Bovine Serum Albumin Protein Detection by a Removable SPR Chip Combined with a Specific MIP Receptor
Abstract
:1. Introduction
2. Materials and Methods
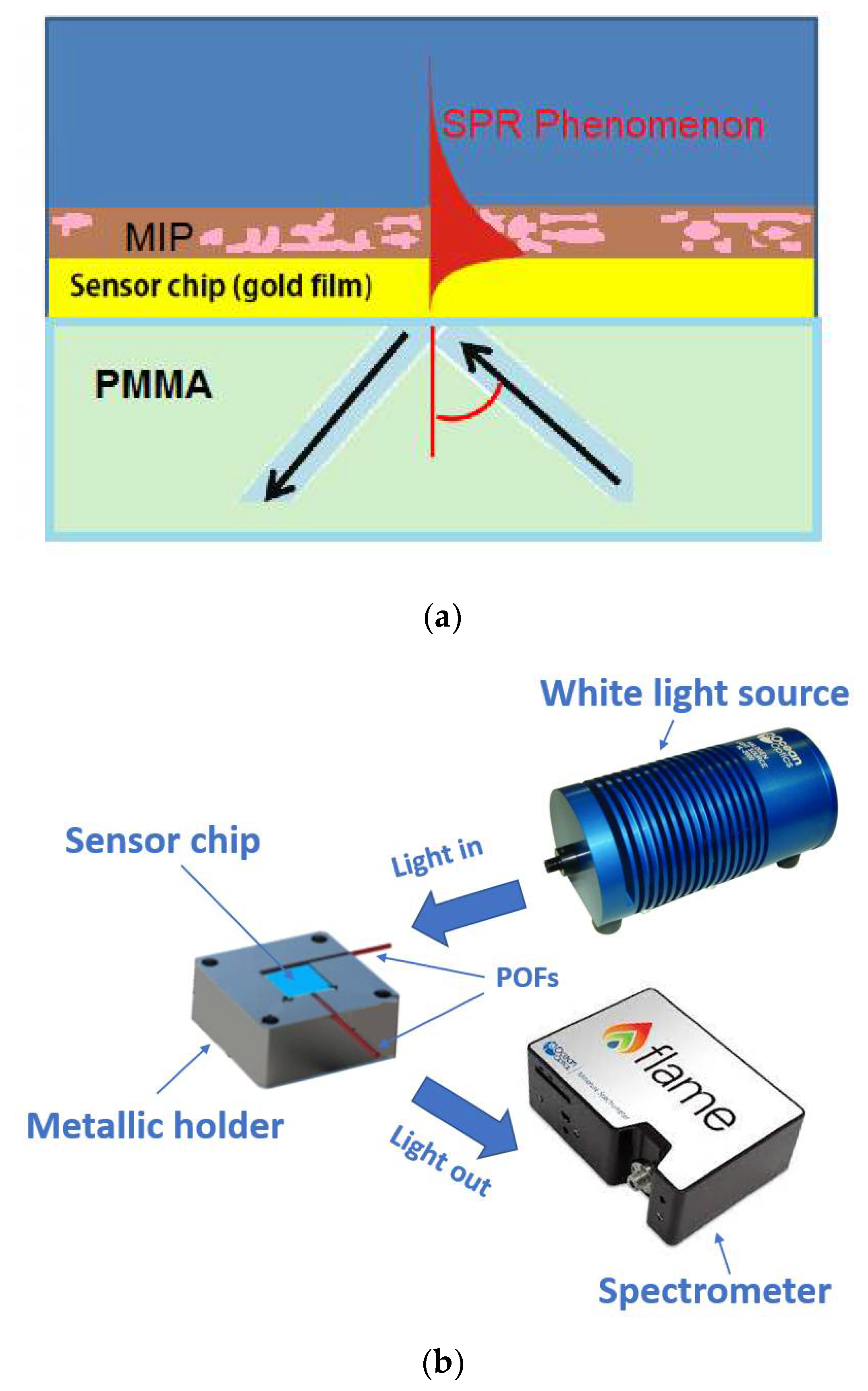
2.1. SPR Sensor Based on a PMMA Slab Waveguide
2.2. Reagents
2.3. MIP Receptor for BSA Protein Detection
2.4. Experimental Setup
2.5. Data Acquisition Protocol
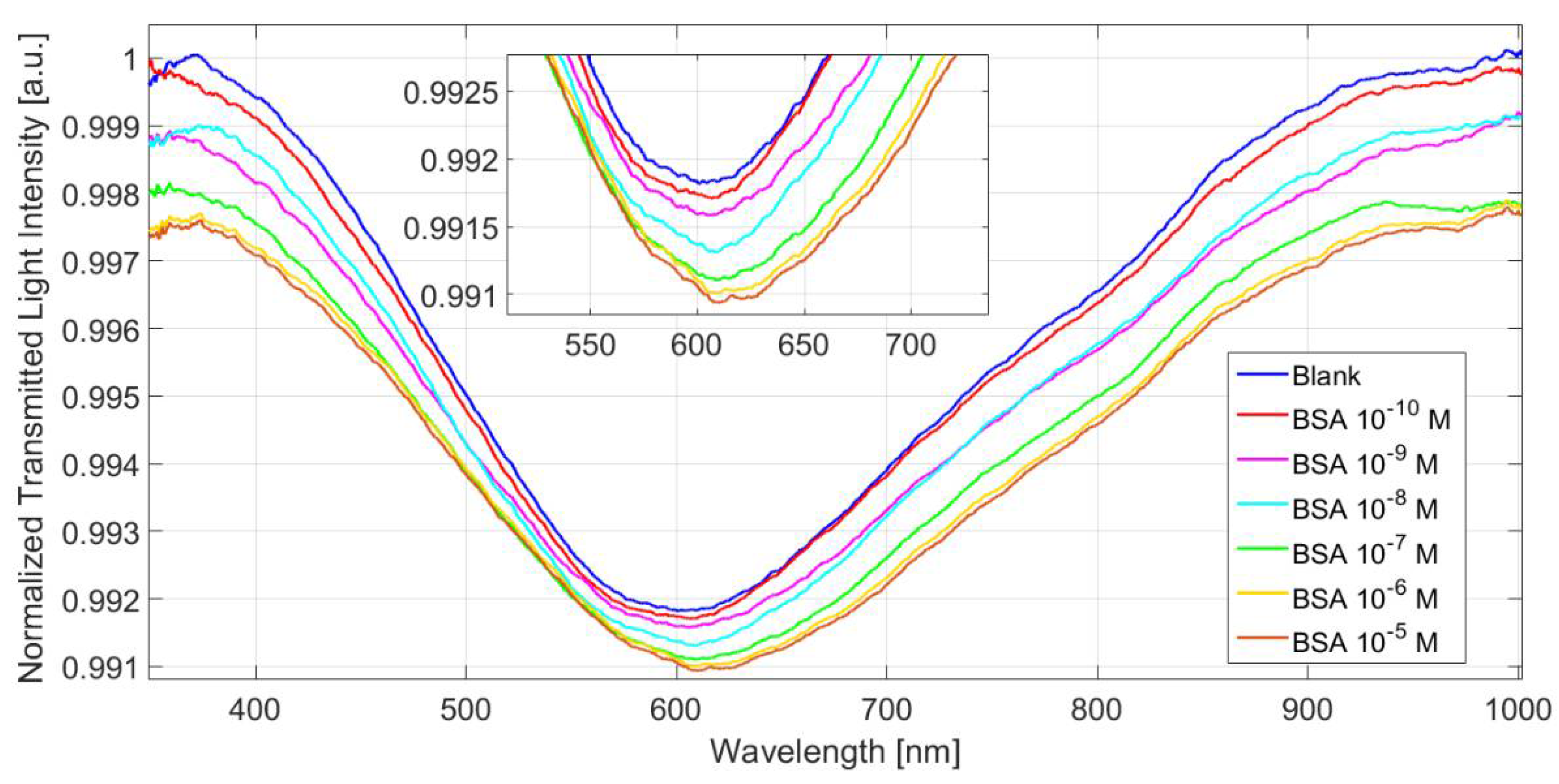
2.6. Binding Experiments
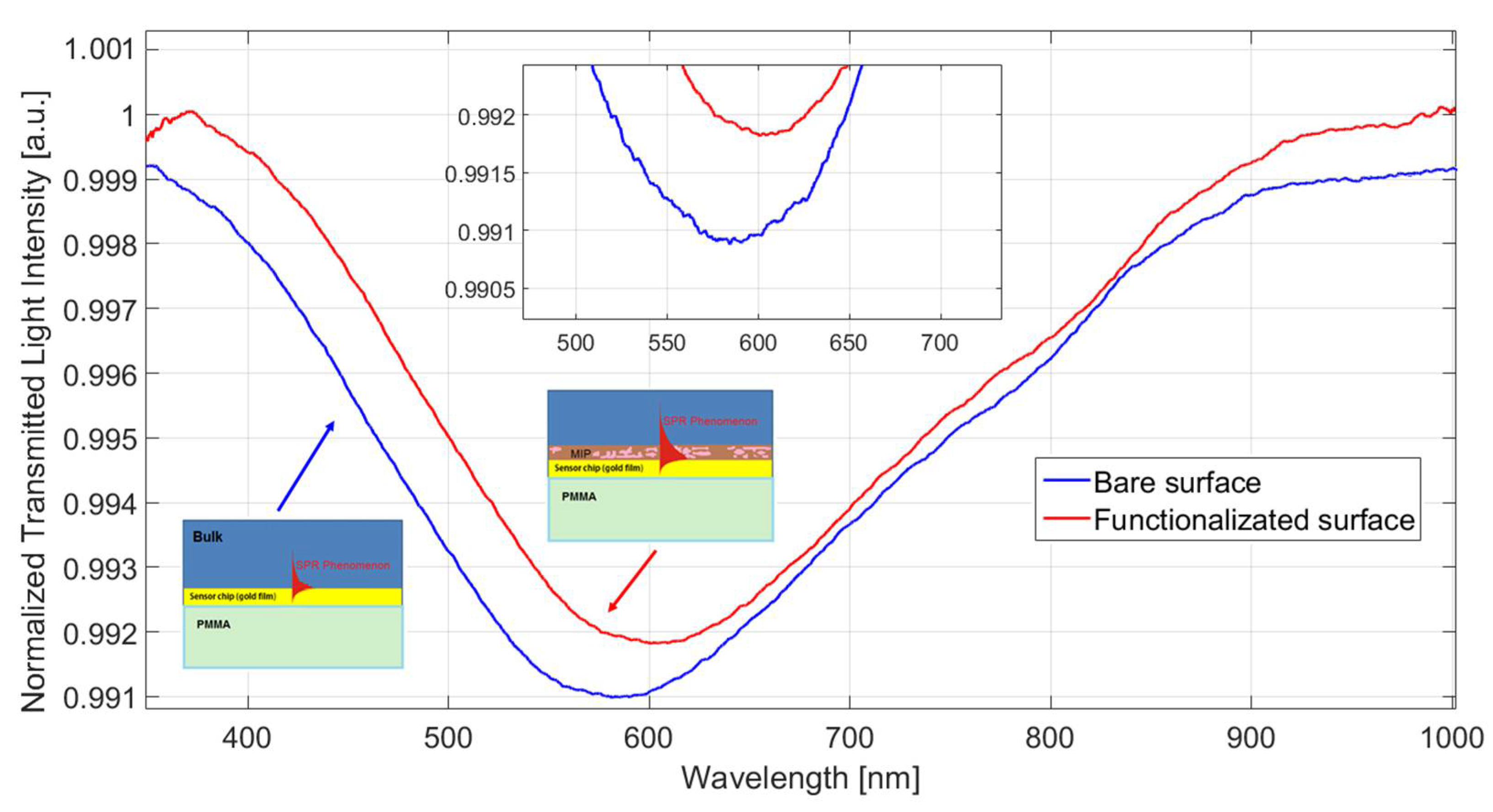
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. Sens. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [Green Version]
- Guo, X. Surface plasmon resonance based biosensor technique: A review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, R.; Li, H.; Kou, J.; Zeng, X.; Huang, H.; Hu, X.; Huang, W. Simultaneous measurement of refractive index and temperature for prism-based surface plasmon resonance sensors. Opt. Express 2019, 27, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Sharma, A.K. High-performance sensor based on surface plasmon resonance with chalcogenide prism and aluminum for detection in infrared. Opt. Lett. 2009, 34, 749–751. [Google Scholar] [CrossRef]
- Maurya, J.B.; Prajapati, Y.K. A comparative study of different metal and prism in the surface plasmon resonance biosensor having MoS2-graphene. Opt. Quant. Electron. 2016, 48, 280. [Google Scholar] [CrossRef]
- Bolduc, O.R.; Live, L.S.; Masson, J.-F. High-resolution surface plasmon resonance sensors based on a dove prism. Talanta 2009, 77, 1680–1687. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-Optic Sensors Based on Surface Plasmon Resonance: A Comprehensive Review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Monfared, Y.E. Overview of recent advances in the design of plasmonic fiber-optic biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, R.-j.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, M.E.; Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Trends in the Implementation of Advanced Plasmonic Materials in Optical Fiber Sensors (2010–2020). Chemosensors 2021, 9, 64. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Z.-q.; Li, J. Photonic crystal fiber based surface plasmon resonance chemical sensors. Sens. Actuators B Chem. 2014, 202, 557–567. [Google Scholar] [CrossRef]
- Klantsataya, E.; Jia, P.; Ebendorff-Heidepriem, H.; Monro, T.M.; François, A. Plasmonic Fiber Optic Refractometric Sensors: From Conventional Architectures to Recent Design Trends. Sensors 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, R.; Gupta, B.D. Fiber-Optic SPR Based Acetylcholine Biosensor Using Enzyme Functionalized Ta2O5 Nanoflakes for Alzheimer’s Disease Diagnosis. J. Lightwave Technol. 2018, 36, 4018–4024. [Google Scholar] [CrossRef]
- Luo, W.; Li, X.; Meng, J.; Wang, Y.; Hong, X. Surface Plasmon Resonance Sensor Based on Side-Polished D-Shaped Photonic Crystal Fiber With Split Cladding Air Holes. IEEE Trans. Instrum. Meas. 2021, 70, 1–11. [Google Scholar]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low Cost Sensors Based on SPR in a Plastic Optical Fiber for Biosensor Implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Jiang, S.Z.; Li, C.H.; Xu, S.C.; Yu, J.; Li, Z.; Wang, M.H.; Liu, A.H.; Man, B.Y. U-bent fiber optic SPR sensor based on graphene/AgNPs. Sens. Actuators B Chem. 2017, 251, 127–133. [Google Scholar] [CrossRef]
- Cennamo, N.; Arcadio, F.; Minardo, A.; Montemurro, D.; Zeni, L. Experimental Characterization of Plasmonic Sensors Based on Lab-Built Tapered Plastic Optical Fibers. Appl. Sci. 2020, 10, 4389. [Google Scholar] [CrossRef]
- Tabassum, R.; Gupta, B.D. Fiber optic manganese ions sensor using SPR and nanocomposite of ZnO–polypyrrole. Sens. Actuators B Chem. 2015, 220, 903–909. [Google Scholar] [CrossRef]
- Si, Y.; Lao, J.; Zhang, X.; Liu, Y.; Cai, S.; González-Vila, Á.; Li, K.; Huang, Y.; Yuan, Y.; Caucheteur, C.; et al. Electrochemical Plasmonic Fiber-optic Sensors for Ultra-Sensitive Heavy Metal Detection. J. Lightwave Technol. 2019, 37, 3495–3502. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Porto, G.; Biasiolo, A.; Perri, C.; Arcadio, F.; Zeni, L. A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water. Sensors 2018, 18, 1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 16904. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, S.; Xu, J.; Li, Z.; Li, C.; Jing, Y.; Zhao, X.; Pan, J.; Zhang, C.; Man, B. Sensitive and selective surface plasmon resonance sensor employing a gold-supported graphene composite film/D-shaped fiber for dopamine detection. J. Phys. D Appl. Phys. J. 2019, 52, 195402. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Vanzetti, L.E.; Bossi, A.M.; Zeni, L. D-shaped plastic optical fibre aptasensor for fast thrombin detection in nanomolar range. Sci. Rep. 2019, 9, 18740. [Google Scholar] [CrossRef]
- Cennamo, N.; Zeni, L.; Ricca, E.; Isticato, R.; Marzullo, V.R.; Capo, A.; Staiano, M.; D’Auria, S.; Varriale, A. Detection of naphthalene in sea-water by a label-free plasmonic optical fiber biosensor. Talanta 2019, 194, 289–297. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Galatus, R.; Bibbò, L.; Pesavento, M.; Zeni, L. Sensors based on surface plasmon resonance in a plastic optical fiber for the detection of trinitrotoluene. Sens. Actuators B Chem. 2013, 188, 221–226. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Perri, C.; Arcadio, F.; Chiaretti, G.; Parisio, E.M.; Camarlinghi, G.; Vettori, C.; Di Marzo, F.; Cennamo, R.; et al. Proof of Concept for a Quick and Highly Sensitive On-Site Detection of SARS-CoV-2 by Plasmonic Optical Fibers and Molecularly Imprinted Polymers. Sensors 2021, 21, 1681. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Zeni, L. A review on simple and highly sensitive plastic optical fiber probes for bio-chemical sensing. Sens. Actuators B Chem. 2021, 331, 129393. [Google Scholar] [CrossRef]
- Taguchi, Y.; Takano, E.; Takeuchi, T. SPR Sensing of Bisphenol A Using Molecularly Imprinted Nanoparticles Immobilized on Slab Optical Waveguide with Consecutive Parallel Au and Ag Deposition Bands Coexistent with Bisphenol A-Immobilized Au Nanoparticles. Langmuir 2012, 28, 7083–7087. [Google Scholar] [CrossRef]
- Cennamo, N.; Mattiello, F.; Zeni, L. Slab Waveguide and Optical Fibers for Novel Plasmonic Sensor Configurations. Sensors 2017, 17, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeni, L.; Pesavento, M.; Marchetti, S.; Cennamo, N. Slab plasmonic platforms combined with Plastic Optical Fibers and Molecularly Imprinted Polymers for chemical sensing. Opt. Laser Technol. 2018, 107, 484–490. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Montemurro, D.; Eramo, C.; Di Ronza, S.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. Biochemical sensing exploiting plasmonic sensors based on gold nanogratings and polymer optical fibers. Photonics Res. 2021, 9, 1397–1408. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Minardo, A.; Eramo, C.; Di Ronza, S.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. A Nanoplasmonic-Based Biosensing Approach for Wide-Range and Highly Sensitive Detection of Chemicals. Nanomaterials 2021, 11, 1961. [Google Scholar] [CrossRef]
- Cui, M.; Xin, Y.; Song, R.; Sun, Q.; Wang, X.; Lu, D. Fluorescence sensor for bovine serum albumin detection based on the aggregation and release of CdS QDs within CMC. Cellulose 2020, 27, 1621–1633. [Google Scholar] [CrossRef]
- Xu, X.J.; Huang, J.; Li, J.J.; Yan, J.W.; Qin, J.G.; Li, Z. A graphene oxide-based AIE biosensor with high selectivity toward bovine serum albumin. Chem. Commun. 2011, 47, 12385–12387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, S.; Tiwari, U.K.; Deep, A.; Sinha, R.K. Two-dimensional transition metal dichalcogenides assisted biofunctionalized optical fiber SPR biosensor for efficient and rapid detection of bovine serum albumin. Sci. Rep. 2019, 9, 6987. [Google Scholar] [CrossRef] [Green Version]
- Jia, K.; Khaywah, M.Y.; Li, Y.; Bijeon, J.L.; Adam, P.M.; Déturche, R.; Guelorget, B.; François, M.; Louarn, G.; Ionescu, R.E. Strong Improvements of Localized Surface Plasmon Resonance Sensitivity by Using Au/Ag Bimetallic Nanostructures Modified with Polydopamine Films. ACS Appl. Mater. Interfaces 2014, 6, 219–227. [Google Scholar] [CrossRef]
- Pathak, A.; Parveen, S.; Gupta, B.D. Ultrasensitive, highly selective, and real-time detection of protein using functionalized CNTs as MIP platform for FOSPR-based biosensor. Nanotechnology 2017, 28, 355503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, T. Surface plasmon resonance sensor chips for the recognition of bovine serum albumin via electropolymerized molecularly imprinted polymers. Chin. Chem. Lett. 2013, 24, 813–816. [Google Scholar] [CrossRef]

| λ0 (nm) | λmax (nm) | K (M) | n | Statistics | ||||
|---|---|---|---|---|---|---|---|---|
| Value | Standard error (stand. err.) | Value | Stand. err. | Value | Stand. err. | Value | Reduced chi-square | Adj. R-square |
| −0.22 | 0.35 | 5.17 | 0.17 | 6.52 × 10−8 | 1.72 × 10−8 | 1 | 1.14 | 0.99 |
| Configuration | Parameter | ||
|---|---|---|---|
| BSA sensor based on SPR probe in a PMMA slab waveguide | Sensitivity at low concentrations (nm/M) | Limit of detection (LOD) (M) | Kaff = 1/K (M−1) |
| 8.27 × 107 | 8.5 × 10−9 | 1.53 × 107 | |
| Configuration | LOD | BSA Detection Range | Reference |
|---|---|---|---|
| SPR in a PMMA slab waveguide | 8.5 nM | 8.5 nM–1 µM | This work |
| Gold nanograting on a PMMA substrate | 37 pM | 37 pM–100 nM | [33] |
| SPR D-shaped POFs | 0.37 µM | 0.37 µM–6.5 µM | [28] |
| GNG-based longitudinal (blue-shift resonance) | 23 pM | 23 pM–10 nM | [34] |
| GNG-based longitudinal (red-shift resonance) | 0.54 µM | 0.54 µM–10 µM | [34] |
| GNG-based orthogonal (blue-shift resonance) | 42 pM | 42 pM–10 nM | [34] |
| Fluorescence sensor | 10 nM | 0.01 μM–2 μM | [35] |
| Aggregation-induced emission biosensor coupled with graphene-oxide | 0.4 μM | 0.4 μM–1.5 μM | [36] |
| SPR-MoS2 optical fiber | 4.36 nM | 4.36 nM–750 nM | [37] |
| LSPR based on bimetallic nanoparticles | 0.15 pM | 0.15 pM–15 pM | [38] |
| Fiber-optic SPR | 5.8 fM | 5.8 fM–5 pM | [39] |
| SPR Kretschmann configuration | 0.3 μM | 0.3 μM–120 μM | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcadio, F.; Zeni, L.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Minardo, A.; Cennamo, N. Bovine Serum Albumin Protein Detection by a Removable SPR Chip Combined with a Specific MIP Receptor. Chemosensors 2021, 9, 218. https://doi.org/10.3390/chemosensors9080218
Arcadio F, Zeni L, Perri C, D’Agostino G, Chiaretti G, Porto G, Minardo A, Cennamo N. Bovine Serum Albumin Protein Detection by a Removable SPR Chip Combined with a Specific MIP Receptor. Chemosensors. 2021; 9(8):218. https://doi.org/10.3390/chemosensors9080218
Chicago/Turabian StyleArcadio, Francesco, Luigi Zeni, Chiara Perri, Girolamo D’Agostino, Giudo Chiaretti, Giovanni Porto, Aldo Minardo, and Nunzio Cennamo. 2021. "Bovine Serum Albumin Protein Detection by a Removable SPR Chip Combined with a Specific MIP Receptor" Chemosensors 9, no. 8: 218. https://doi.org/10.3390/chemosensors9080218
APA StyleArcadio, F., Zeni, L., Perri, C., D’Agostino, G., Chiaretti, G., Porto, G., Minardo, A., & Cennamo, N. (2021). Bovine Serum Albumin Protein Detection by a Removable SPR Chip Combined with a Specific MIP Receptor. Chemosensors, 9(8), 218. https://doi.org/10.3390/chemosensors9080218









