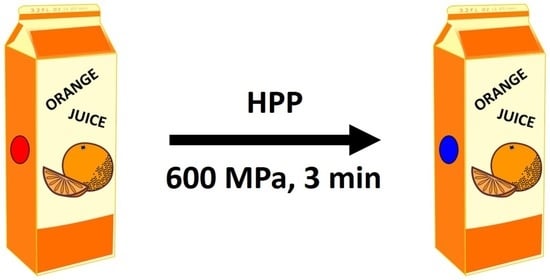
Novel Colour-Based, Prototype Indicator for Use in High-Pressure Processing (HPP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The HPP Indicator
- The pH indicator film disc
- The water-permeable barrier layer
- The acidified water-releasing pressed disc
- Assembling the HPP indicator
2.2.2. High-Pressure Processing/Pasteurisation (HPP)
2.2.3. Other Methods
3. Results and Discussion
3.1. The pH Indicator Film Disc
3.2. The Acidified Water-Releasing Pressed Disc
3.3. The Water-Permeable Barrier Layer
3.4. The HPP Indicator
3.5. HPP Indicator Shelf-Life
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abera, G. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Queiroz, C.; Moreira, C.F.; Lavinas, F.C.; Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Effect of high hydrostatic pressure on phenolic compounds, ascorbic acid and antioxidant activity in cashew apple juice. High Press. Res. 2010, 30, 507–513. [Google Scholar] [CrossRef]
- Farkas, D.F.; Hoover, D.G. High pressure processing. J. Food Sci. 2000, 65, 47–64. [Google Scholar] [CrossRef]
- Sun, D.-W. Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Monteagudo, S.M.; Balasubramaniam, V.M. Fundamentals and Applications of High-Pressure Processing Technology. In Engineering Foods for Bioactives Stability and Delivery; Springer: Berlin, Germany, 2016; pp. 3–17. [Google Scholar]
- Pal, M.; Devrani, M. Application of Various Techniques for Meat Preservation. J. Exp. Food Chem. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.L.; Sun, D.-W. High Pressure Processing of Foods: An Overview. In Emerging Technologies for Food Pro-cessing; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3–32. [Google Scholar]
- Rastogi, N.K.; Knorr, D. Recent Developments in High Pressure Processing of Foods; Springer: Berlin, Germany, 2013. [Google Scholar]
- Koutchma, T. Adapting High Hydrostatic Pressure (HPP) for Food Processing Operations; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Elamin, W.; Endan, J.B.; Yosuf, Y.A.; Shamsudin, R.; Ahmedov, A.A. High Pressure Processing Technology and Equipment Evolution: A Review. J. Eng. Sci. Technol. Rev. 2015, 8, 75–83. [Google Scholar] [CrossRef]
- Global High Pressure Processing (HPP) Food Market—Analysis By Product Type, Distribution Channel, By Region, By Country (2020 Edition): Market Insights, Outlook Post Covid-19 Pandemic (2020–2025). Available online: https://www.reportlinker.com/p05993453/Global-High-Pressure-Processing-HPP-Food-Market-Analysis-By-Product-Type-Distribution-Channel-By-Region-By-Country-Edition-Market-Insights-Outlook-Post-Covid-19-Pandemic.html?utm_source=GNW (accessed on 30 June 2021).
- Minerich, P.L.; Labuza, T.P. Development of a pressure indicator for high hydrostatic pressure processing of foods. Innov. Food Sci. Emerg. Technol. 2003, 4, 235–243. [Google Scholar] [CrossRef]
- Bauer, B.; Knorr, D. The impact of pressure, temperature and treatment time on starches: Pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- García, A.F.; Butz, P.; Corrales, M.; Lindauer, R.; Picouet, P.; Rodrigo, G.; Tauscher, B. A simple coloured indicator for moni-toring ultra high pressure processing conditions. J. Food Eng. 2009, 92, 410–415. [Google Scholar] [CrossRef]
- Koutchma, T.; Guo, B.; Patazca, E.; Parisi, B. High pressure-high temperature sterilization: From kinetic analysis to process verification+. J. Food Process. Eng. 2005, 28, 610–629. [Google Scholar] [CrossRef]
- Multi-Color Corporation: High Pressure Processing (HPP) Labels that Can Withstand the High-Pressure Process. Available online: https://www.mcclabel.com/en/food-dairy/innovations/functional-labels/high-pressure-processing-hpp (accessed on 30 June 2021).
- Bushman, A.C. High Pressure Processing Indicator. U.S. Patent 2020/0037639 A1, 6 February 2020. [Google Scholar]
- McCann, B. HPP Indicator Technology Helps Food Brands know for Certain. Available online: https://www.ctiinks.com/post/2019/05/30/hpp-indicator-technology-for-food (accessed on 30 June 2021).
- Sensor Indicator Products. Available online: http://sensorindicator.com/ (accessed on 30 June 2021).
- Wu, C.; Scott, J.; Shea, J.-E. Binding of Congo Red to Amyloid Protofibrils of the Alzheimer Aβ9–40 Peptide Probed by Molecular Dynamics Simulations. Biophys. J. 2012, 103, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cricut Explore Air 2. Available online: https://cricut.com/en_us/machines/cricut-explore-air-2.html (accessed on 30 June 2021).
- Whatman Nuclepore Membrane. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/wha110410?lang=en®ion=GB (accessed on 30 June 2021).
- Avure. Available online: www.avure.com (accessed on 30 June 2021).
- Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214–1222. [Google Scholar] [CrossRef]
- Mergel, D.; Buschendorf, D.; Eggert, S.; Grammes, R.; Samset, B.H. Density and refractive index of TiO2 films prepared by reactive evaporation. Thin Solid Film. 2000, 371, 218–224. [Google Scholar] [CrossRef]
- Bergna, H.E. Colloid Chemistry of Silica: An Overview; ACS Publications: Washington, DC, USA, 1994. [Google Scholar]
- Greenwood, N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 1984; p. 911. [Google Scholar]
- Sillanpää, M.; Bhatnagar, A. NOM Removal by Adsorption. Nat. Org. Matter Water 2015, 213–238. [Google Scholar] [CrossRef]
- Bathen, D.; Breitbach, M. Adsorptionstechnik; Springer: Berlin, Germany, 2013. [Google Scholar]
- Costa, T.; Gallas, M.; Benvenutti, E.; da Jornada, J. Infrared and thermogravimetric study of high pressure consolidation in alkoxide silica gel powders. J. Non-Cryst. Solids 1997, 220, 195–201. [Google Scholar] [CrossRef]
- Tran, A.T.; Tomlin, J.; Lam, P.H.; Stinger, B.L.; Miller, A.D.; Walczyk, D.J.; Cruz, O.; Vaden, T.D.; Yu, L. Conductivity, Vis-cosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering 2019, 3, 81. [Google Scholar] [CrossRef] [Green Version]
- Andrés, V.; Villanueva, M.-J.; Tenorio, M.-D. Influence of high pressure processing on microbial shelf life, sensory profile, soluble sugars, organic acids, and mineral content of milk- and soy-smoothies. LWT 2016, 65, 98–105. [Google Scholar] [CrossRef]
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of high pressure processing on the microbial inactivation in fruit prepa-rations and other vegetable based beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Kebede, B.T.; Dang, D.N.H.; Buvé, C.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Reyes, J.E.; Guanoquiza, M.I.; Tabilo-Munizaga, G.; Vega-Galvez, A.; Miranda, M.; Pérez-Won, M. Microbiological stabilization of Aloe vera (Aloe barbadensis Miller) gel by high hydrostatic pressure treatment. Int. J. Food Microbiol. 2012, 158, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; López, J.; Torres-Ossandón, M.J.; Galotto, M.J.; Puente-Díaz, L.; Quispe-Fuentes, I.; Di Scala, K. High hy-drostatic pressure effect on chemical composition, color, phenolic acids and antioxidant capacity of Cape gooseberry pulp (Physalis peruviana L.). LWT 2014, 58, 519–526. [Google Scholar] [CrossRef]





| Basis of Indicators | Form | Property Measured | Detecting Range (MPa) | Temperature (°C) | Ref |
|---|---|---|---|---|---|
| Compression | Copper tablet | Density | 400–600 | 7–24 | [12] |
| Gelation | Starch suspension (liquid) | Gelatinization (loss of birefringence via a microscope) | 100–700 | 0–70 | [13] |
| Enzyme | Liquid | UV/vis spectrometer (decreased absorbance at 420 nm) | Above 600 | Above 60 | [14] |
| Microbe survival | Coloured strips | Colour change of the strips after 24 h incubation | 600–800 | 91–108 | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusufu, D.; Bingham, M.; Mills, A. Novel Colour-Based, Prototype Indicator for Use in High-Pressure Processing (HPP). Chemosensors 2021, 9, 164. https://doi.org/10.3390/chemosensors9070164
Yusufu D, Bingham M, Mills A. Novel Colour-Based, Prototype Indicator for Use in High-Pressure Processing (HPP). Chemosensors. 2021; 9(7):164. https://doi.org/10.3390/chemosensors9070164
Chicago/Turabian StyleYusufu, Dilidaer, Michael Bingham, and Andrew Mills. 2021. "Novel Colour-Based, Prototype Indicator for Use in High-Pressure Processing (HPP)" Chemosensors 9, no. 7: 164. https://doi.org/10.3390/chemosensors9070164