Concentration-Dependent Fluorescence Emission of Quercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Computational Details
3. Results and Discussion
3.1. FL Spectra of Q in Solutions
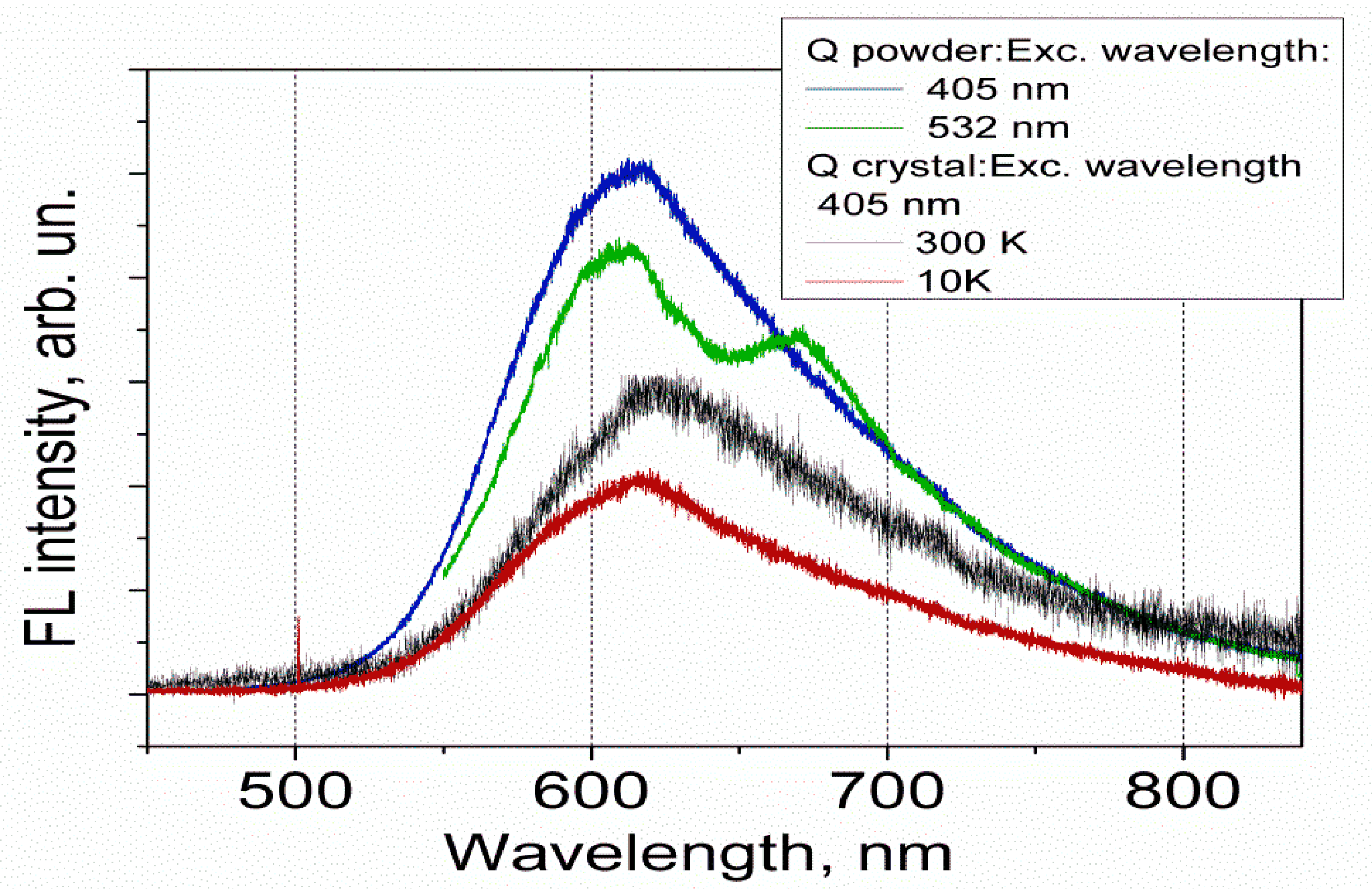
3.2. FL Spectra of Q in the Solid State
3.3. Computation of FL Characteristic Wavelengths
4. Conclusions
- The FL spectra were measured for Q solutions in different types of solvents (non-polar, polar protic and polar aprotic), for Q crystals and Q anhydrous powder, using excitation laser-line of 405 nm. Only one peak with maximum at 505 nm was observed for low concentration solutions for all the solvents used. For powder and solutions with high concentrations the same spectral features at 615 and 670 nm were observed in the FL emission spectra.
- The TDDFT-M06-2X/6-31++G(d,p) approach was successfully used to calculate the characteristic wavelengths of the FL emission of Q molecule in solvents and vacuum. As far as we know, this methodology was applied for the first time to find the absorption and emission energies of Q molecule in methanol, acetone, and chloroform solvents. Besides, the spontaneous transition of –OH5 group proton in the first excited state for Q molecule in vacuum was not reported before.
- The FL emission peak observed for all Q solutions in methanol, acetone, and chloroform with low concentration (505 nm) is due to the Q_M keto OH3 transition with calculated values of 485–487 nm.
- Computational results of the FL emission wavelength for Q molecule in the solid state using the molecule configuration corresponding to Q_A keto O5 form in vacuum (630 nm), show a good agreement with the experimental data (615 nm). The spectral feature at 615 nm observed in the FL emission spectra of the solutions with high concentrations, can be related to the presence of undissolved clusters of Q molecules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, A.T.; Kamli, M.R.; Murtaza, I.; Singh, J.B.; Ali, A.; Haq, Q.M.R. Dietary Flavonoid Quercetin and Associated Health Benefits—An Overview. Food Rev. Int. 2010, 26, 302–317. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Drent, M.; de Boer, V.C.J.; Bast, A.; Haenen, G.R.M.M. Quercetin Reduces Markers of Oxidative Stress and Inflammation in Sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The Flavonoid Quercetin in Disease Prevention and Therapy: Facts and Fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Wiseman, R.L.; Zhang, Y.; Lee, K.P.K.; Harding, H.P.; Haynes, C.M.; Price, J.; Sicheri, F.; Ron, D. Flavonol Activation Defines an Unanticipated Ligand-Binding Site in the Kinase-RNase Domain of IRE1. Mol. Cell 2010, 38, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Solnier, J.; Fladerer, J.-P. Flavonoids: A Complementary Approach to Conventional Therapy of COVID-19? Phytochem. Rev. 2021, 20, 773–795. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.-S. Inhibition of SARS-CoV 3CL Protease by Flavonoids. J. Enzym. Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. IJGM 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Tong, C.; Shi, F.; Tong, X.; Shi, S.; Ali, I.; Guo, Y. Shining Natural Flavonols in Sensing and Bioimaging. TrAC Trends Anal. Chem. 2021, 137, 116222. [Google Scholar] [CrossRef]
- Alva-Ensastegui, J.C.; Palomar-Pardavé, M.; Romero-Romo, M.; Ramírez-Silva, M.T. Quercetin Spectrofluorometric Quantification in Aqueous Media Using Different Surfactants as Fluorescence Promoters. RSC Adv. 2018, 8, 10980–10986. [Google Scholar] [CrossRef] [Green Version]
- Nghia, N.N.; Huy, B.T.; Lee, Y.-I. Highly Sensitive and Selective Optosensing of Quercetin Based on Novel Complexation with Yttrium Ions. Analyst 2020, 145, 3376–3384. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Novel Quercetin Aggregation-Induced Emission Luminogen (AIEgen) with Excited-State Intramolecular Proton Transfer for In Vivo Bioimaging. Adv. Funct. Mater. 2018, 28, 1706196. [Google Scholar] [CrossRef]
- Parr, R.G.; Weitao, Y. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1994; ISBN 978-0-19-535773-8. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of Electronic Excitations within the Adiabatic Approximation of Time Dependent Density Functional Theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 2003, 54, 724. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-Row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self--consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1998, 80, 3265. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian∼09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Simkovitch, R.; Huppert, D. Excited-State Intramolecular Proton Transfer of the Natural Product Quercetin. J. Phys. Chem. B 2015, 119, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Protti, S.; Lapouge, C.; Cornard, J.-P. Protic Equilibria as the Key Factor of Quercetin Emission in Solution. Relevance to Biochemical and Analytical Studies. Phys. Chem. Chem. Phys. 2011, 13, 6858–6864. [Google Scholar] [CrossRef]
- Simkovitch, R.; Huppert, D. Intramolecular Excited-State Hydrogen Transfer in Rutin and Quercetin. Isr. J. Chem. 2017, 57, 393–402. [Google Scholar] [CrossRef]
- Olejniczak, S.; Potrzebowski, M.J. Solid State NMR Studies and Density Functional Theory (DFT) Calculations of Conformers of Quercetin. Electronic Supplementary Information (ESI) Available: TGA Profiles for Samples 1 and 2 and 13C NMR Shielding Parameters. Org. Biomol. Chem. 2004, 2, 2315. [Google Scholar] [CrossRef] [PubMed]
- Vasisht, K.; Chadha, K.; Karan, M.; Bhalla, Y.; Jena, A.K.; Chadha, R. Enhancing Biopharmaceutical Parameters of Bioflavonoid Quercetin by Cocrystallization. CrystEngComm 2016, 18, 1403–1415. [Google Scholar] [CrossRef]
- Guharay, J.; Sengupta, P.K. Excited-State Proton-Transfer and Dual Fluorescence of Robinetin in Different Environments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 905–912. [Google Scholar] [CrossRef]
- Sengupta, P.K. Pharmacologically Active Plant Flavonols as Proton Transfer Based Multiparametric Fluorescence Probes Targeting Biomolecules: Perspectives and Prospects. In Reviews in Fluorescence 2016; Geddes, C.D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 45–70. ISBN 978-3-319-48259-0. [Google Scholar]
- Yang, Y.; Chen, Y.; Zhao, Y.; Shi, W.; Ma, F.; Li, Y. Under Different Solvents Excited-State Intramolecular Proton Transfer Mechanism and Solvatochromic Effect of 2-(2-Hydroxyphenyl) Benzothiazole Molecule. J. Lumin. 2019, 206, 326–334. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Li, Y. Theoretical Study of the ESIPT Process for a New Natural Product Quercetin. Sci. Rep. 2016, 6, 32152. [Google Scholar] [CrossRef] [Green Version]


| ES0 (a.u) S0 opt | ES1 (a.u) S0 opt | ES1–ES0 (eV) | λab (nm) | fab | ES1* (a.u) S1 opt | ES0* (a.u) S1 opt | ES1*–ES0* (eV) | λem (nm) | fem | |
|---|---|---|---|---|---|---|---|---|---|---|
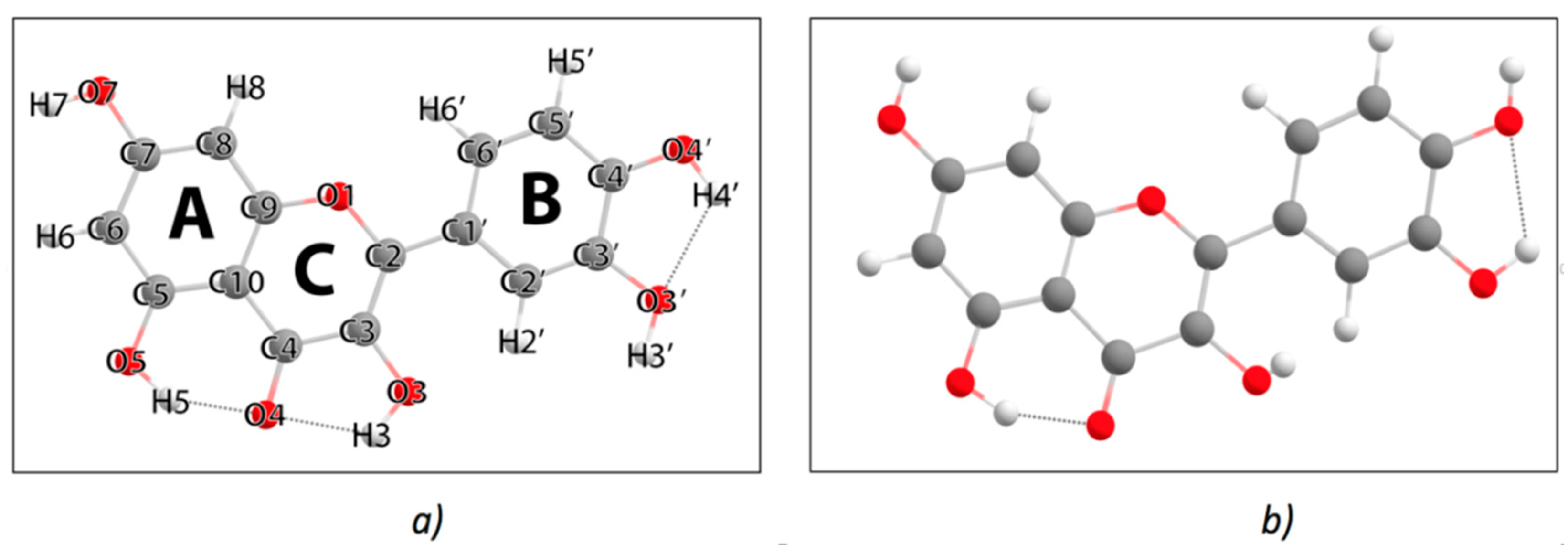
| Q_M enol (Figure 4a) | ||||||||||
| Methanol | −1103.8499 | −1103.7112 | 3.775 | 328.46 | 0.69 | −1103.7212 | −1103.8385 | 3.194 | 388.27 | 1.00 |
| Acetone | −1103.8494 | −1103.7107 | 3.773 | 328.65 | 0.70 | −1103.7205 | −1103.8382 | 3.201 | 387.41 | 0.99 |
| Chloroform | −1103.8453 | −1103.7068 | 3.769 | 329.01 | 0.73 | −1103.7155 | −1103.8351 | 3.252 | 381.31 | 0.91 |
| Q_M keto OH3 (Figure 5a) | ||||||||||
| Methanol | −1103.8229 | −1103.7132 | 2.983 | 415.63 | 0.78 | −1103.7215 | −1103.8154 | 2.554 | 485.52 | 0.78 |
| Acetone | −1103.8223 | −1103.7130 | 2.976 | 416.67 | 0.78 | −1103.7210 | −1103.8149 | 2.553 | 485.66 | 0.78 |
| Chloroform | −1103.8175 | −1103.7100 | 2.925 | 423.88 | 0.71 | −1103.7174 | −1103.8109 | 2.544 | 487.45 | 0.71 |
| Q_M keto OH5 (Figure 5b) | ||||||||||
| Methanol Acetone Chloroform | −1103.8310 | −1103.7162 | 3.122 | 397.17 | 0.46 | −1103.7354 | −1103.8180 | 2.249 | 551.29 | 0.46 |
| −1103.8296 | −1103.7154 | 3.105 | 399.29 | 0.45 | −1103.7349 | −1103.8175 | 2.250 | 551.62 | 0.45 | |
| −1103.8257 | −1103.7134 | 3.057 | 405.59 | 0.32 | −1103.7307 | −1103.8123 | 2.221 | 558.25 | 0.31 | |
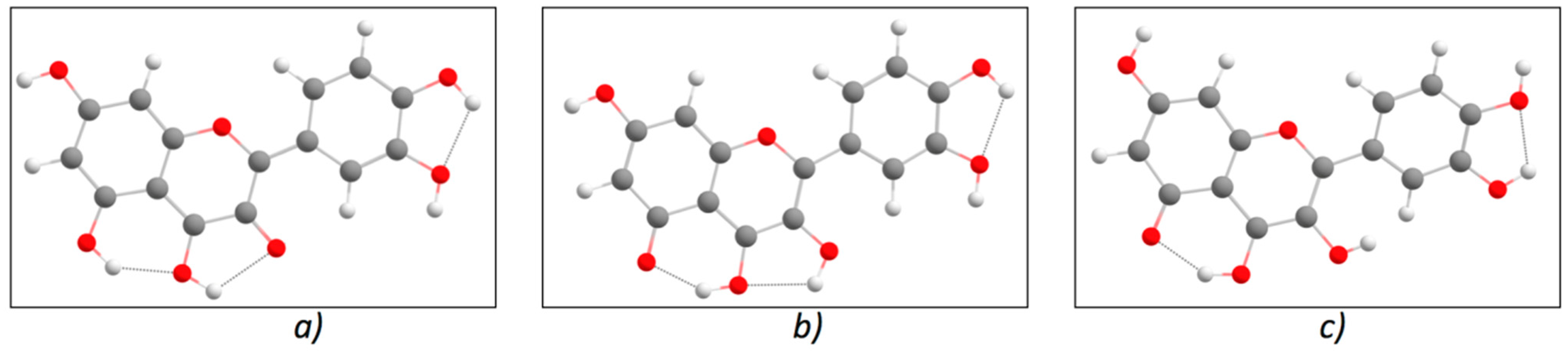
| Q_A enol (Figure 4b) | ||||||||||
| Vacuum | −1103.8183 | −1103.6667 | 4.124 | 300.65 | 0.35 | - | - | - | - | - |
| Q_A keto OH5 (Figure 5c) | ||||||||||
| Vacuum | −1103.8001 | −1103.6862 | 3.098 | 400.23 | 0.20 | −1103.7045 | −1103.7768 | 1.965 | 630.87 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prutskij, T.; Deriabina, A.; Melendez, F.J.; Castro, M.E.; Castillo Trejo, L.; Vazquez Leon, G.D.; Gonzalez, E.; Perova, T.S. Concentration-Dependent Fluorescence Emission of Quercetin. Chemosensors 2021, 9, 315. https://doi.org/10.3390/chemosensors9110315
Prutskij T, Deriabina A, Melendez FJ, Castro ME, Castillo Trejo L, Vazquez Leon GD, Gonzalez E, Perova TS. Concentration-Dependent Fluorescence Emission of Quercetin. Chemosensors. 2021; 9(11):315. https://doi.org/10.3390/chemosensors9110315
Chicago/Turabian StylePrutskij, Tatiana, Alexandra Deriabina, Francisco J. Melendez, María Eugenia Castro, Leticia Castillo Trejo, German D. Vazquez Leon, Eduardo Gonzalez, and Tatiana S. Perova. 2021. "Concentration-Dependent Fluorescence Emission of Quercetin" Chemosensors 9, no. 11: 315. https://doi.org/10.3390/chemosensors9110315
APA StylePrutskij, T., Deriabina, A., Melendez, F. J., Castro, M. E., Castillo Trejo, L., Vazquez Leon, G. D., Gonzalez, E., & Perova, T. S. (2021). Concentration-Dependent Fluorescence Emission of Quercetin. Chemosensors, 9(11), 315. https://doi.org/10.3390/chemosensors9110315







