.; Martínez-Máñez, R.; Soto, J. A selective chromogenic reagent for cyanide determination. Chem. Commun. 2002, 2248–22249. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Valencia, M.A.; Sui, B.; Zhang, Y.; Belfield, K.D. Far-red-emitting TEG-substituted squaraine dye: Synthesis, optical properties, and selective detection of cyanide in aqueous solution. Eur. J. Org. Chem. 2017, 2017, 3957–3964. [Google Scholar] [CrossRef]
- Wright, A.T.; Anslyn, E.V. Differential receptor arrays and assays for solution-based molecular recognition. Chem. Soc. Rev. 2006, 35, 14–28. [Google Scholar] [CrossRef]
- Severin, K. Pattern-based sensing with simple metal-dye complexes. Curr. Opin. Chem. Biol. 2010, 14, 737–742. [Google Scholar] [CrossRef]
- Yu, H.; Fu, M.; Xiao, Y. Switching off FRET by analyte-induced decomposition of squaraine energy acceptor: A concept to transform ‘turn off’ chemodosimeter into ratiometirc sensors. Phys. Chem. Chem. Phys. 2010, 12, 7386–7391. [Google Scholar] [CrossRef]
- Bacher, E.P.; Lepore, A.J.; Pena-Romero, D.; Smith, D.B.; Ashfeld, B. Nucleophilic addition of phosphorus (III) derivatives to squaraines: Colorimetric detection of transition metal-mediated or thermal reversion. Chem. Commun. 2019, 55, 3286–3289. [Google Scholar] [CrossRef] [PubMed]
- Bacher, E.P.; Koh, K.J.; Lepore, A.J.; Oliver, A.G.; Wiest, O.; Ashfeld, B.L. A phosphine-mediated dearomative skeletal rearrangement of dianiline squaraine dyes. Org. Lett. 2021, 23, 2853–2857. [Google Scholar] [CrossRef] [PubMed]
- Gassensmith, J.J.; Baumes, J.M.; Smith, B.D. Discovery and early development of squaraine rotaxane. Chem. Commun. 2009, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.L.; Zhai, C.; Dempsey, J.M.; McGarraugh, H.H.; Matthews, B.P.; Christmann, C.R.; Smith, B.D. Paired agent fluorescence imaging of cancer in a living mouse using preassembled squaraine molecular probes with emission wavelengths of 690 and 830 nm. Bioconjugate Chem. 2020, 31, 214–223. [Google Scholar] [CrossRef]
- Shaw, S.K.; Liu, W.; Gomez Duran, C.F.A.; Schreiber, C.L.; de Lourdes Batacount Mendiola, M.; Zhai, C.; Roland, F.M.; Padanilam, S.J.; Smith, B.D. Non-covalently pre-assembled high-performance near-infrared fluorescent molecular probes. Chem. Eur. J. 2018, 24, 13821–13829. [Google Scholar] [CrossRef]
- Lee, J.-J.; White, A.G.; Rice, D.R.; Smith, B.D. In vivo imaging using polymeric nanoparticles stained with near-infrared chemiluminescent and fluorescent squaraine catenane endoperoxide. Chem. Commun. 2013, 49, 3016–3018. [Google Scholar] [CrossRef] [Green Version]
- Diehl, K.; Bachman, J.L.; Anslyn, E.V. Tuning thiol addition to squaraines by ortho-substitution and the use of serum albumin. Dyes Pigments 2017, 141, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Karpenko, I.A.; Klymchenko, A.S.; Gioria, S.; Kreder, R.; Shulov, L.; Villa, P.; Mély, Y.; Hibert, M.; Bonnet, D. Squaraine as a bright, stable and environment-sensitive far-red label for receptor-specific cellular imaging. Chem. Commun. 2015, 51, 2960–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yu, Y.; Zhao, Q.; Tang, C.; Zhang, H.; Qin, Y.; Feng, X.; Zhang, J. Fluorescent probes based on nucleophilic aromatic substitution reactions for reactive sulfur and selenium species: Recent progress, applications, and design strategies. Coord. Chem. Rev. 2021, 427, 213601. [Google Scholar] [CrossRef]
- Peng, H.; Chen, W.; Cheng, Y.; Hakuna, L.; Strongin, R.; Wang, B. Thiol reactive probes and chemosensors. Sensors 2012, 12, 15907–15946. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.; Abe, H.; Ito, M.; Kondo, Y.; Shimizu, S.; Aikawa, K.; Ito, Y. DNA template nucleophilic aromatic substitution reactions for fluorogenic sensing of oligonucleotides. Chem. Commun. 2009, 6586–6588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shibata, A.; Ito, M.; Shuto, S.; Ito, Y.; Mannervik, B.; Abe, H.; Morgenstern, R. Synthesis and characterization of a series of highly fluorogenic substrates for glutathione transferases, a general strategy. J. Am. Chem. Soc. 2011, 133, 14109–14119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-D.; Sun, R.; Ge, J.-F.; Xu, Y.-J.; Xu, Y.; Lu, J.-M. A squaraine-based red emission off-on chemosensor for biothiols and its application in living cells imaging. Org. Biomol. Chem. 2013, 11, 4258–4264. [Google Scholar] [CrossRef]
- Xiong, L.; Ma, J.; Huang, Y.; Wang, Z.; Lu, Z. Highly sensitive squaraine-based water-soluble far-red/near-infrared chromofluorogenic thiophenol probe. ACS Sens. 2017, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fu, Q.; Fan, H.; Ho, J.; Wang, W. A highly selective fluorescent probe for thiophenols. Angew. Chem. Int. Ed. 2007, 46, 8445–8448. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, Y.; Liu, Y.; Wang, W. Rational design of a highly selective and sensitive fluorescent PET probe for discrimination of thiophenols and aliphatic thiols. Chem. Commun. 2010, 46, 1944–1946. [Google Scholar] [CrossRef]
- Wang, G.; Xu, W.; Yang, H.; Fu, N. Highly sensitive and selective strategy for imaging Hg2+ using near-infrared squaraine dye in live cells and zebrafish. Dyes Pigments 2018, 157, 369–376. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s reaction in organic synthesis. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Philips, D.S.; Ghosh, S.; Sudheesh, K.V.; Suresh, C.H.; Ajayaghosh, A. An unsymmetrical squaraine-dye-based chemical platform for multiple analyte recognition. Chem. Eur. J. 2017, 23, 17973–17980. [Google Scholar] [CrossRef] [PubMed]


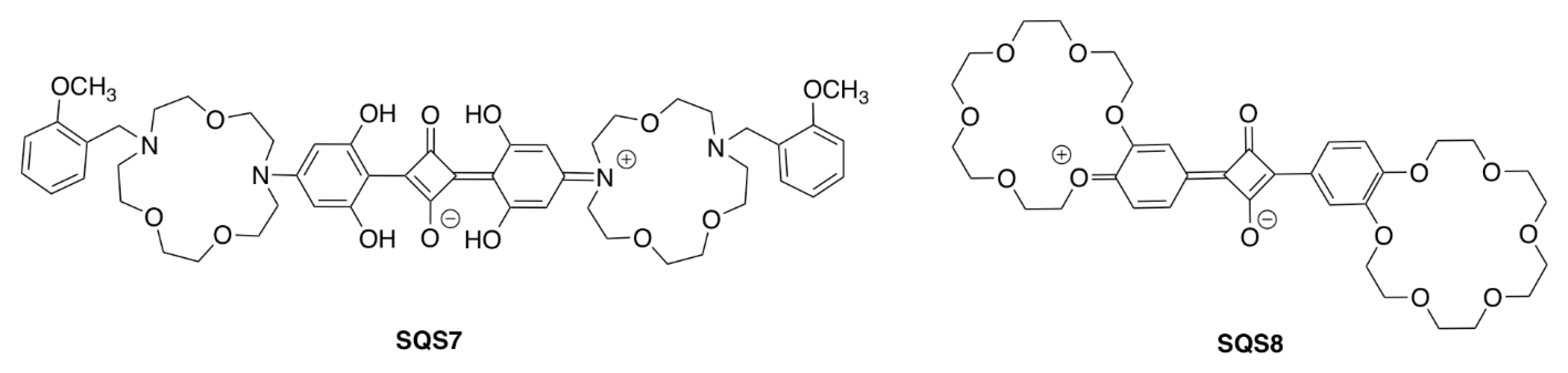
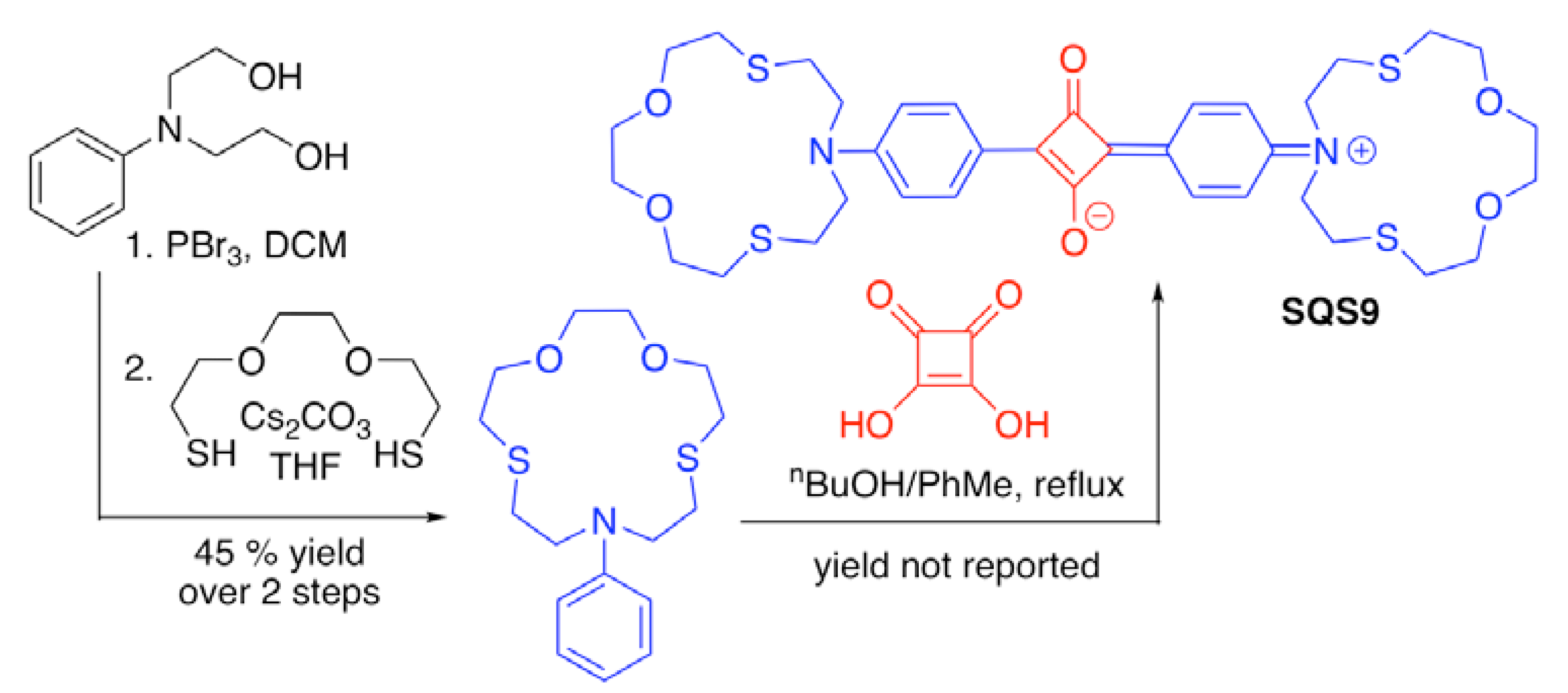
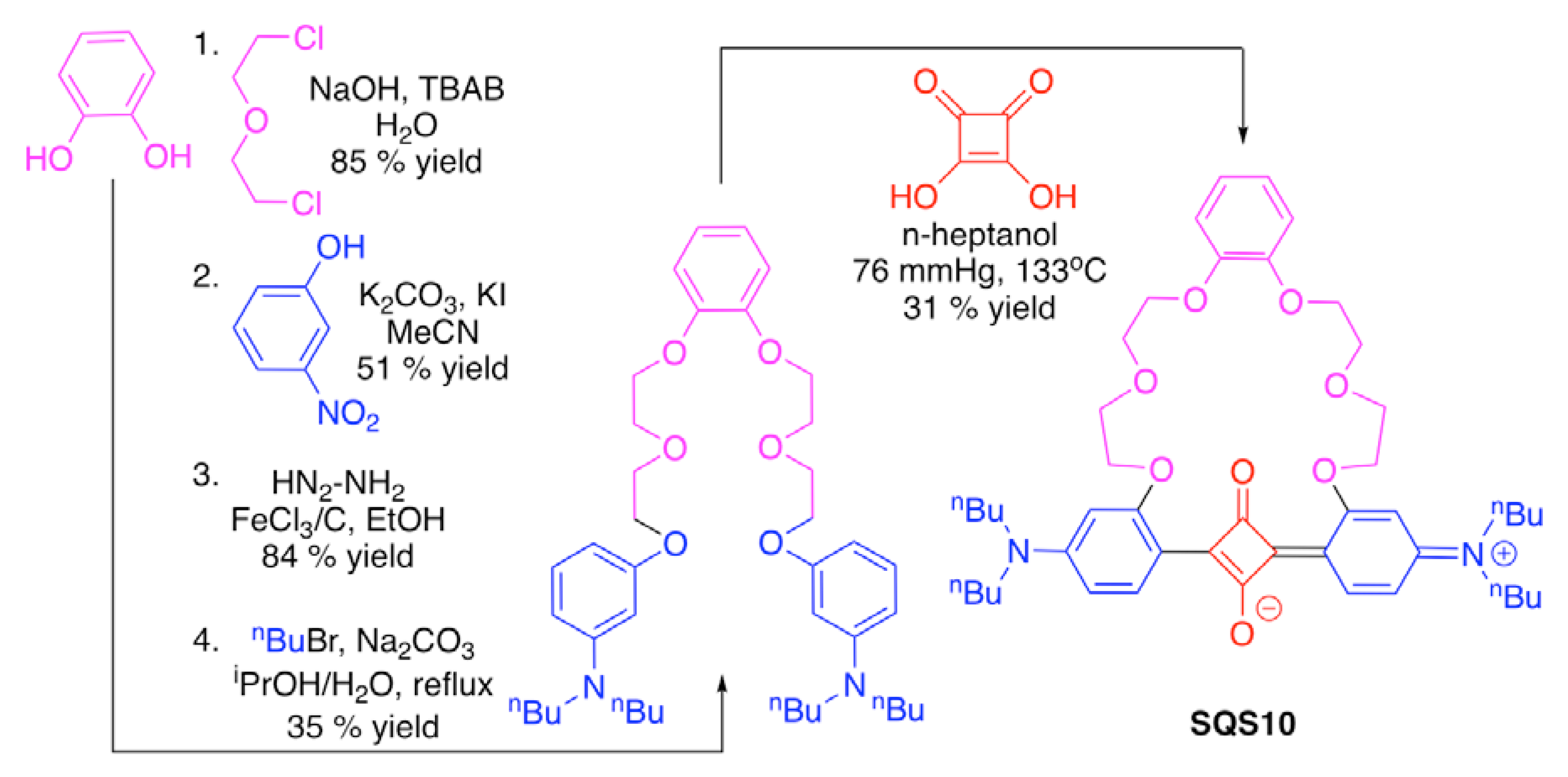
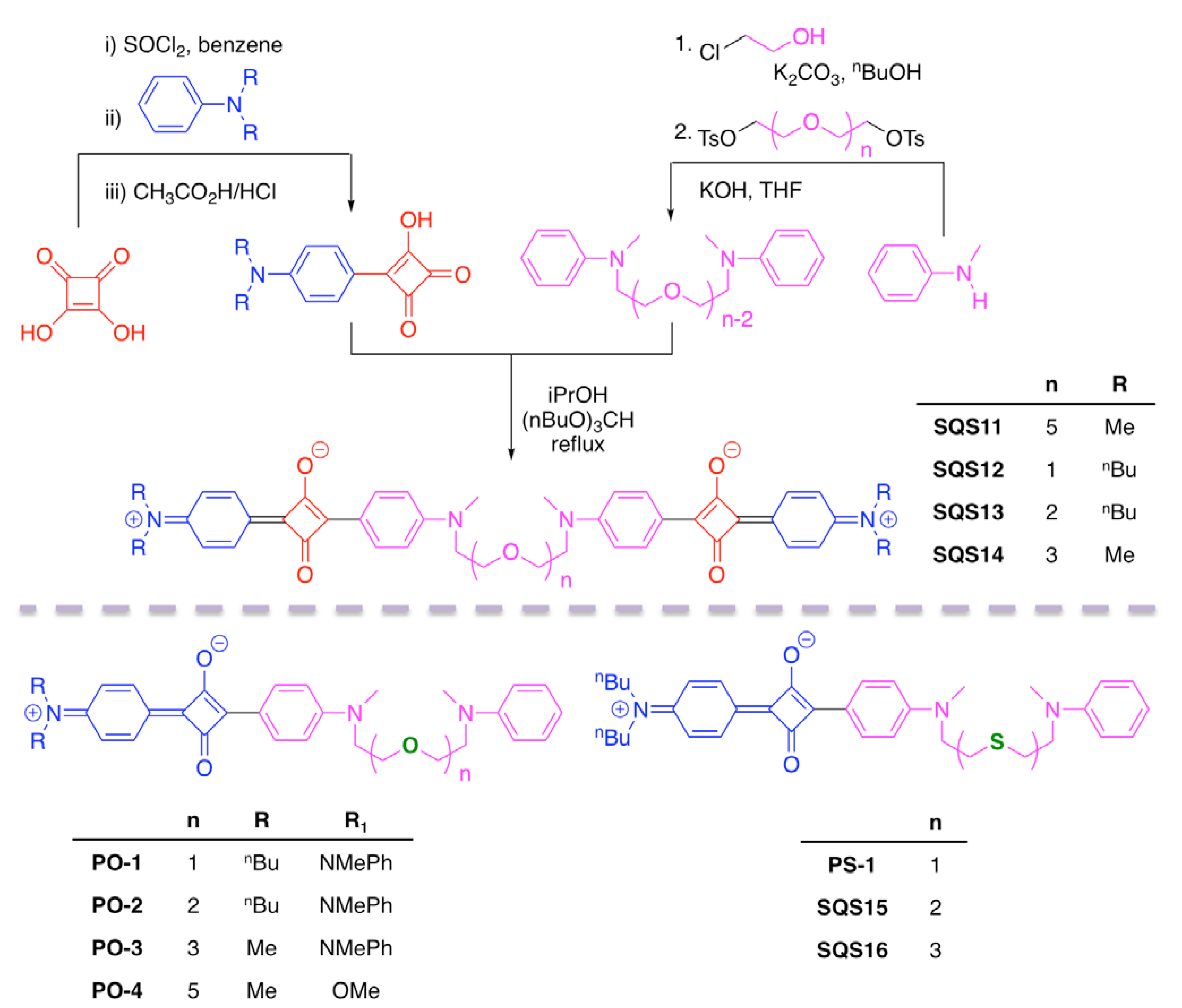





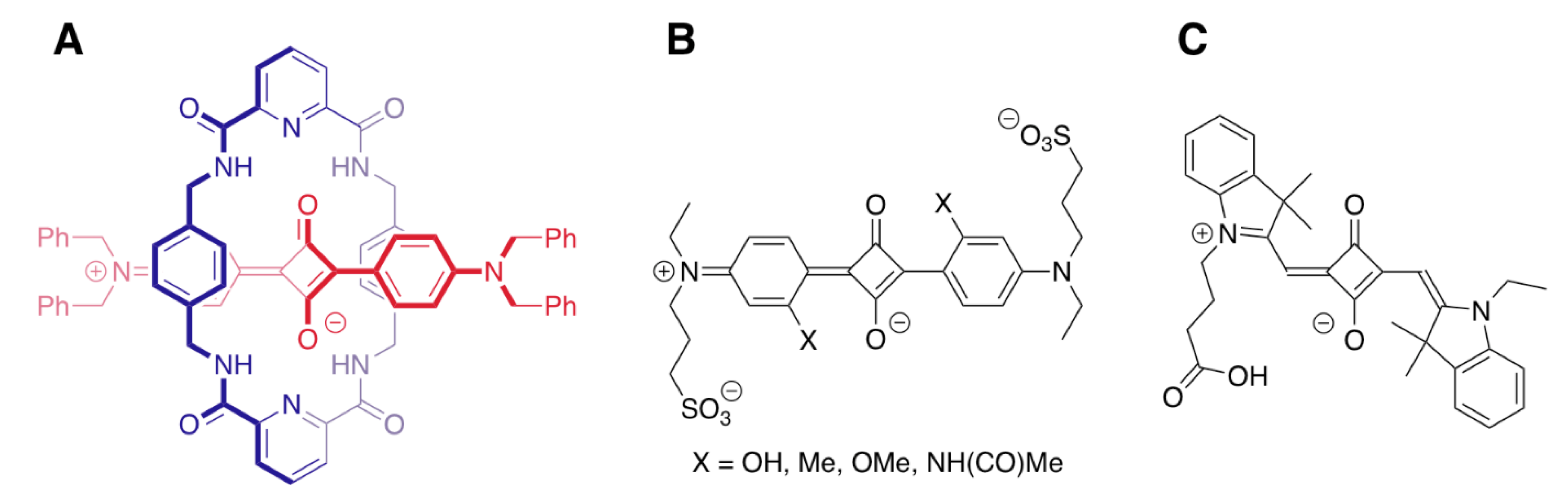
| Recognition Motif | SQS# | Analyte | Refs. |
|---|---|---|---|
| crown ether | |||
| SQS1 | Li+ | [53] | |
| SQS2 | Li+, Na+ | [53] | |
| SQS3 | Ba2+, Pb2+, Cu2+, Fe3+, Hg2+ | [58] | |
| SQS4 | Mg2+, Ba2+ | [59] | |
| SQS5 | – | [60] | |
| SQS6 | Li+ | [61] | |
| SQS7 | Na+ | [62] | |
| SQS8 | Hg2+ | [63] | |
| SQS9 | Hg2+, Ag+, Fe3+, Cu2+ | [58,66] | |
| SQS10 | Pb2+, Cu2+ | [67] | |
| podand | |||
| SQS11 | Ca2+ | [71] | |
| SQS12 | Ca2+, Mg2+ | [72] | |
| SQS13 | Ca2+, Mg2+ | [72] | |
| SQS14 | Ca2+, Mg2+ | [72] | |
| SQS15 | Hg2+ | [74] | |
| SQS16 | Hg2+ | [74] | |
| SQS17 | Hg2+ | [75] | |
| SQS18 | Hg2+ | [76] | |
| SQS19 | Hg2+ | [77] | |
| SQS20 | Hg2+ | [78] | |
| SQS21 | Hg2+ | [79] | |
| SQS22 | Hg2+, Cu2+, Fe3+ | [80] | |
| SQS23 | Hg2+, Cys, Hcys, GSH | [81] | |
| SQS24 | Hg2+, Ag+, Cys, His, Trp | [82] | |
| tetracarboxylate | |||
| SQS25 | Ca2+ | [86] | |
| nitrogen-containing heterocycle | |||
| SQS26 | Hg2+ | [94] | |
| SQS27 | Cu2+ | [96] | |
| SQS28 | Cu2+ | [96] | |
| SQS29 | Hg2+ | [97] | |
| SQS30 | Zn2+, Cd2+, Cu2+, Co2+, Ni2+, Ag+ | [98] | |
| acidic NH | |||
| SQS31 | F−, CO2 | [99] | |
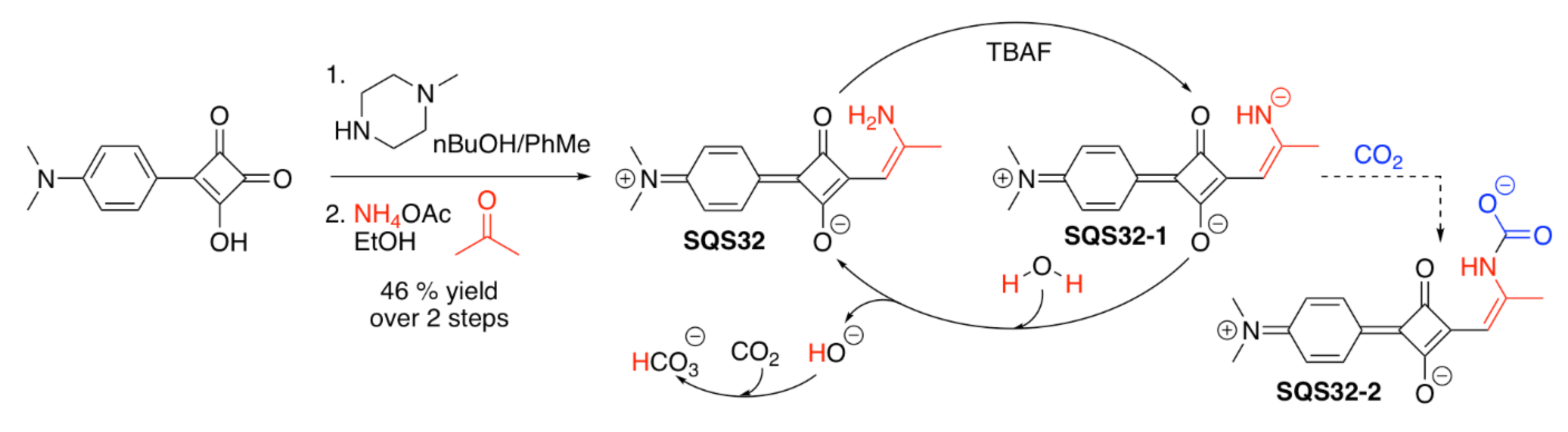
| SQS32 | F−, CO2 | [100] | |
| squaraine-ring as electrophile | |||
| SQS33 | propane-1-thiol, 3-mercapto-propionic acid, 2-acetylamino-3-mercapto-propionic acid methyl ester, 2,3-dimercapto-propane-1-ol, naphthalene-2-thiol; Pd2+, Hg2+, Ni2+, Cu2+, Fe2+ | [103] | |
| SQS34 | 4-acetamidothiophenol, N-acetyl-L-Cys, L-Cys methyl ether, L-Cys, 2-(dimethylamino)ethanethiol; Hg2+ | [104] | |
| SQS35 | propanethiol, Cys, Cys-Gly, GSH | [105] | |
| CN− | [108] | ||
| SQS36 | Cys, GSH | [106] | |
| SQS37 | NH2NH2, HO−, ethanolamine, methylamine, β-phenylethylamine; glyoxal, glutaric dialdehyde, propionaldehyde, pyridine-2-carboxaldehyde, formaldehyde, acetaldehyde | [107] | |
| SQS38 | CN− | [109] | |
| SQS39 | F−, CN− | [112] | |
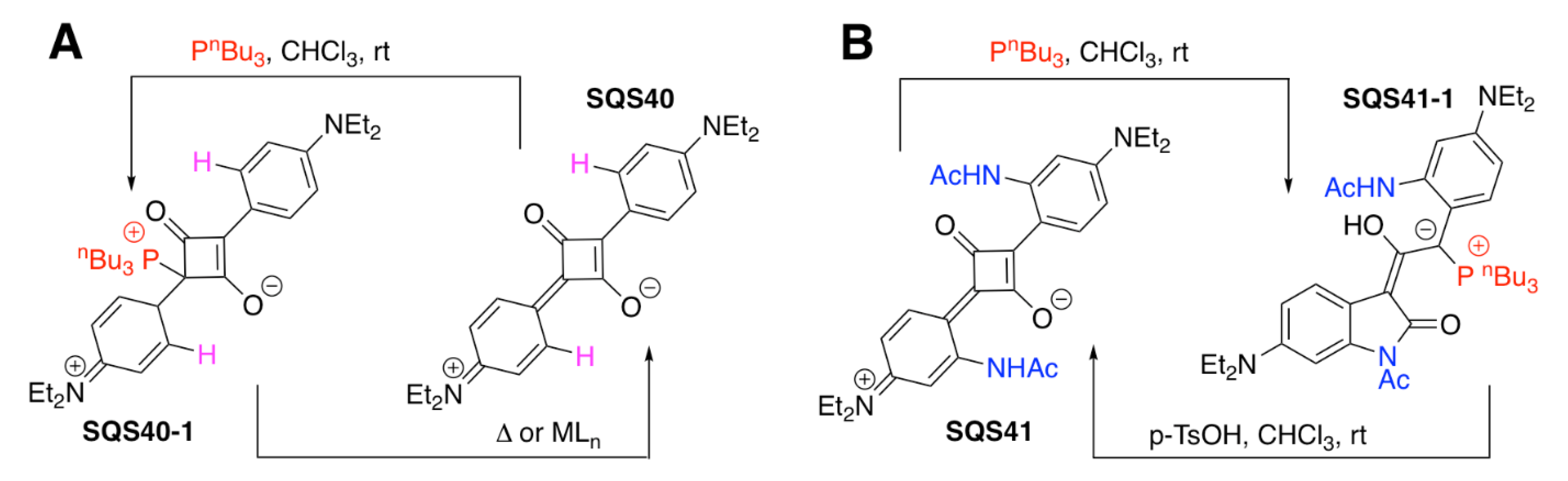
| SQS40 | PnBu3, PN(Me2)3, P(p-MeO-C6H4)3; Pd2+, Rh+, Ag+, Au3+, Cu+, Cu2+ | [113] | |
| SQS41 | PnBu3; TsOH | [114] | |
| caging group | |||
| SQS42 | Cys, Hcys | [125] | |
| SQS43 | PhSH | [126] | |
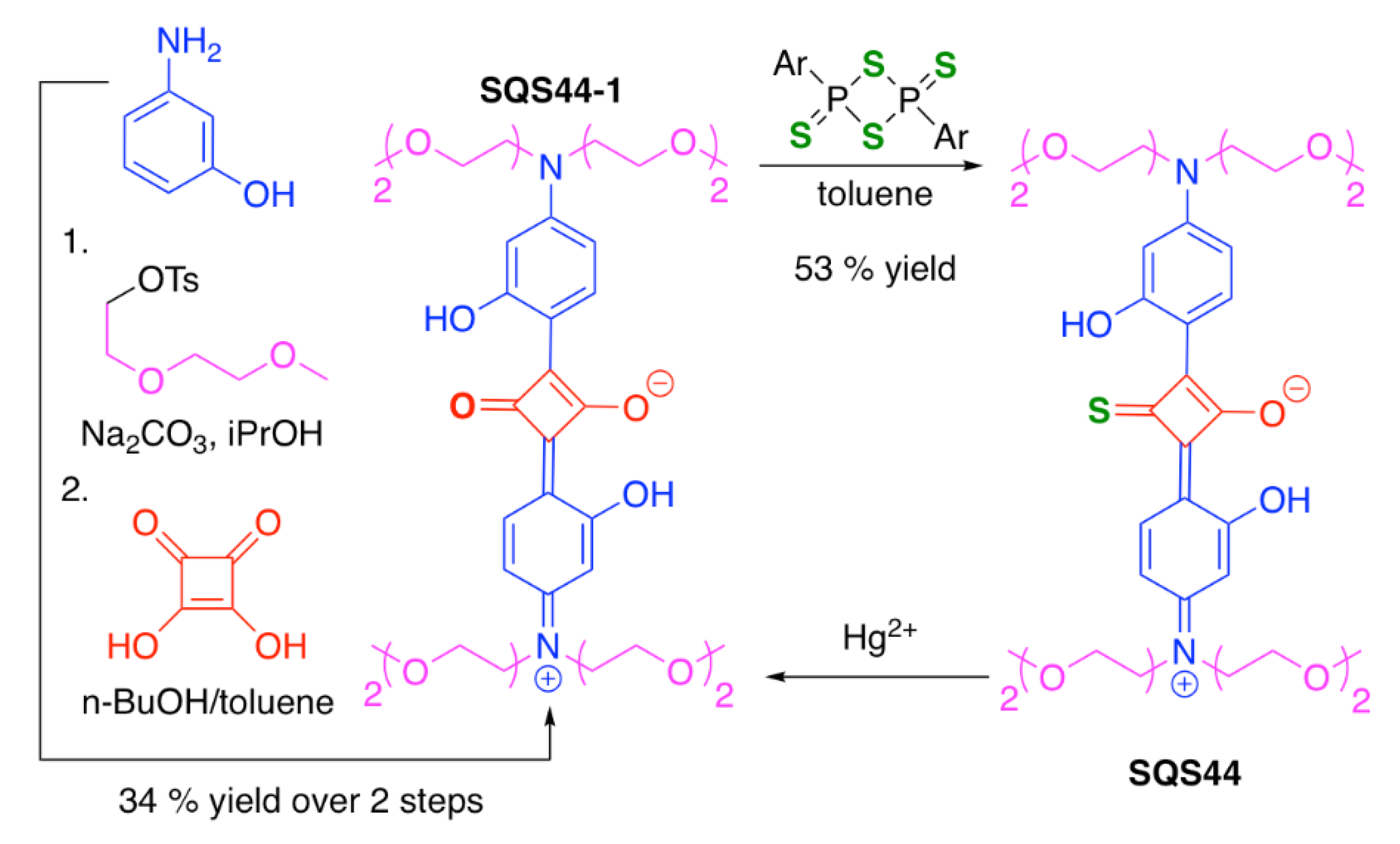
| SQS44 | Hg2+ | [129] | |
| metal-containing scaffold | |||
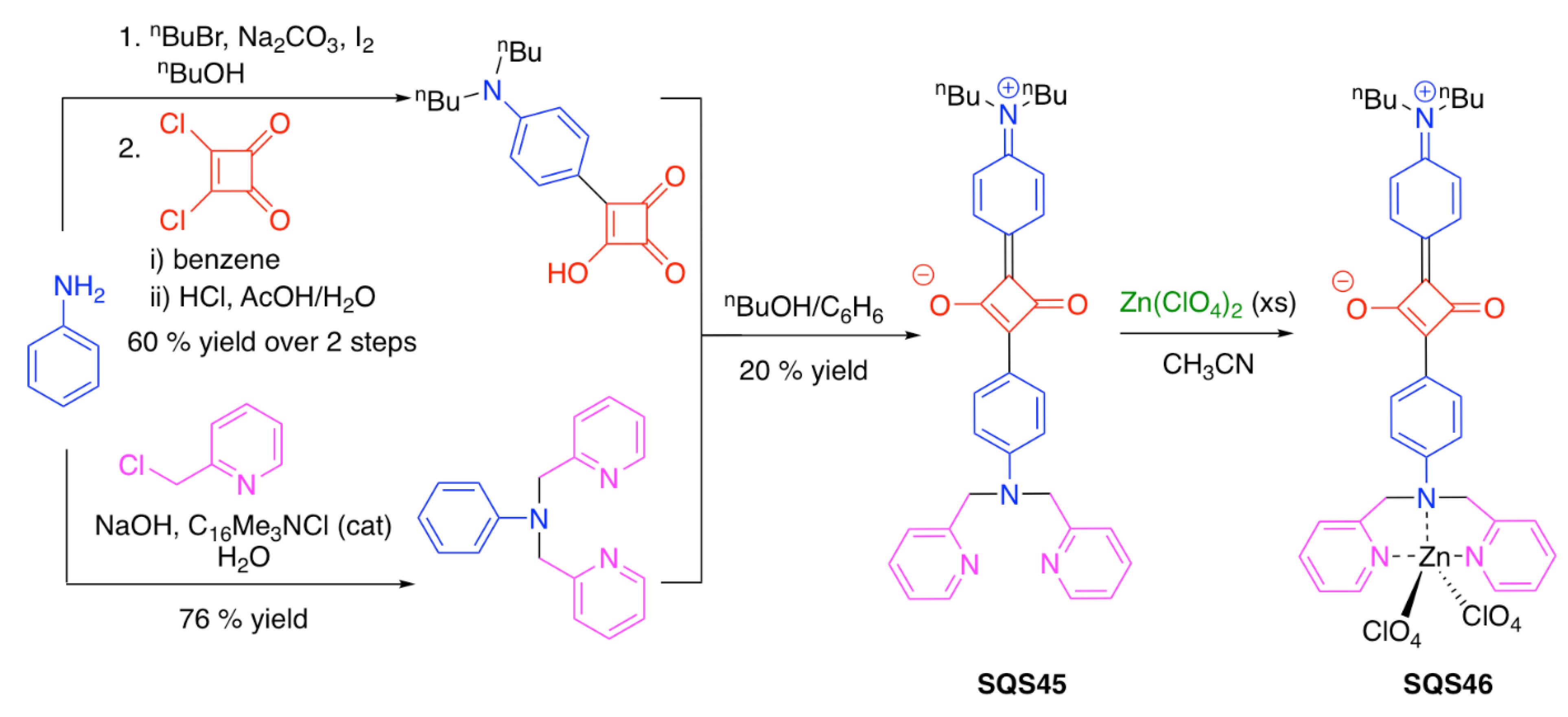
| SQS45 | Zn2+ | [131] | |
| SQS46 | CO2/CO32−; GMP, c-GMP; picric acid | [131] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, D.D.; Dzyuba, S.V. Squaraine-Based Optical Sensors: Designer Toolbox for Exploring Ionic and Molecular Recognitions. Chemosensors 2021, 9, 302. https://doi.org/10.3390/chemosensors9110302
Ta DD, Dzyuba SV. Squaraine-Based Optical Sensors: Designer Toolbox for Exploring Ionic and Molecular Recognitions. Chemosensors. 2021; 9(11):302. https://doi.org/10.3390/chemosensors9110302
Chicago/Turabian StyleTa, Daniel D., and Sergei V. Dzyuba. 2021. "Squaraine-Based Optical Sensors: Designer Toolbox for Exploring Ionic and Molecular Recognitions" Chemosensors 9, no. 11: 302. https://doi.org/10.3390/chemosensors9110302
APA StyleTa, D. D., & Dzyuba, S. V. (2021). Squaraine-Based Optical Sensors: Designer Toolbox for Exploring Ionic and Molecular Recognitions. Chemosensors, 9(11), 302. https://doi.org/10.3390/chemosensors9110302