A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
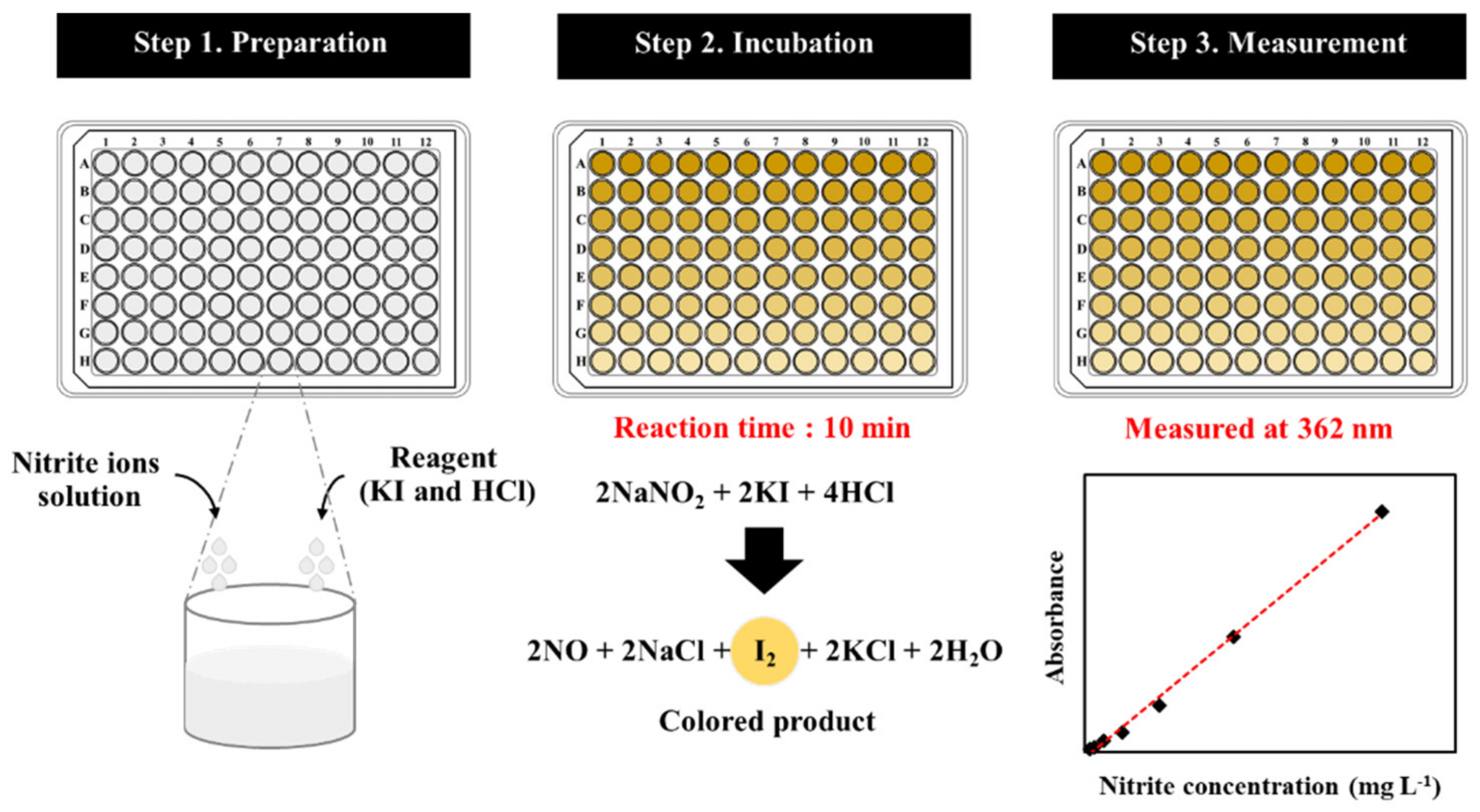
2.2. Analytical Procedure
2.3. Optimization
2.4. Analytical Performance
2.5. Interference Effect
2.6. Application for Nitrite Sensing
3. Results and Discussion
3.1. Absorption Spectra
3.2. Optimal Conditions
3.2.1. Concentration of Iodide Ions
3.2.2. Concentration of Hydrochloric Acid
3.2.3. Reaction Time
3.3. Analytical Performance
3.3.1. Linearity, Limit of Detection, and Limit of Quantification
3.3.2. Repeatability
3.3.3. Interference Study
3.4. Application for Nitrite Sensing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalby, O.; Butler, D.; Birkett, J.W. Analysis of gunshot residue and associated materials—A review. J. Forensic Sci. 2010, 55, 924–943. [Google Scholar] [CrossRef] [PubMed]
- Garofano, L.; Capra, M.; Ferrari, F.; Bizzaro, G.P.; Di Tullio, D.; Dell’Olio, M.; Ghitti, A. Gunshot residue: Further studies on particles of environmental and occupational origin. Forensic Sci. Int. 1999, 103, 1–21. [Google Scholar] [CrossRef]
- Saverio Romolo, F.; Margot, P. Identification of gunshot residue: A critical review. Forensic Sci. Int. 2001, 119, 195–211. [Google Scholar] [CrossRef]
- Lucena, M.A.M.; Ordonez, C.; Weber, I.T.; Torre, M.; Garcia-Ruiz, C.; Lopez-Lopez, M. Investigation of the use of luminescent markers as gunshot residue indicators. Forensic Sci. Int. 2017, 280, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Goudsmits, E.; Sharples, G.P.; Birkett, J.W. Preliminary classification of characteristic organic gunshot residue compounds. Sci. Justice 2016, 56, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Brozek-Mucha, Z. On the prevalence of gunshot residue in selected populations—An empirical study performed with SEM-EDX analysis. Forensic Sci. Int. 2014, 237, 46–52. [Google Scholar] [CrossRef]
- Turillazzi, E.; Di Peri, G.P.; Nieddu, A.; Bello, S.; Monaci, F.; Neri, M.; Pomara, C.; Rabozzi, R.; Riezzo, I.; Fineschi, V. Analytical and quantitative concentration of gunshot residues (Pb, Sb, Ba) to estimate entrance hole and shooting-distance using confocal laser microscopy and inductively coupled plasma atomic emission spectrometer analysis: An experimental study. Forensic Sci. Int. 2013, 231, 142–149. [Google Scholar] [CrossRef]
- Aliste, M.; Chavez, L.G. Analysis of gunshot residues as trace in nasal mucus by GFAAS. Forensic Sci. Int. 2016, 261, 14–18. [Google Scholar] [CrossRef]
- Goudsmits, E.; Sharples, G.P.; Birkett, J.W. Recent trends in organic gunshot residue analysis. Trac. Trends Anal. Chem. 2015, 74, 46–57. [Google Scholar] [CrossRef]
- Aksoy, C.; Bora, T.; Senocak, N.; Aydin, F. A new method to reduce false positives due to antimony in detection of gunshot residues. Forensic Sci. Int. 2015, 250, 87–90. [Google Scholar] [CrossRef]
- Maitre, M.; Kirkbride, K.P.; Horder, M.; Roux, C.; Beavis, A. Current perspectives in the interpretation of gunshot residues in forensic science: A review. Forensic Sci. Int. 2017, 270, 1–11. [Google Scholar] [CrossRef]
- Petraco, N.; Yander, M.; Sardone, J. A method for the quantitative determination of nitrites in gunshot residue cases. Forensic Sci. Int. 1981, 18, 85–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, M.; He, H.; Cai, Z.; Gao, N.; He, C.; Chang, G.; Wang, X.; He, Y. Highly sensitive nitrite sensor based on AuNPs/RGO nanocomposites modified graphene electrochemical transistors. Biosens. Bioelectron. 2019, 146, 111751. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef]
- Murray, E.; Roche, P.; Harrington, K.; McCaul, M.; Moore, B.; Morrin, A.; Diamond, D.; Paull, B. Low cost 235 nm ultra-violet light-emitting diode-based absorbance detector for application in a portable ion chromatography system for nitrite and nitrate monitoring. J. Chromatogr. A 2019, 1603, 8–14. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.Q.; Zhu, Y. A novel ion chromatography cycling-column-switching system for the determination of low-level chlorate and nitrite in high salt matrices. J. Hazard. Mater. 2012, 235, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.B.; Moreira, R.C.; de Oliveira Tavares, M.G.; Coltro, W.K.T. Monitoring of nitrite, nitrate, chloride and sulfate in environmental samples using electrophoresis microchips coupled with contactless conductivity detection. Talanta 2016, 147, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Troška, P.; Chudoba, R.; Danč, L.; Bodor, R.; Horčičiak, M.; Tesařová, E.; Masár, M. Determination of nitrite and nitrate in cerebrospinal fluid by microchip electrophoresis with microsolid phase extraction pre-treatment. J. Chromatogr. B 2013, 930, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Mašić, A.; Santos, A.T.L.; Etter, B.; Udert, K.M.; Villez, K. Estimation of nitrite in source-separated nitrified urine with UV spectrophotometry. Water Res. 2015, 85, 244–254. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Y.; Fan, C.; Wang, J.; Yang, Y. Development of a cloud point extraction and spectrophotometry-based microplate method for the determination of nitrite in human urine and blood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Senra-Ferreiro, S.; Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Griess micro-assay for the determination of nitrite by combining fibre optics-based cuvetteless UV-vis micro-spectrophotometry with liquid-phase microextraction. Anal. Chim Acta 2010, 668, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Okab, R.A.; Syed, A.A. Novel reactions for simple and sensitive spectrophotometric determination of nitrite. Talanta 2007, 72, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Fat’hi, M.R.; Hatami, A. Indirect cloud point extraction and spectrophotometric determination of nitrite in water and meat products. Microchem. J. 2012, 104, 22–25. [Google Scholar] [CrossRef]
- Raman, V.; Dabbas, M.S. Some observations on the use of P-rosaniline hydrochloride/phloroglucinol for the spectrophotometric determination of nitrite. Microchem. J. 1989, 40, 242–245. [Google Scholar] [CrossRef]
- Rathore, H.P.S.; Tiwari, S.K. Spectrophotometric determination of nitrite in polluted waters using 3-nitroaniline. Anal. Chim. Acta 1991, 242, 225–228. [Google Scholar] [CrossRef]
- Tsao, F.-P.; Underwood, A.L. Spectrophotometric determination of nitrite with p-nitroaniline and 2-methyl-8-quinolinol in hexadecyl-trimethylammonium bromide solution. Anal. Chim. Acta 1982, 136, 129–134. [Google Scholar] [CrossRef]
- Sulaiman, S.T.; Al-Nuri, I.J. Coulometric microdetermination of nitrite via diazotization of 1-amino-4-naphthalenesulfonic acid. Microchem. J. 1986, 33, 112–115. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Xue, Z.; Shinger, M.I.; Abdu, H.I.; Xiong, L.; Shan, D.; Lu, X. A simple yet sensitive colorimetric nitrite ions assay based on diazotization with p-Aminobenzoic and coupling with phloroglucinol in acidic medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Amin, D. Determination of nitrite ion using the reaction with 4-aminobenzotrifluoride and 1-naphthol. Analyst 1986, 111, 1335–1337. [Google Scholar] [CrossRef]
- Tarafder, P.K.; Rathore, D.P.S. Spectrophotometric determination of nitrite in water. Analyst 1988, 113, 1073–1076. [Google Scholar] [CrossRef]
- Filik, H.; Giray, D.; Ceylan, B.; Apak, R. A novel fiber optic spectrophotometric determination of nitrite using Safranin O and cloud point extraction. Talanta 2011, 85, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shi, W.J.; Yang, N.F. Study and spectrophotometric determination of nitrite with sulfanilamide and N-phenyl J-Acid system. Guang Pu Xue Yu Guang Pu Fen Xi 2007, 27, 573–576. [Google Scholar]
- Nouroozi, S.; Mirshafian, R. Flow injection kinetic spectrophotometric method for the determination of trace amounts of nitrite. Talanta 2009, 79, 1149–1153. [Google Scholar] [CrossRef]
- Shanmugam, R.; Sathiyanarayanan, K.; Kumar, A.S. A Simple Colorimetric Screening of Nitrite Using Iodide in an Acidic pH Solution. Austin J. Anal. Pharm. Chem. 2014, 1, 1–3. [Google Scholar]
- Miura, Y.; Hamada, H. Ion chromatography of nitrite at the ppb level with photometric measurement of iodine formed by post-column reaction of nitrite with iodide. J. Chromatogr. A 1999, 850, 153–160. [Google Scholar] [CrossRef]
- Grgur, B.N.; Gvozdenović, M.M.; Stevanović, J.S.; Jugović, B.Z.; Trišović, L.T. Electrochemical oxidation of iodide in aqueous solution. Chem. Eng. J. 2006, 124, 47–54. [Google Scholar] [CrossRef]
- Ozmen, H.; Polat, F.; Cukurovali, A. Spectrophotometric Determination of Nitrite in Water Samples with 4-(1-Methyl-1-Mesitylcyclobutane-3-yl) -2-Aminothiazole. Anal. Lett. 2006, 39, 823–833. [Google Scholar] [CrossRef]
- Afkhami, A.; Masahi, S.; Bahram, M. Spectrophotometric Determination of Nitrite Based on Its Reaction with p-Nitroaniline in the Presence of Diphenylamine in Micellar Media. Bull. Korean Chem. Soc. 2004, 25, 1009–1011. [Google Scholar]
- Zatar, N.A.; Abu-Eid, M.A.; Eid, A.F. Spectrophotometric determination of nitrite and nitrate using phosphomolybdenum blue complex. Talanta 1999, 50, 819–826. [Google Scholar] [CrossRef]
- Satake, M.; Wang, G.-F. Spectrophotometric determination of nitrite in natural waters using diazotization-coupling method with column preconcentration on naphthalene supported with ion-pair of tetradecyldimethylbenzyl-ammonium and iodide. Fresenius J. Anal. Chem. 1997, 357, 433–438. [Google Scholar] [CrossRef]
- Rastegarzadeh, S.; Kalantaripour, M. Development of an optical redox chemical sensor for nitrite determination. Química Nova 2012, 35, 766–770. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ma, Q.; Wang, C.-H.; Hu, X.; Gao, Y.; Wang, H.; Qin, D. A Simple Fluorescence sensor for detecting of Nitrite (NO2-) in Real Samples using Water-dispersible Graphite-like Carbon Nitride (w-g-C3N4) Nanomaterials. New J. Chem. 2017, 41, 7171–7176. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.; Yang, C.; Xu, J.; Liu, H.; Zhu, B. A colorimetric fluorescent probe for rapid and specific detection of nitrite. Luminescence 2019, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Badiee, H.; Zanjanchi, M.; Zamani, A.; Fashi, A. Solvent stir bar microextraction technique with three-hollow fiber configuration for trace determination of nitrite in river water samples. Environ. Sci. Pollut. Res. 2019, 26, 32967–32976. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Appendix F: Guidelines for Standard Method Performance Requirements. AOAC Int. 2016, 1–18. [Google Scholar]



| Reagent | λmax | Linear Range (mg L−1) | LOD (µg L−1) | Reaction Time (min) | References |
|---|---|---|---|---|---|
| 4-(1-Methyl-1-mesitylcyclobutan-3-yl)-2-aminothiazole + N,N-dimethyl aniline | 482 | 0.0500–2.00 | 12 | 15 | [37] |
| p-Nitroaniline + diphenylamine | 500 | 0.050–0.80 | 10 | 11 | [38] |
| Phosphomolybdenum blue complex | 814 | 0.200–3.60 | 200 | 30 | [39] |
| Sulphanilic acid + 1-naphthol | 418 | 0.020–0.87 | 14 | 20 | [40] |
| p-Aminobenzoic + phloroglucinol | 434 | 0.0500–1.00 | 24 | 24 | [28] |
| Iodide ion (0.070 mol L−1) | 363 | 0.300–4.00 | 30 | 6 | [41] |
| Iodide ion (0.1 mol L−1) | 365 | 0.008–0.120 | 6 | 30 | [23] |
| Iodide ion (0.1 mol L−1) | 410 | 0.0053–1.0 | 1.6 | 20 | [44] |
| Iodide ion (0.050 mol L−1) | 362 | 0.0625–4.00 | 25 | 10 | This work |
| Fluorescent method | λem | ||||
| Water-dispersible graphite-like carbon nitride | 435 | 0–4.04 | 0.011 | 10 | [42] |
| 1,2-Diaminoanthraquinone (probe P-N) | 639 | 0–1.10 | 3.7 | 8 | [43] |
| Interference Species | Interference (mg L−1) | Tolerance Ratio Interference/Nitrite 2 mg L−1 | %Relative Error |
|---|---|---|---|
| TNT | 100 | 50 | −4.31 |
| DNB | 100 | 50 | −3.75 |
| DNT | 100 | 50 | −1.31 |
| Urea | 10,000 | 5000 | +3.24 |
| Zn2+ | 10,000 | 5000 | +3.50 |
| Ni2+ | 10,000 | 5000 | −1.31 |
| Mg2+ | 10,000 | 5000 | −1.50 |
| Ba2+ | 10,000 | 5000 | +1.85 |
| SO42− | 10,000 | 5000 | −1.31 |
| Cl− | 10,000 | 5000 | +1.85 |
| Fe2+ | 300 | 150 | −4.95 |
| Pb2+ | 100 | 50 | −4.61 |
| NO3− | 100 | 50 | −4.61 |
| Surfaces | Nitrite Added (g) | Nitrite Measurement (g) | %RSD | %Recovery |
|---|---|---|---|---|
| Hands | 0.153 | 0.142 ± 0.004 | 2.82 | 93 ± 3 |
| 0.261 | 0.24 ± 0.02 | 6.67 | 90 ± 4 | |
| 0.329 | 0.325 ± 0.001 | 0.31 | 98.8 ± 0.3 | |
| 0.595 | 0.552 ± 0.008 | 1.45 | 93 ± 1 | |
| 0.760 | 0.64 ± 0.01 | 1.56 | 97 ± 2 | |
| 0.991 | 0.952 ± 0.005 | 0.53 | 96.1 ± 0.5 | |
| Clothing | 0.153 | 0.147 ± 0.003 | 2.04 | 96 ± 2 |
| 0.261 | 0.241 ± 0.009 | 3.73 | 92 ± 3 | |
| 0.329 | 0.308 ± 0.004 | 1.30 | 94 ± 1 | |
| 0.594 | 0.523 ± 0.007 | 1.34 | 97 ± 1 | |
| 0.760 | 0.619 ± 0.004 | 0.65 | 98.6 ± 0.5 | |
| 0.990 | 0.970 ± 0.007 | 0.72 | 98.0 ± 0.7 | |
| Table | 0.152 | 0.149 ± 0.003 | 2.01 | 98 ± 2 |
| 0.262 | 0.238 ± 0.008 | 3.36 | 91 ± 3 | |
| 0.329 | 0.311 ± 0.004 | 1.29 | 95 ± 1 | |
| 0.595 | 0.51 ± 0.01 | 1.96 | 97 ± 2 | |
| 0.760 | 0.640 ± 0.008 | 1.25 | 97 ± 1 | |
| 0.991 | 0.96 ± 0.02 | 2.08 | 96 ± 2 | |
| Telephone | 0.152 | 0.147 ± 0.001 | 0.68 | 96.7 ± 0.7 |
| 0.261 | 0.244 ± 0.003 | 1.23 | 94 ± 1 | |
| 0.329 | 0.310 ± 0.008 | 2.58 | 94 ± 2 | |
| 0.594 | 0.540 ± 0.006 | 1.11 | 91 ± 1 | |
| 0.760 | 0.739 ± 0.006 | 0.81 | 97.2 ± 0.8 | |
| 0.991 | 0.96 ± 0.01 | 1.04 | 97 ± 1 | |
| Notebook computer | 0.152 | 0.138 ± 0.002 | 1.45 | 91 ± 1 |
| 0.262 | 0.257 ± 0.003 | 1.17 | 98 ± 1 | |
| 0.329 | 0.305 ± 0.008 | 2.62 | 93 ± 2 | |
| 0.598 | 0.549 ± 0.008 | 1.46 | 92 ± 1 | |
| 0.760 | 0.628 ± 0.003 | 0.48 | 82.6 ± 0.4 | |
| 0.990 | 0.985 ± 0.004 | 0.41 | 99.5 ± 0.4 | |
| Tiled floor | 0.152 | 0.134 ± 0.003 | 2.24 | 88 ± 2 |
| 0.261 | 0.231 ± 0.007 | 3.03 | 96 ± 3 | |
| 0.329 | 0.305 ± 0.006 | 1.97 | 92 ± 2 | |
| 0.595 | 0.477 ± 0.004 | 0.84 | 97.0 ± 0.7 | |
| 0.761 | 0.610 ± 0.008 | 1.31 | 93 ± 1 | |
| 0.991 | 0.880 ± 0.004 | 0.45 | 98.9 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thipwimonmas, Y.; Jaidam, J.; Samoson, K.; Khunseeraksa, V.; Phonchai, A.; Thiangchanya, A.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes. Chemosensors 2021, 9, 161. https://doi.org/10.3390/chemosensors9070161
Thipwimonmas Y, Jaidam J, Samoson K, Khunseeraksa V, Phonchai A, Thiangchanya A, Chang KH, Abdullah AFL, Limbut W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes. Chemosensors. 2021; 9(7):161. https://doi.org/10.3390/chemosensors9070161
Chicago/Turabian StyleThipwimonmas, Yudtapum, Janjira Jaidam, Kritsada Samoson, Vacharachai Khunseeraksa, Apichai Phonchai, Adul Thiangchanya, Kah Haw Chang, Ahmad Fahmi Lim Abdullah, and Warakorn Limbut. 2021. "A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes" Chemosensors 9, no. 7: 161. https://doi.org/10.3390/chemosensors9070161





