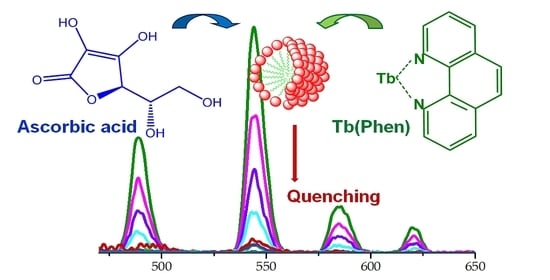
Terbium(III) as a Fluorescent Probe for Molecular Detection of Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Detection of Ascorbic Acid in Tablets
2.4. UV/Vis
2.5. Fluorescence
2.6. DLS
2.7. pH
3. Results and Discussion
3.1. Complexation of Tb(III) Ions with 1,10-Phenanthroline and Ascorbic Acid
3.2. The Effect of Surfactants on the Fluorescence of Tb(III) Ions
3.3. The Effect of pH
3.4. Quenching Effect Calibration Graph and Detection Limits of Ascorbic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-Activated Nanoparticles: A Toolbox for Bioimaging, Therapeutics, and Neuromodulation. Acc. Chem. Res. 2020, 53, 2692–2704. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medina, A.; Llorent-Martínez, E.J.; Ortega-Barrales, P.; Córdova, M.L.F.-D.; Pilar, O.-B. Lanthanide-Sensitized Luminescence as a Promising Tool in Clinical Analysis. Appl. Spectrosc. Rev. 2011, 46, 561–580. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total. Environ. 2021, 754, 142363. [Google Scholar] [CrossRef]
- Ortega-Algar, S.; Ramos-Martos, N.; Molina-Díaz, A. Flow-injection solid surface lanthanide-sensitized luminescence sensor for determination of p-aminobenzoic acid. Anal. Bioanal. Chem. 2008, 391, 715–719. [Google Scholar] [CrossRef]
- Dong, Y.; Suo, H.; Yang, X.; Zhao, X.; Zhou, X. Eu(III) complex based on nonsteroidal anti-inflammatory drugs loxoprofen as the ligand: A novel low-toxic luminescent material for cell imaging. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2020, 229, 118014. [Google Scholar] [CrossRef]
- Selivanova, N.; Vasilieva, K.; Galyametdinov, Y. Luminescent complexes of terbium ion for molecular recognition of ibuprofen. Luminescence 2013, 29, 202–210. [Google Scholar] [CrossRef]
- Shtykov, S.N.; Smirnova, T.D.; Bylinkin, Y.G.; Zhemerichkin, D.A. Fluorimetric determination of tetracyclines with the europium chelate of 1,10-phenanthroline in micellar solutions of anionic surfactants. J. Anal. Chem. 2005, 60, 24–28. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Bingham, S.; Welch, A.; Luben, R.; Wareham, N.; Oakes, S.; Day, N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: A prospective population study. Lancet 2001, 357, 657–663. [Google Scholar] [CrossRef]
- Güçlü, K.; Sözgen, K.; Tütem, E.; Ozyürek, M.; Apak, R. Spectrophotometric determination of ascorbic acid using copper(II)–neocuproine reagent in beverages and pharmaceuticals. Talanta 2005, 65, 1226–1232. [Google Scholar] [CrossRef]
- Gökmen, V.; Kahraman, N.; Demir, N.; Acar, J. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. J. Chromatogr. A 2000, 881, 309–316. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Hu, B.; Wang, J.-H. Label-free colorimetric sensing of ascorbic acid based on Fenton reaction with unmodified gold nanoparticle probes and multiple molecular logic gates. Anal. Chim. Acta 2012, 717, 127–133. [Google Scholar] [CrossRef]
- Kong, W.; Wu, D.; Li, G.; Chen, X.; Gong, P.; Sun, Z.; Chen, G.; Xia, L.; You, J.; Wu, Y. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta 2017, 165, 677–684. [Google Scholar] [CrossRef]
- Smirnova, T.D.; Shtykov, S.N.; Zhelobitskaya, E.A. Energy transfer in liquid and solid nanoobjects: Application in luminescent analysis. Phys. Sci. Rev. 2018, 4, 4. [Google Scholar] [CrossRef]
- Mohammadian, E.; Rahimpour, E.; Alizadeh-Sani, M.; Foroumadi, A.; Jouyban, A. An overview on terbium sensitized based-optical sensors/nanosensors for determination of pharmaceuticals. Appl. Spectrosc. Rev. 2020, 1–38. [Google Scholar] [CrossRef]
- Tan, H.; Wu, J.; Chen, Y. Terbium(III) based coordination polymer microparticles as a luminescent probe for ascorbic acid. Microchim. Acta 2014, 181, 1431–1437. [Google Scholar] [CrossRef]
- Wang, L.; Bian, G.; Dong, L.; Xia, Y.; Hong, S. Preparation of a Novel Fluorescence Probe of Terbium Composite Nanoparticles and its Application in the Determination of Ascorbic Acid. Microchim. Acta 2005, 150, 291–296. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Osipova, V.V.; Galyametdinov, Y.G. Effect of a lanthanide ion on the micellation and self-organization of lyotropic liquid crystal systems. Russ. J. Phys. Chem. A 2006, 80, 649–653. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Roses, M.; Bosch, E. Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Anal. Chim. Acta 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Galeeva, A.I.; Gubaydullin, A.T.; Lobkov, V.S.; Galyametdinov, Y.G. Mesogenic and Luminescent Properties of Lyotropic Liquid Crystals Containing Eu(III) and Tb(III) Ions. J. Phys. Chem. B 2011, 116, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, N.M.; Konov, A.B.; Romanova, K.A.; Gubaidullin, A.; Galyametdinov, Y.G. Lyotropic La-containing lamellar liquid crystals: Phase behaviour, thermal and structural properties. Soft Matter 2015, 11, 7809–7816. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, T.D.; Shtykov, S.N.; Nevryueva, N.V.; Zhemerichkin, D.A.; Parashchenko, I.I. Fluorimetric assay of flumequine using sensitized terbium fluorescence in organized media. Pharm. Chem. J. 2011, 44, 635–638. [Google Scholar] [CrossRef]
- Eksperiandova, L.P.; Belikov, K.; Khimchenko, S.V.; Blank, T. Once again about determination and detection limits. J. Anal. Chem. 2010, 65, 223–228. [Google Scholar] [CrossRef]


 ); SDS (
); SDS (  ); C12EO10 (
); C12EO10 (  );Tb(Phen)-AA-HTAB (
);Tb(Phen)-AA-HTAB (  );Tb(Phen)-AA-SDS (
);Tb(Phen)-AA-SDS (  ); Tb(Phen)-AA-C12EO10 (
); Tb(Phen)-AA-C12EO10 (  ).
).
 ); SDS (
); SDS (  ); C12EO10 (
); C12EO10 (  );Tb(Phen)-AA-HTAB (
);Tb(Phen)-AA-HTAB (  );Tb(Phen)-AA-SDS (
);Tb(Phen)-AA-SDS (  ); Tb(Phen)-AA-C12EO10 (
); Tb(Phen)-AA-C12EO10 (  ).
).





| Surfactant | Working Concentration Сsurf, mol·L−1 | CMC, mol·L−1 | d, nm | Tb(Phen)-AA Complex Diameter, nm |
|---|---|---|---|---|
| C12EO10 | 1.6 × 10−3 | 9.1 × 10−5 [19] | 3 ± 0.92 | 7.5 ± 1.60 |
| SDS | 8.3 × 10−2 | 8.1 × 10−3 [20] | 5 ± 1.07 | 5.5 ± 1.12 |
| HTAB | 3.0 × 10−2 | 9.1 × 10−4 [20] | 6 ± 1.27 | 1.7 ± 0.32 |
| Surfactant | Water | C12EO10 | SDS | HTAB |
|---|---|---|---|---|
| τ, μs | 160 ± 16 | 194 ± 19 | 40 ± 4 | 187 ± 19 |
| η | 1.83 ± 0.09 | 1.98 ± 0.1 | 1.98 ± 0.1 | 1.91 ± 0.09 |
| Samples | Added, μM | Detected, μM | Recovery, % |
|---|---|---|---|
| Tablet 1 | 0 | not dedected | - |
| Tablet 2 | 50 | 52.1 ± 0.60 | 100.4 |
| Tablet 3 | 100 ± 16 | 98.4 ± 0.91 | 98.4 |
| Tablet 4 | 150 ± 0.09 | 151.2 ± 0.12 | 100.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selivanova, N.; Galyametdinov, Y. Terbium(III) as a Fluorescent Probe for Molecular Detection of Ascorbic Acid. Chemosensors 2021, 9, 134. https://doi.org/10.3390/chemosensors9060134
Selivanova N, Galyametdinov Y. Terbium(III) as a Fluorescent Probe for Molecular Detection of Ascorbic Acid. Chemosensors. 2021; 9(6):134. https://doi.org/10.3390/chemosensors9060134
Chicago/Turabian StyleSelivanova, Natalia, and Yuriy Galyametdinov. 2021. "Terbium(III) as a Fluorescent Probe for Molecular Detection of Ascorbic Acid" Chemosensors 9, no. 6: 134. https://doi.org/10.3390/chemosensors9060134
APA StyleSelivanova, N., & Galyametdinov, Y. (2021). Terbium(III) as a Fluorescent Probe for Molecular Detection of Ascorbic Acid. Chemosensors, 9(6), 134. https://doi.org/10.3390/chemosensors9060134