Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study
Abstract
:1. Introduction
2. Materials and Methods
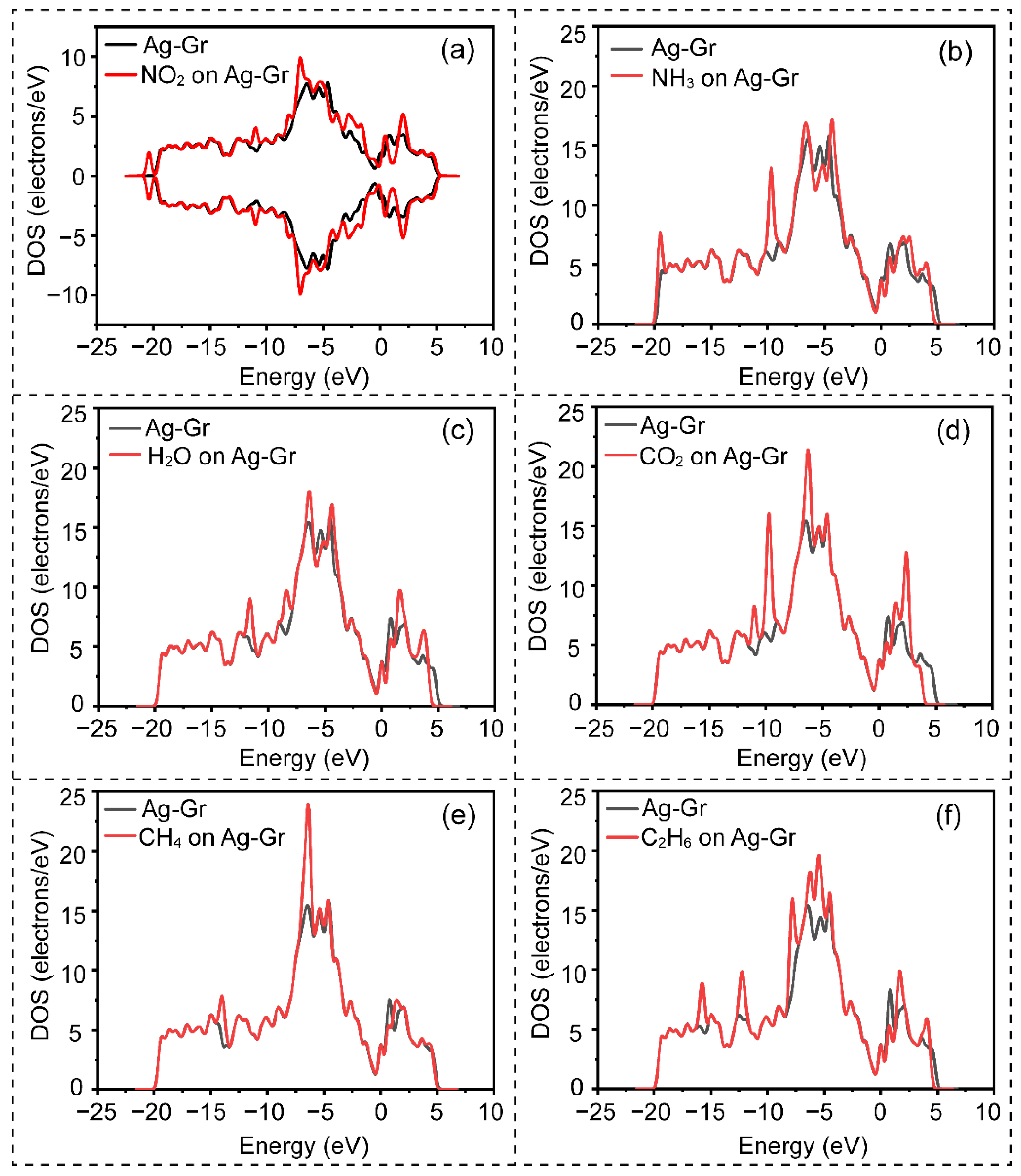
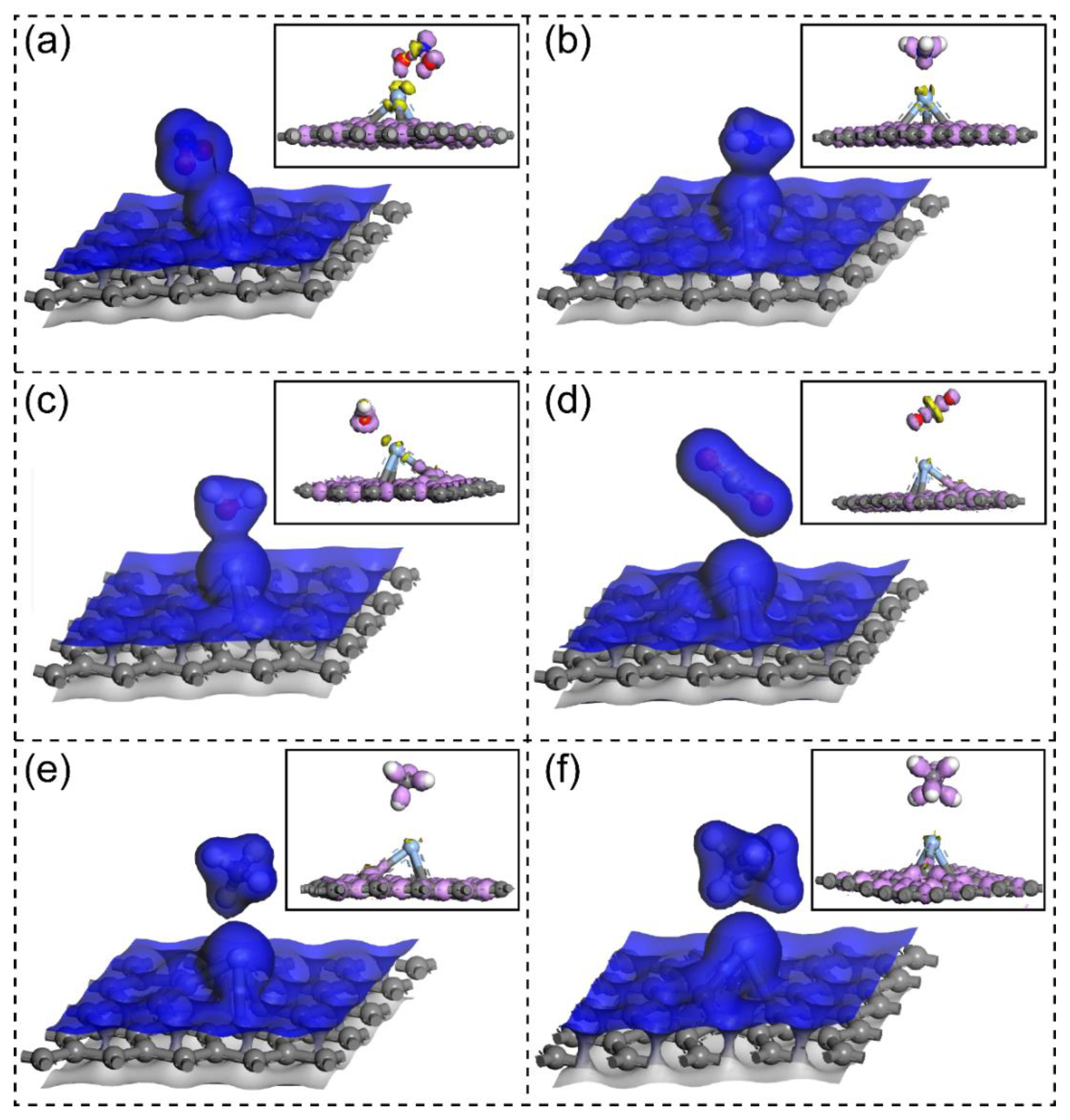
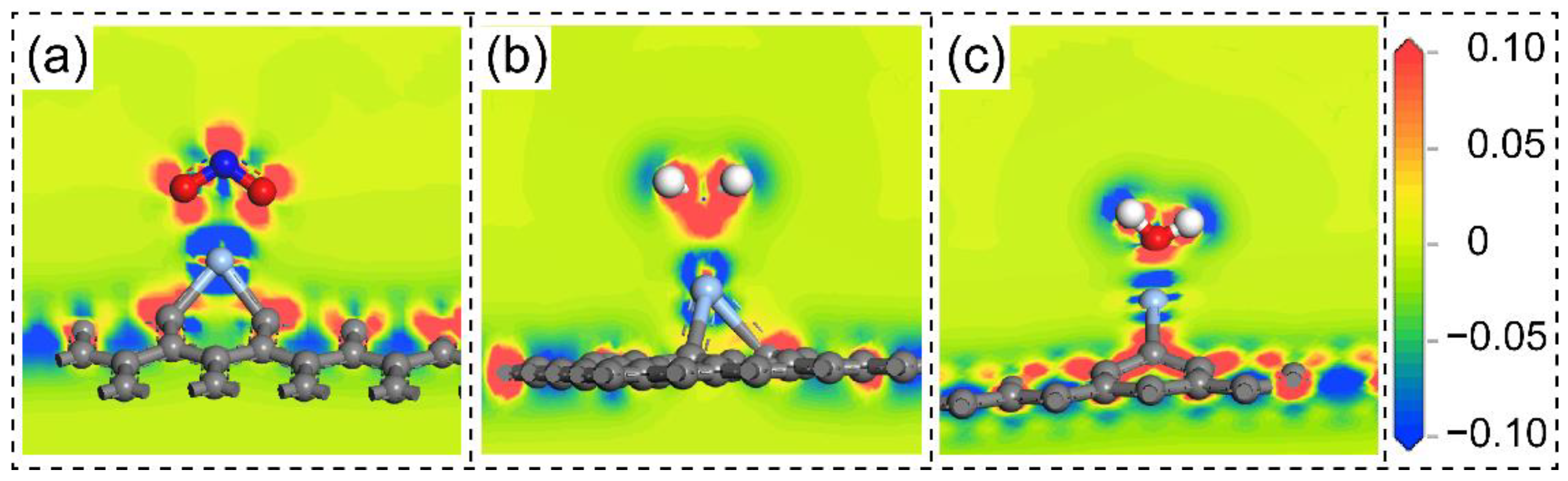
3. Results
4. Conclusions and Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhou, L.; Liu, Y.; Liu, F.; Liang, X.; Liu, F.; Gao, Y.; Yan, X.; Lu, G. UV-activated ultrasensitive and fast reversible ppb NO2 sensing based on ZnO nanorod modified by constructing interfacial electric field with In2O3 nanoparticles. Sens. Actuators B Chem. 2020, 305, 127498. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Pang, M.; Xue, Q. Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 2019, 65. [Google Scholar] [CrossRef]
- Hu, F.-F.; Tang, H.-Y.; Tan, C.-J.; Ye, H.-Y.; Chen, X.-P.; Zhang, G.-Q. Nitrogen Dioxide Gas Sensor Based on Monolayer SnS: A First-Principle Study. IEEE Electron Device Lett. 2017, 38, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-G.; Tan, C.-J.; Yang, Q.; Xu, Y.-X.; Li, S.-L.; Chen, X.-P. A Novel Ultra-Sensitive Nitrogen Dioxide Sensor Based on Germanium Monosulfide Monolayer. IEEE Electron Device Lett. 2017, 38, 1590–1593. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Flexible, 3D SnS2/Reduced graphene oxide heterostructured NO2 sensor. Sens. Actuators B Chem. 2020, 305, 127445. [Google Scholar] [CrossRef]
- Liao, N.; Zhou, H.; Zheng, B.; Xue, W. Silicon Oxycarbide-Derived Carbon as Potential NO2 Gas Sensor: A First Principles’ Study. IEEE Electron Device Lett. 2018, 39, 1760–1763. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Joshi, M.; Myung, J.-h. Sputtered SnO2/ZnO Heterostructures for Improved NO2 Gas Sensing Properties. Chemosensors 2020, 8, 67. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, K.C.; Kang, S.; Kim, C.; Kim, T.H.; Hong, S.-P.; Park, S.Y.; Suh, J.M.; Choi, M.-J.; Han, S.; et al. Two-Dimensional NbS2 Gas Sensors for Selective and Reversible NO2 Detection at Room Temperature. ACS Sens. 2019, 4, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Camargo Moreira, Ó.L.; Cheng, W.-Y.; Fuh, H.-R.; Chien, W.-C.; Yan, W.; Fei, H.; Xu, H.; Zhang, D.; Chen, Y.; Zhao, Y.; et al. High Selectivity Gas Sensing and Charge Transfer of SnSe2. ACS Sens. 2019, 4, 2546–2552. [Google Scholar] [CrossRef]
- Panigrahi, P.; Hussain, T.; Karton, A.; Ahuja, R. Elemental Substitution of Two-Dimensional Transition Metal Dichalcogenides (MoSe2 and MoTe2): Implications for Enhanced Gas Sensing. ACS Sens. 2019, 4, 2646–2653. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Nádherná, M.; Opekar, F.; Reiter, J. Ionic liquid–polymer electrolyte for amperometric solid-state NO2 sensor. Electrochim. Acta 2011, 56, 5650–5655. [Google Scholar] [CrossRef]
- Gebicki, J.; Kloskowski, A.; Chrzanowski, W.; Stepnowski, P.; Namiesnik, J. Application of Ionic Liquids in Amperometric Gas Sensors. Crit. Rev. Anal. Chem. 2016, 46, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Nádherná, M.; Opekar, F.; Reiter, J.; Štulík, K. A planar, solid-state amperometric sensor for nitrogen dioxide, employing an ionic liquid electrolyte contained in a polymeric matrix. Sens. Actuators B Chem. 2012, 161, 811–817. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent advances in carbon material-based NO2 gas sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Chen, D.; Miao, J.; Lin, S.; Cui, D. Highly Sensitive and Flexible Piezoresistive Pressure Sensors Based on 3D Reduced Graphene Oxide Aerogel. IEEE Electron Device Lett. 2021, 42, 589–592. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Three-Dimensional Graphene Hydrogel Decorated with SnO2 for High-Performance NO2 Sensing with Enhanced Immunity to Humidity. ACS Appl. Mater. Interfaces 2020, 12, 2634–2643. [Google Scholar] [CrossRef]
- Niu, F.; Shao, Z.-W.; Gao, H.; Tao, L.-M.; Ding, Y. Si-doped graphene nanosheets for NOx gas sensing. Sens. Actuators B Chem. 2021, 328. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Niu, W.; Fam, D.W.H.; Palaniappan, A.; Larisika, M.; Faulkner, S.H.; Nowak, C.; Nimmo, M.A.; Liedberg, B.; et al. Highly manufacturable graphene oxide biosensor for sensitive Interleukin-6 detection. RSC Adv. 2015, 5, 39245–39251. [Google Scholar] [CrossRef]
- Cruz-Martinez, H.; Rojas-Chavez, H.; Montejo-Alvaro, F.; Pena-Castaneda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Sun, Y.e.; Jiang, C.; Yao, Y.; Wang, D.; Zhang, Y. Room-temperature highly sensitive CO gas sensor based on Ag-loaded zinc oxide/molybdenum disulfide ternary nanocomposite and its sensing properties. Sens. Actuators B Chem. 2017, 253, 1120–1128. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Gui, Y.; Hu, W. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Manna, B.; Chakrabarti, I.; Guha, P.K. Platinum Nanoparticles Decorated Graphene Oxide Based Resistive Device for Enhanced Formaldehyde Sensing: First-Principle Study and its Experimental Correlation. IEEE Trans. Electron Devices 2019, 66, 1942–1949. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.; Xu, L.; Zeng, W. Adsorption of SO2 molecule on Ni-doped and Pd-doped graphene based on first-principle study. Appl. Surf. Sci. 2020, 517, 146180. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H. A first-principles study on the interaction of biogas with noble metal (Rh, Pt, Pd) decorated nitrogen doped graphene as a gas sensor: A DFT study. Appl. Surf. Sci. 2018, 435, 1199–1212. [Google Scholar] [CrossRef]
- Li, Q.; Chen, D.; Miao, J.; Lin, S.; Yu, Z.; Han, Y.; Yang, Z.; Zhi, X.; Cui, D.; An, Z. Ag-Modified 3D Reduced Graphene Oxide Aerogel-Based Sensor with an Embedded Microheater for a Fast Response and High-Sensitive Detection of NO2. ACS Appl. Mater. Interfaces 2020, 12, 25243–25252. [Google Scholar] [CrossRef] [PubMed]
- Makoś-Chełstowska, P.; Słupek, E.; Gębicki, J. Deep eutectic solvent-based green absorbents for the effective removal of volatile organochlorine compounds from biogas. Green Chem. 2021, 23, 4814–4827. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Gębicki, J. Extractive detoxification of feedstocks for the production of biofuels using new hydrophobic deep eutectic solvents—Experimental and theoretical studies. J. Mol. Liq. 2020, 308. [Google Scholar] [CrossRef]
- Gutierrez, A.; Atilhan, M.; Aparicio, S. A theoretical study on lidocaine solubility in deep eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 27464–27473. [Google Scholar] [CrossRef]
- Atilhan, M.; Altamash, T.; Aparicio, S. Quantum Chemistry Insight into the Interactions Between Deep Eutectic Solvents and SO2. Molecules 2019, 24, 2963. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-J.; Lee, D.-M.; Yu, H.; Jang, J.-S.; Kim, M.-H.; Kang, J.-Y.; Jeong, H.S.; Kim, I.-D. All-carbon fiber-based chemical sensor: Improved reversible NO2 reaction kinetics. Sens. Actuators B Chem. 2019, 290, 293–301. [Google Scholar] [CrossRef]
- Paul, R.K.; Badhulika, S.; Saucedo, N.M.; Mulchandani, A. Graphene nanomesh as highly sensitive chemiresistor gas sensor. Anal. Chem. 2012, 84, 8171–8178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Tao, K.; Miao, J.; Norford, L.K. Improved Selectivity and Sensitivity of Gas Sensing Using a 3D Reduced Graphene Oxide Hydrogel with an Integrated Microheater. ACS Appl. Mater. Interfaces 2015, 7, 27502–27510. [Google Scholar] [CrossRef] [PubMed]
- Piloto, C.; Notarianni, M.; Shafiei, M.; Taran, E.; Galpaya, D.; Yan, C.; Motta, N. Highly NO2 sensitive caesium doped graphene oxide conductometric sensors. Beilstein J. Nanotechnol. 2014, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, B.; Yoon, J.; Hahm, M.G.; Kim, D.-H.; Kim, A.R.; Kahng, Y.H.; Park, S.-W.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; et al. Graphene-based gas sensor: Metal decoration effect and application to a flexible device. J. Mater. Chem. C 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Huang, J.; Du, Y.; Ruan, S.C. Reduced Graphene Oxide/Au Nanocomposite for NO(2) Sensing at Low Operating Temperature. Sensors 2016, 16, 1152. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wu, Z.; Ding, H.; Yang, X.; Wei, Y.; Xiao, M.; Yang, Z.; Yang, B.R.; Liu, C.; Lu, X.; et al. Three-Dimensional-Structured Boron- and Nitrogen-Doped Graphene Hydrogel Enabling High-Sensitivity NO2 Detection at Room Temperature. ACS Sens. 2019, 4, 1889–1898. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-dimensional mesoporous graphene aerogel-supported SnO2 nanocrystals for high-performance NO2 gas sensing at low temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, M.; Liu, G.; Gao, Y.; Zhao, L.; Li, S.; Wang, Y.; Liu, F.; Liang, X.; Zhang, T.; et al. Room temperature NO2 gas sensor based on porous Co3O4 slices/reduced graphene oxide hybrid. Sens. Actuators B Chem. 2018, 263, 387–399. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.H.; Park, S.G.; Kwon, J.D.; Lee, Y.J.; Lee, K.H.; Lee, B.H.; et al. Chemical Sensing of 2D Graphene/MoS2 Heterostructure device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Nemade, H.B.; Giri, P.K. Adsorption of Small Molecules on Niobium Doped Graphene: A Study Based on Density Functional Theory. IEEE Electron Device Lett. 2018, 39, 296–299. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, K.; Zhang, Y.; Ye, H.; Chen, X. Superior Selectivity and Sensitivity of C3N Sensor in Probing Toxic Gases NO2 and SO2. IEEE Electron Device Lett. 2018, 39, 284–287. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Y.; Yang, Z.; Wu, J. Nanoheterostructure Construction and DFT Study of Ni-Doped In2O3 Nanocubes/WS2 Hexagon Nanosheets for Formaldehyde Sensing at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 11979–11989. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, D.; Miao, J.; Lin, S.; Yu, Z.; Cui, D.; Yang, Z.; Chen, X. Highly sensitive sensor based on ordered porous ZnO nanosheets for ethanol detecting application. Sens. Actuators B Chem. 2021, 326. [Google Scholar] [CrossRef]
- Luo, H.-C.; Meng, R.-S.; Gao, H.L.; Sun, X.; Xiao, J.; Ye, H.-Y.; Zhang, G.-Q.; Chen, X.-P. First-Principles Study of Nitric Oxide Sensor Based on Blue Phosphorus Monolayer. IEEE Electron Device Lett. 2017, 38, 1139–1142. [Google Scholar] [CrossRef]
- Jin, C.; Tang, X.; Tan, X.; Smith, S.C.; Dai, Y.; Kou, L. A Janus MoSSe monolayer: A superior and strain-sensitive gas sensing material. J. Mater. Chem. A 2019, 7, 1099–1106. [Google Scholar] [CrossRef]
- Tang, X.; Shang, J.; Gu, Y.; Du, A.; Kou, L. Reversible gas capture using a ferroelectric switch and 2D molecule multiferroics on the In2Se3 monolayer. J. Mater. Chem. A 2020, 8, 7331–7338. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wei, Y.; Ding, H.; Wu, Z.; Yang, X.; Li, Z.; Huang, W.; Xie, X.; Tao, K.; Wang, X. Green Synthesis of 3D Chemically Functionalized Graphene Hydrogel for High-Performance NH3 and NO2 Detection at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20623–20632. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS(2), WS(2) and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wu, J.; Qi, X.; He, Q.; Liusman, C.; Lu, G.; Zhou, X.; Zhang, H. Graphene oxide scrolls on hydrophobic substrates fabricated by molecular combing and their application in gas sensing. Small 2013, 9, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhanen, T. Ultrafast Graphene Oxide Humidity Sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef] [PubMed]
| Sensing Materials | Detection Range (ppm) | |Response| (%) a | Response/Recovery Time (s) | LOD (ppb) | References |
|---|---|---|---|---|---|
| Ag-rGO aerogel | 0.05–5 | 8.6 (0.08 ppm) | 75/89.5 | 6.9 | Our previous work [28] |
| 1D rGO fiber | 1–20 | 0.39 (20 ppm) | 180/180 | 813 | [33] |
| graphene nanomesh | 1–10 | 6 (1 ppm) | 900/1200 | 15 | [34] |
| rGO hydrogel | 1–10 | 3.24 (10 ppm) | 600/6180 | 187 | [35] |
| Cs-GO | 0.18–12.2 | 1.5 (0.732 ppm) | 240/540 | 90 | [36] |
| Al-graphene | 1.2–5 | 8 (1.2 ppm) | 360/1200 | - | [37] |
| Au-rGO | 0.5–5 | 33 (5 ppm) | 132/386 | - | [38] |
| Si-graphene | 0.018–300 | 21.5 (50 ppm) | 126/378 | 18 | [18] |
| N-rGO | 0.02–0.8 | 11.7 (0.8 ppm) | 151/10 | 14 | [39] |
| SnO2-rGO | 14–110 | 11.5 (110 ppm) | 480/480 | 2000 | [40] |
| Co3O4-rGO | 0.05–10 | 26.8 (5 ppm) | 90/2400 | 50 | [41] |
| MoS2-Graphene | 1.2–100 | 6.83 (5 ppm) | 300/1800 | 1200 | [42] |
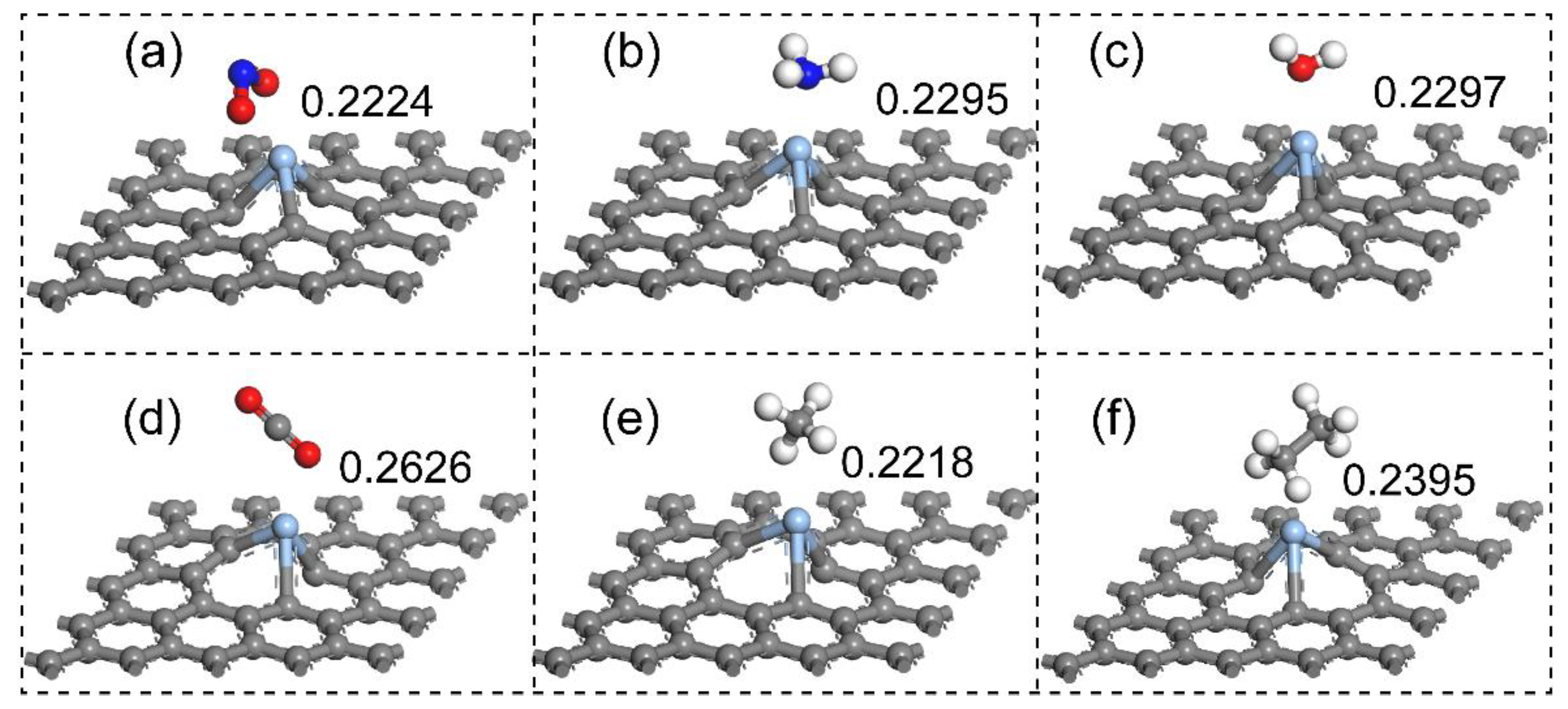
| Gas | d (nm) | Ead (eV) | Q (e) |
|---|---|---|---|
| NO2 | 0.2224 (Ag-O) | −2.209 | −0.450 |
| NH3 | 0.2295 (Ag-N) | −1.115 | 0.136 |
| H2O | 0.2297 (Ag-O) | −0.930 | 0.122 |
| CO2 | 0.2626 (Ag-O) | −0.360 | 0.018 |
| CH4 | 0.2218 (Ag-H) | −0.335 | 0.031 |
| C2H6 | 0.2395 (Ag-H) | −0.514 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, Y.; Chen, D.; Miao, J.; Zhi, X.; Deng, S.; Lin, S.; Jin, H.; Cui, D. Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study. Chemosensors 2021, 9, 227. https://doi.org/10.3390/chemosensors9080227
Li Q, Liu Y, Chen D, Miao J, Zhi X, Deng S, Lin S, Jin H, Cui D. Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study. Chemosensors. 2021; 9(8):227. https://doi.org/10.3390/chemosensors9080227
Chicago/Turabian StyleLi, Qichao, Yamin Liu, Di Chen, Jianmin Miao, Xiao Zhi, Shengwei Deng, Shujing Lin, Han Jin, and Daxiang Cui. 2021. "Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study" Chemosensors 9, no. 8: 227. https://doi.org/10.3390/chemosensors9080227
APA StyleLi, Q., Liu, Y., Chen, D., Miao, J., Zhi, X., Deng, S., Lin, S., Jin, H., & Cui, D. (2021). Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study. Chemosensors, 9(8), 227. https://doi.org/10.3390/chemosensors9080227









