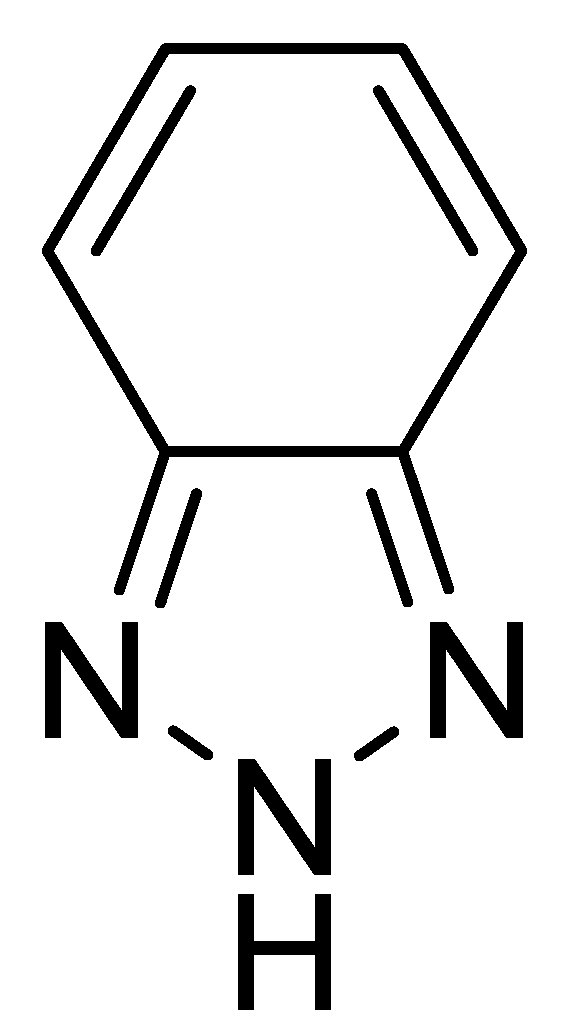
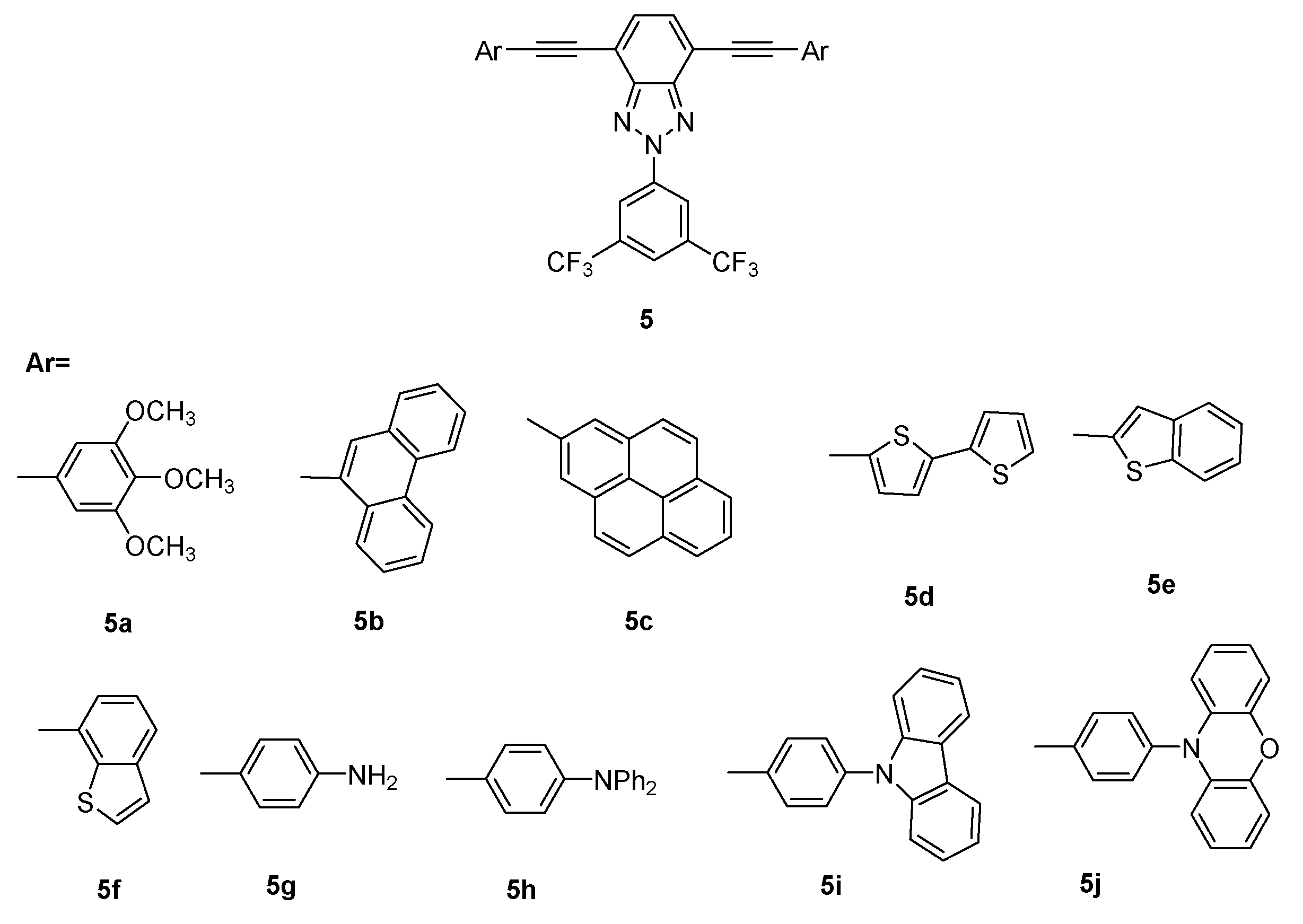
New Organic Materials Based on Multitask 2H-benzo[d]1,2,3-triazole Moiety
Abstract
:1. Introduction
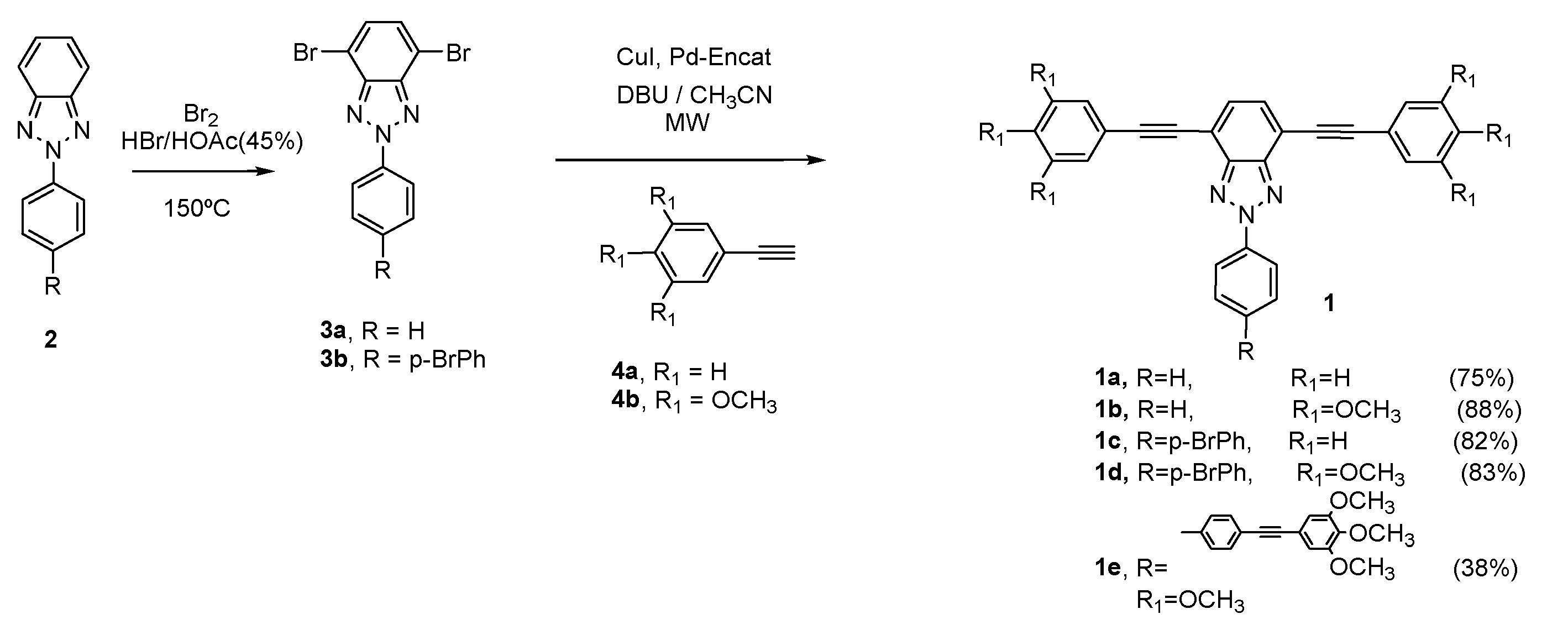
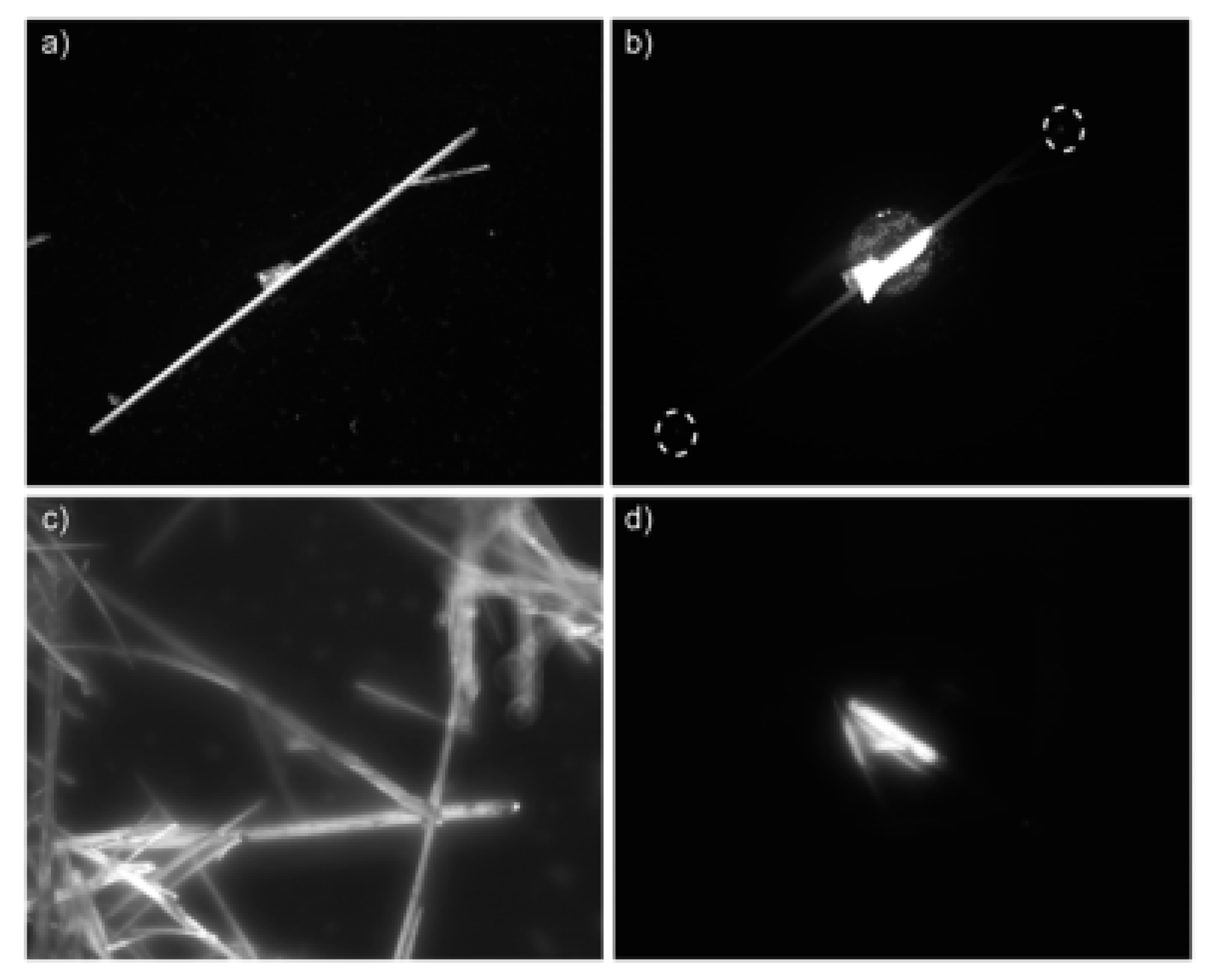
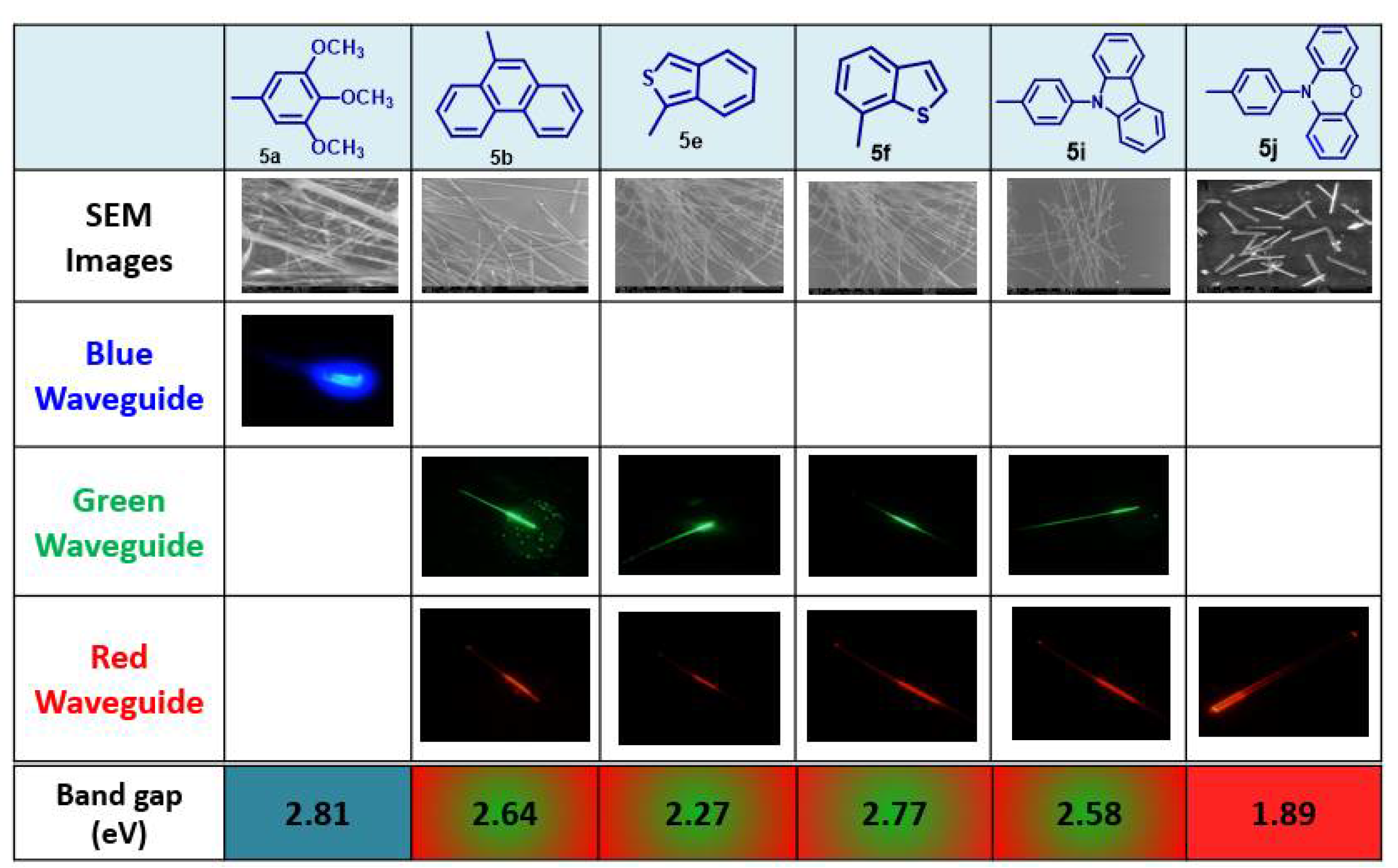
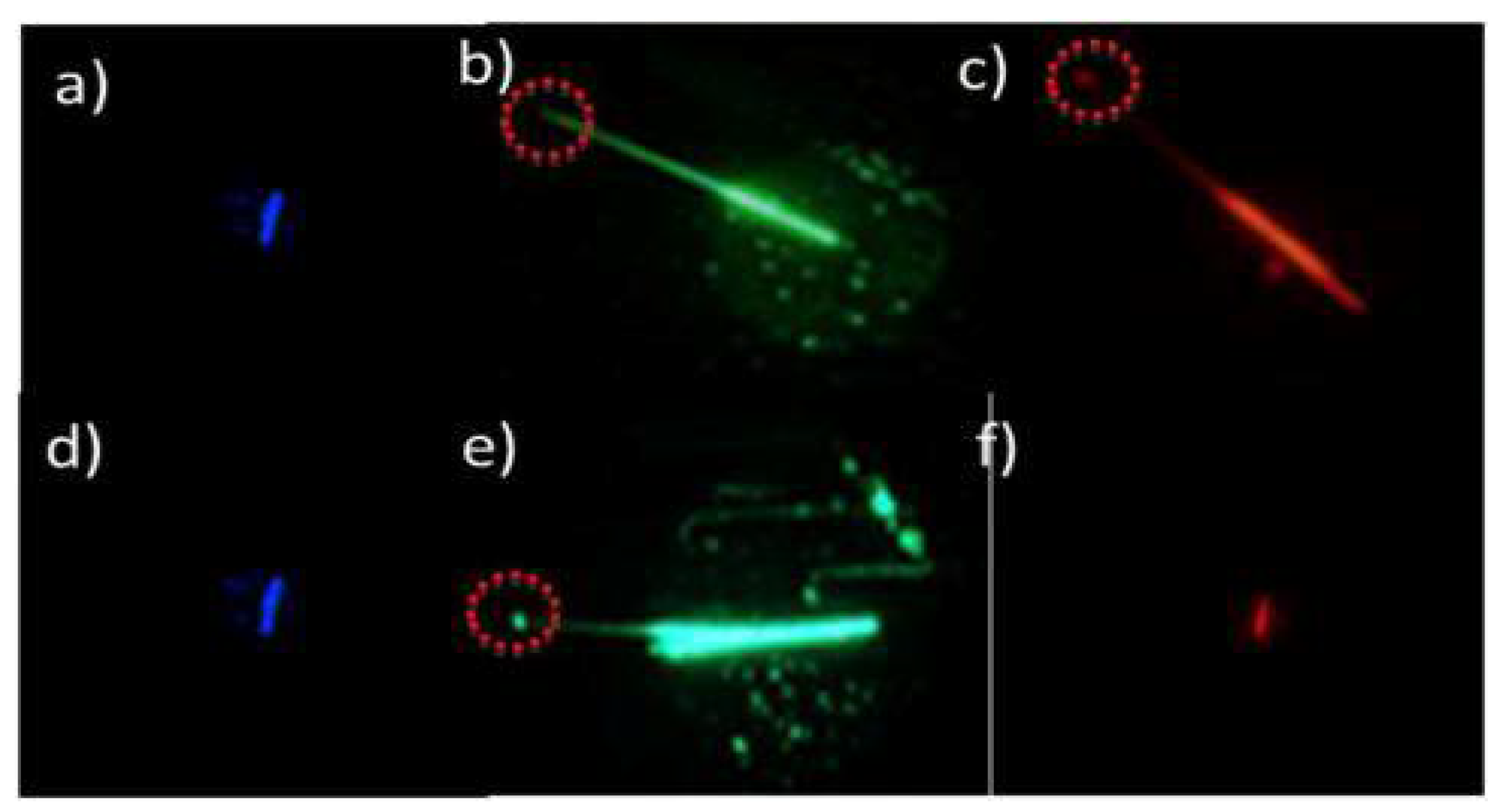
2. BTz as Optical Waveguides
3. BTz as Semiconductor in Organic Field-Effect Transistors (OFETs)
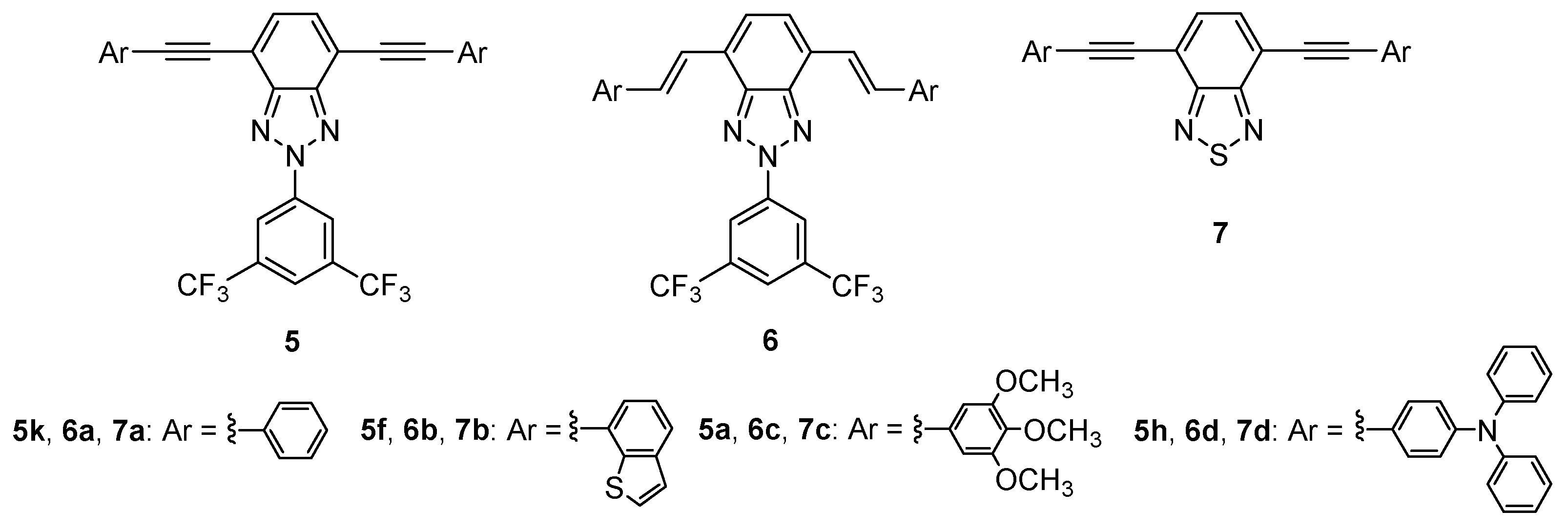
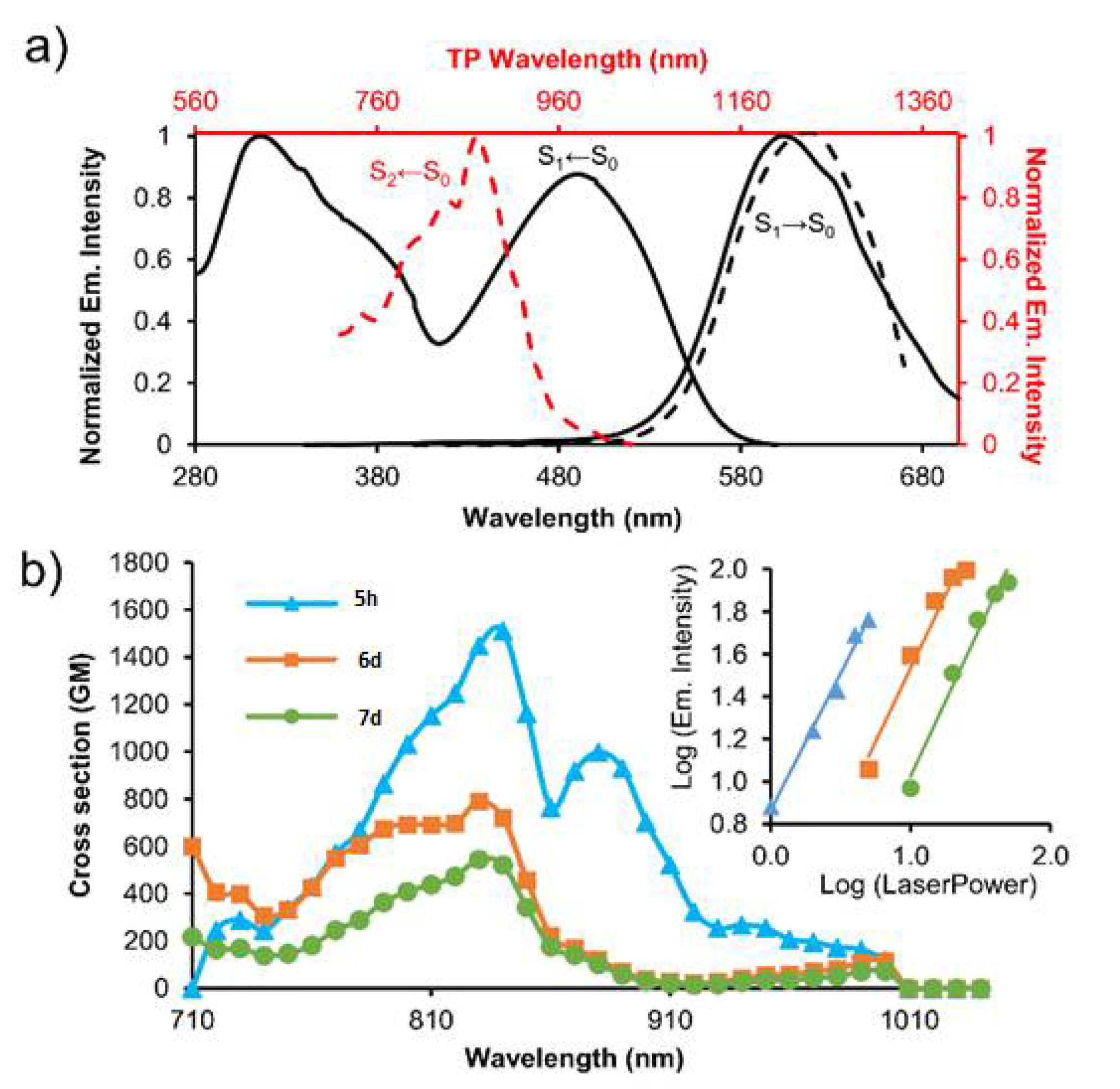
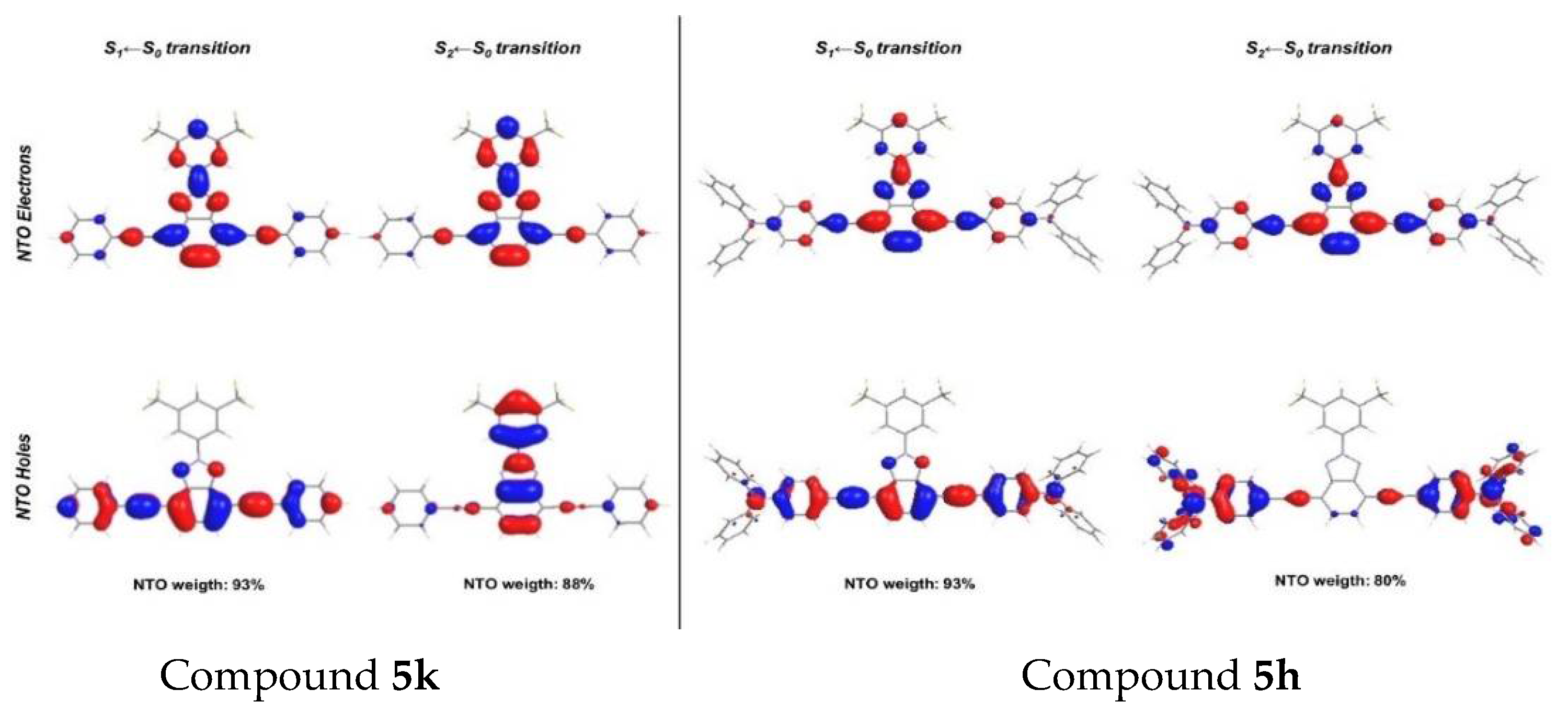
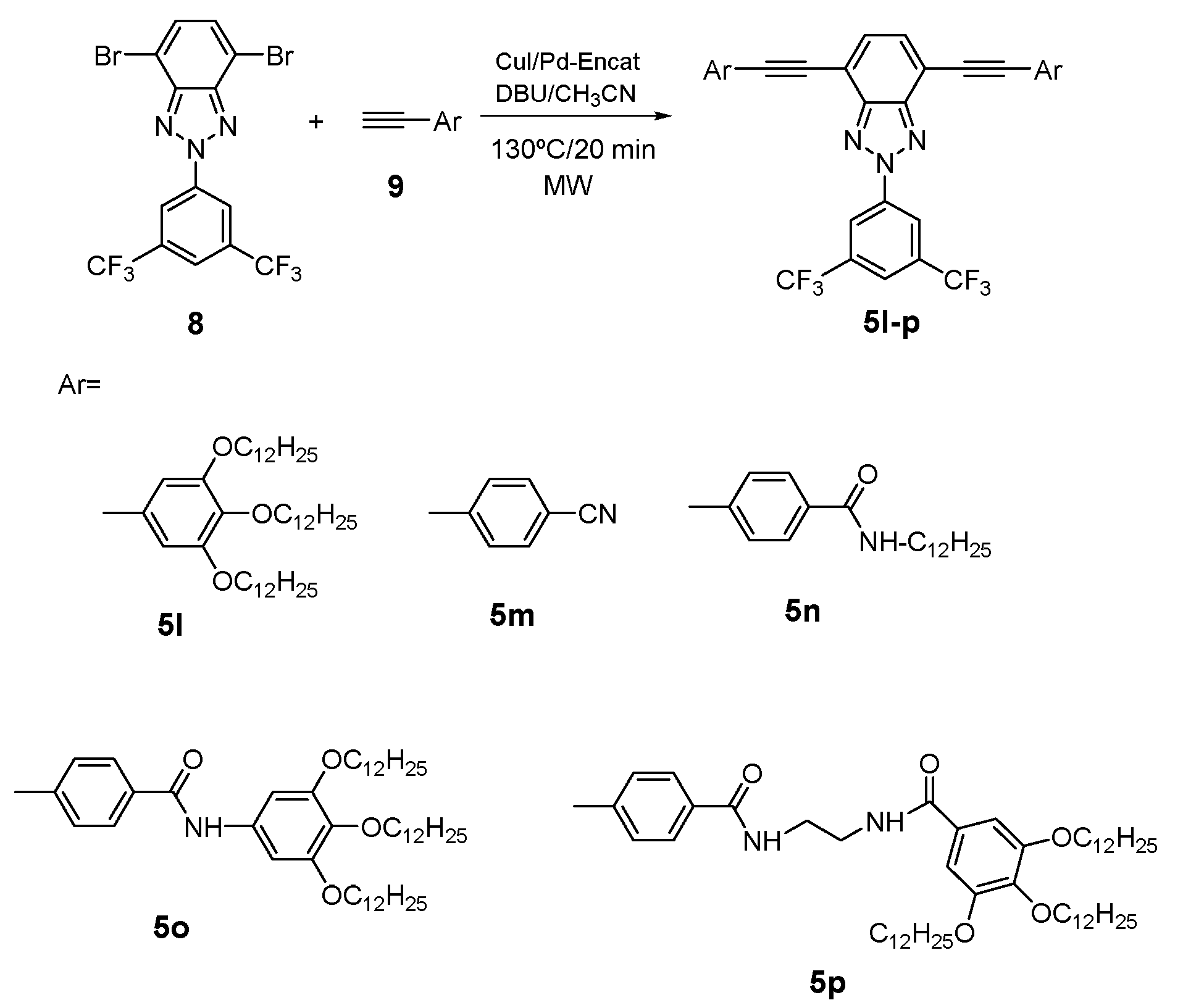
4. BTz as Two Photon Absorption (TPA)
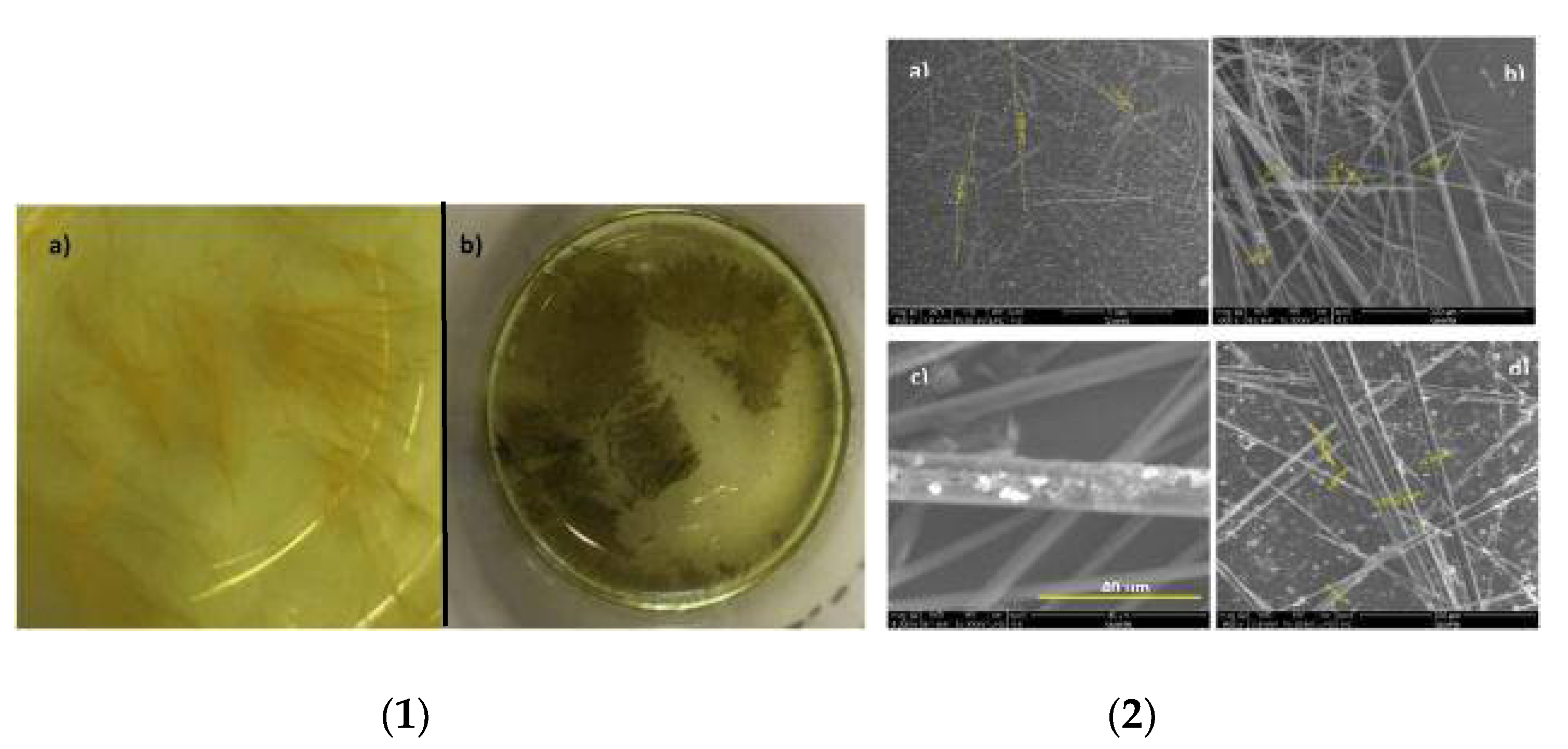
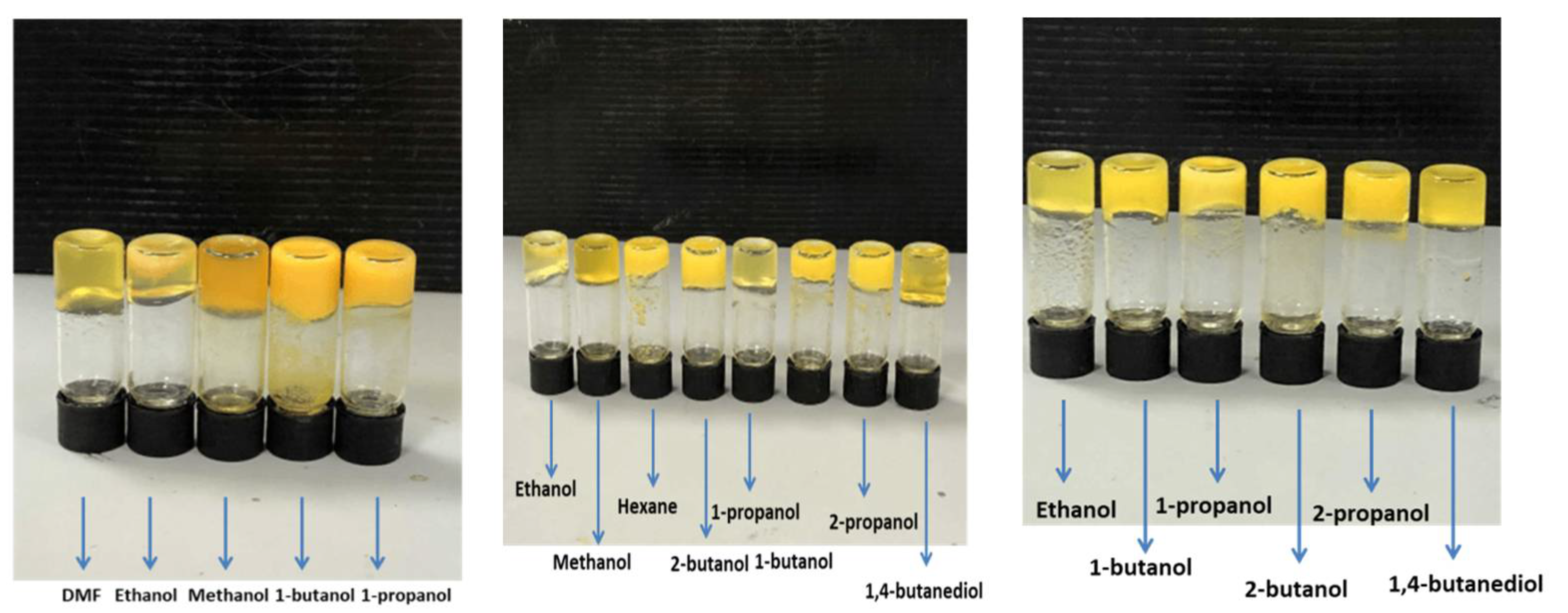
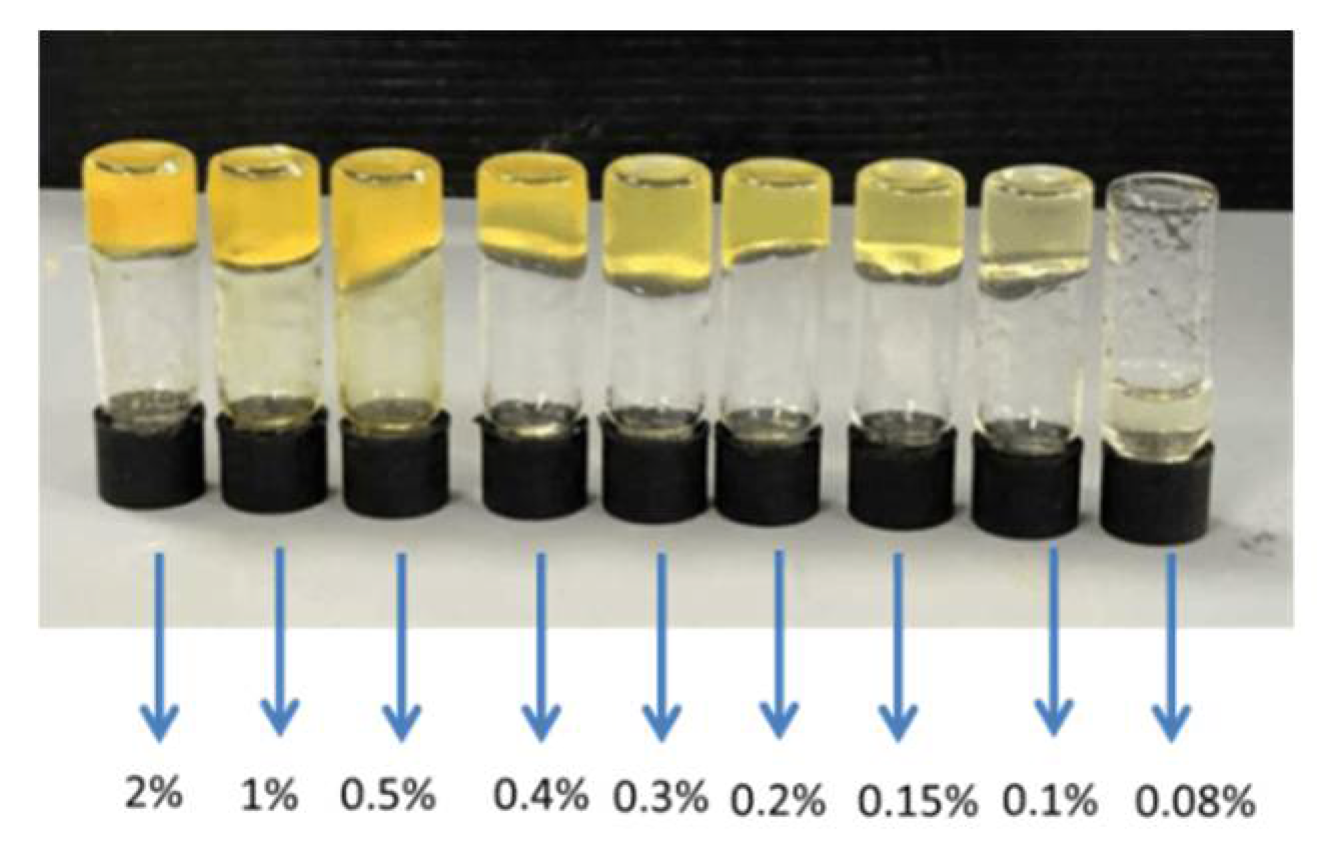
5. BTz as Organogels
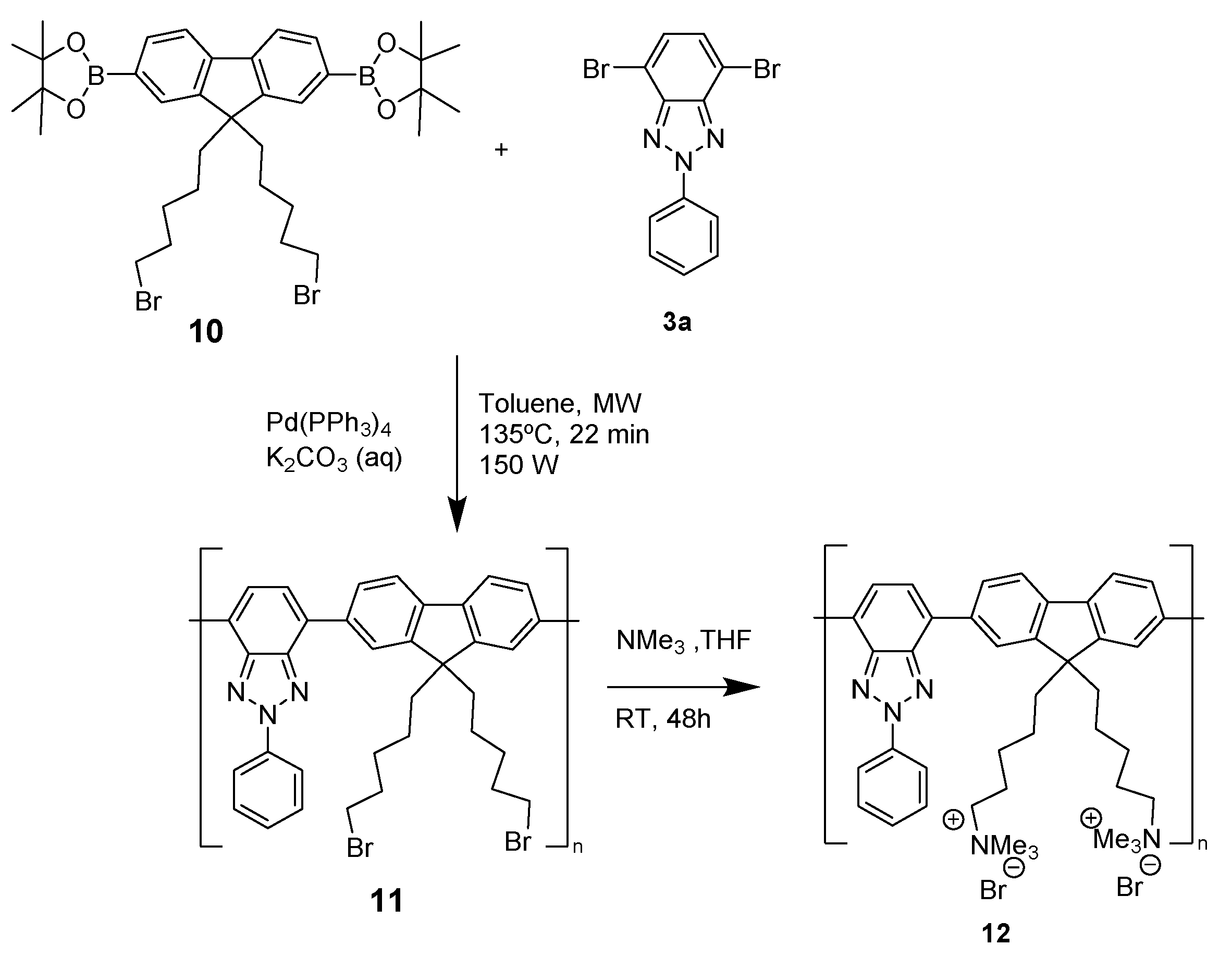
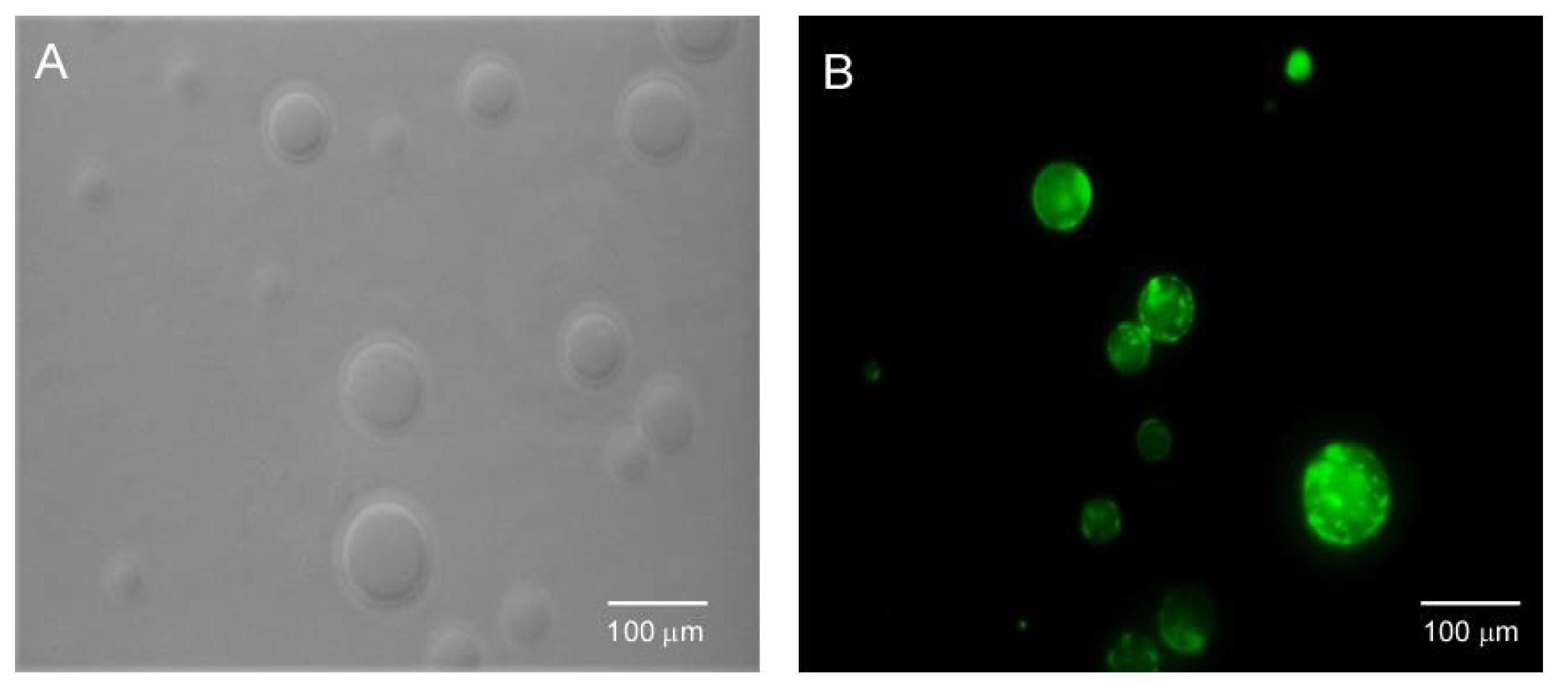
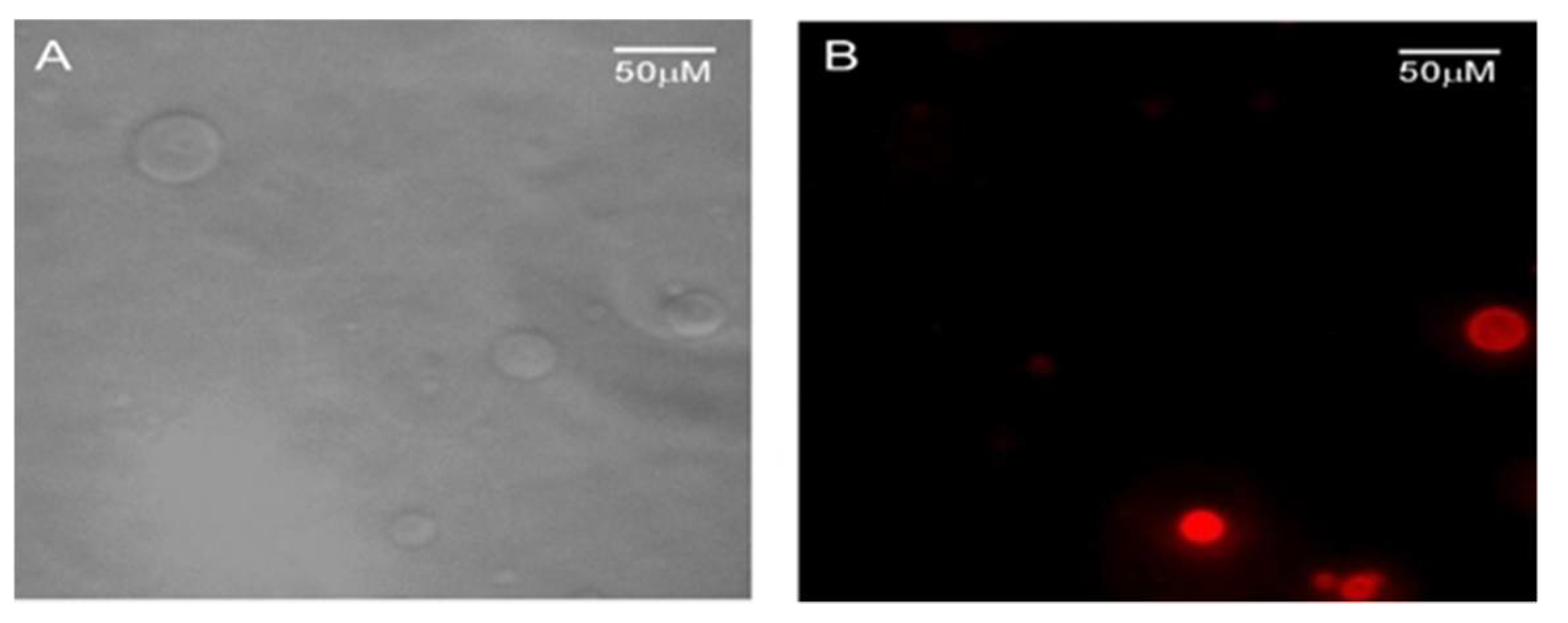
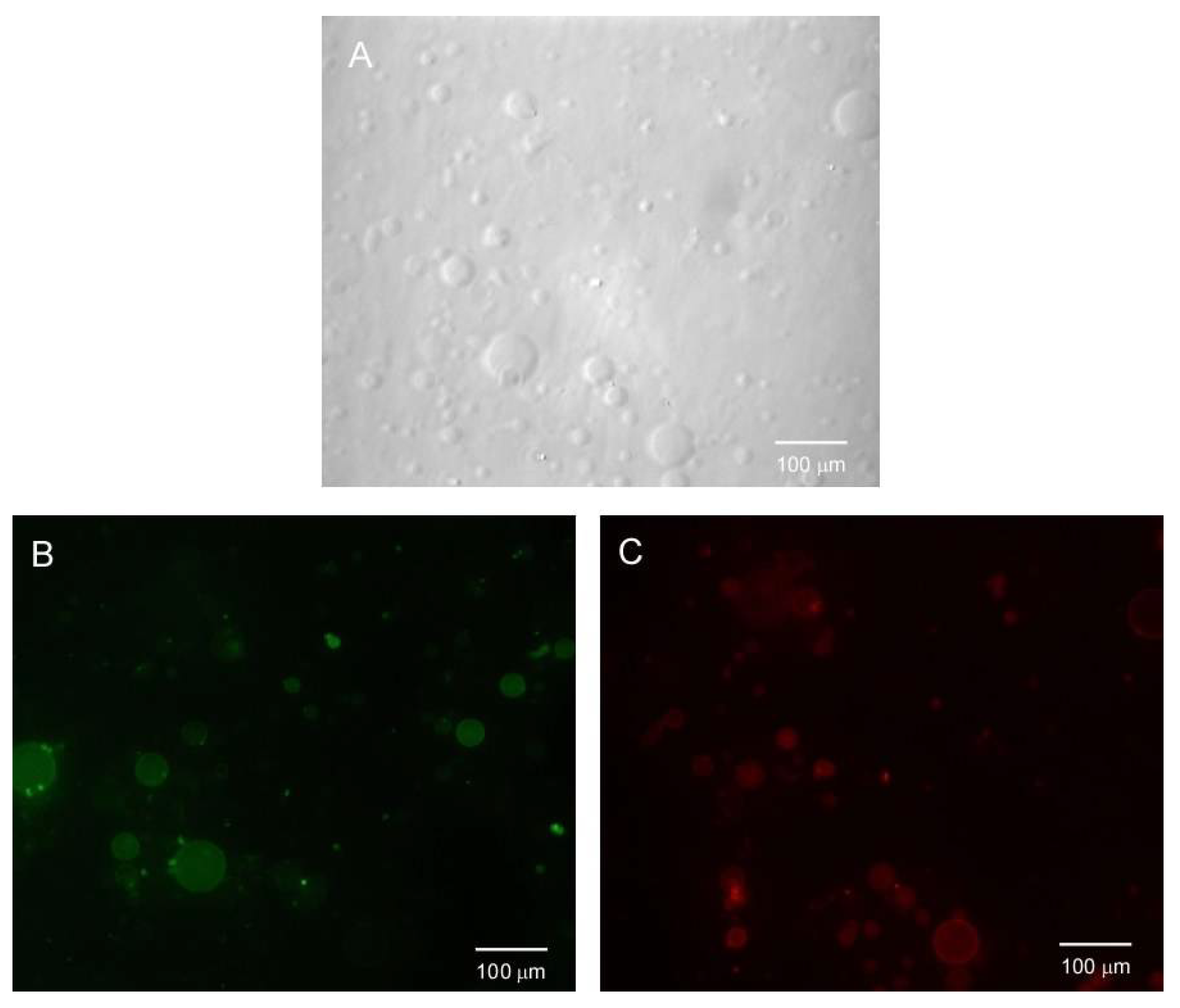
6. BTz as Biomarker
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraxedas, J. Moleular Organic Materials. From Molecules to Crystalline Solid; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Neena, K.K.; Sudhakar, P.; Dipak, K.; Thilagar, P. Diarylboryl-phenothiazine based multifunctional molecular siblings. Chem. Commun. 2017, 53, 3641–3644. [Google Scholar] [CrossRef]
- Sinha, S.; Chowdhury, B.; Ghoraib, U.K.; Ghosh, P. Multitasking behaviour of a small organic compound: Solid state bright white-light emission, mechanochromism and ratiometric sensing of Al(iii) and pyrophosphate. Chem. Commun. 2019, 55, 5127–5130. [Google Scholar] [CrossRef]
- Tanimoto, A.; Yamamoto, T. Nickel-2,2’-Bipyridyl and Palladium-TriphenylphosphineComplex Promoted Synthesis of Newp-Conjugated Poly(2-hexylbenzotriazole)s and Characterization of the Polymers. Adv. Synth. Catal. 2004, 346, 1818–1823. [Google Scholar] [CrossRef]
- Balan, A.; Baran, D.; Toppare, L. Benzotriazole containing conjugated polymers for multipurpose organic electronic applications. Polym. Chem. 2011, 2, 1029–1043. [Google Scholar] [CrossRef]
- Balan, A.; Gunbas, G.; Durmus, A.; Toppare, L. Donor−Acceptor Polymer with Benzotriazole Moiety: Enhancing the Electrochromic Properties of the Donor Unit. Chem. Mater. 2008, 20, 7510–7513. [Google Scholar] [CrossRef]
- Patel, D.; Fude, F.; Ohnishi, Y.; Abboud, K.; Hirata, S.H.; Schanze, K.S.; Reynolds, J.R. It Takes More Than an Imine: The Role of the Central Atom on the Electron-Accepting Ability of Benzotriazole and Benzothiadiazole Oligomers. J. Am. Chem. Soc. 2012, 134, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ye, S.; Zou, Y.; Peng, B.; He, Y.; Zhou, H. A Dithienyl Benzotriazole-based Polyfluorene: Synthesis and Applications in Polymer Solar Cells and Red Light-Emitting Diodes. Chem. Phys. 2011, 212, 1489–1496. [Google Scholar] [CrossRef]
- Dong, Y.; Cal, W.; Wang, M.; Li, Q.; Yilg, L.; Huang, F.; Cao, Y. [1,2,5]Thiadiazolo[3,4-f]benzotriazole based narrow band gapconjugated polymers with photocurrent response up to 1.1μm. Org. Electron. 2013, 14, 2459–2467. [Google Scholar] [CrossRef]
- Dong, Y.; Cai, W.; Hu, X.; Zhong, C.; Huang, F.; Cao, Y. Synthesis of novel narrow-band-gap copolymers based on [1,2,5]thiadiazolo[3,4-f]benzotriazole and their application in bulk-heterojunction photovoltaic devices. Polymers 2012, 53, 1465–1471. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Ghosh, S.; Pati, P.B.; Zade, S.S. Effect of the change of heteroatom on phenyl capped benzazole: Photophysical and electrochemical properties from the structural viewpoint. J. Lumin. 2018, 194, 164–169. [Google Scholar] [CrossRef]
- Minella, M.; De Laurentiis, E.; Pellegrino, F.; Prozzi, M.; Dal Bello, F.; Maurin, V.; Minero, C. Photocatalytic Transformations of 1H-Benzotriazole and Benzotriazole Derivates. Nanomaterials 2020, 10, 1835. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, B.; Du, M.; Li, G.; Tang, A.; Zhou, E. A2–A1–D–A1–A2 type non-fullerene acceptors based on methoxy substituted benzotriazole with three different end-capped groups for P3HT-based organic solar cells. J. Mater. Chem. C 2018, 6, 10902–10909. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, K.; Jiang, X.F.; Ying, L.; Huang, F.; Cao, Y. High-Performance Nonfullerene Polymer Solar Cells based on Imide-Functionalized Wide-Bandgap Polymers. Adv. Mater. 2017, 29, 1606396. [Google Scholar] [CrossRef]
- Jessop, I.A.; Bustos, M.; Hidalgo, D.; Terraza, C.A.; Tundidor-Camba, A.; Pardo, M.A.; Fuentealba, D.; Hssein, M.; Bernede, J.C. Synthesis of 2H-benzotriazole based donor-acceptor polymers bearing carbazole derivative as pendant groups: Optical, electronical and photovoltaic properties. Int. J. Electrochem. Sci. 2016, 11, 9822–9838. [Google Scholar] [CrossRef]
- Yuan, J.T.; Hou, J.J.; Liu, X.L.; Feng, Y.R.; Zhang, X.M. Optimized trimetallic benzotriazole-5-carboxylate MOFs with coordinately unsaturated active sites as an efficient electrocatalyst for the oxygen evolution reaction. Dalton Trans. 2020, 49, 750–756. [Google Scholar] [CrossRef]
- Kuznetsor, Y.I. Triazoles as a class of multifunctional corrosion inhibitors. A review. Part, I. 1,2,3-Benzotriazole and its derivatives. Copper, zinc and their alloys. Int. J. Corros. Scale Inhib. 2018, 7, 271–307. [Google Scholar]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, G.; Piras, S.; Paglietti, G.; Ferrone, M.; Fermeglia, M.; Pricl, S.; La Colla, P.; Secci, B.; Collu, G.; et al. Synthesis and in vitro evaluation of the anti-viral activity of N-[4-(1H(2H) benzotriazol-1(2)-yl)phenyl]alkylcarboxamides. Med. Chem. 2006, 2, 577–589. [Google Scholar] [CrossRef]
- Carta, A.; Briguglio, I.; Piras, S.; Corona, P.; Boatto, G.; Nieddu, M.; Giunchedi, P.; Marongiu, M.E.; Giliberti, G.; Iuliano, F.; et al. Quinoline tricyclic derivatives. Design, synthesis and evaluation of the antiviral activity of three new classes of RNA-dependent RNA polymerase inhibitors. Bioorg. Med. Chem. 2011, 19, 7070–7084. [Google Scholar] [CrossRef]
- Yan, R.; Gargas, D.; Yang, P. Nanowire Photonics. Nat. Photon. 2009, 3, 569–576. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, Y.S. Organic nanophotonics: From controllable assembly of functional molecules to low-dimensional materials with desired photonic properties. Chem. Soc. Rev. 2014, 43, 4325–4340. [Google Scholar] [CrossRef]
- Hunsperger, R.G. Integrated Optics: Theory and Technology; Springer: Berlin, Germany, 2002. [Google Scholar]
- Lal, S.; Link, S.; Halas, N.J. Nano-optics from sensing to waveguiding. Nat. Photon. 2007, 1, 641–648. [Google Scholar] [CrossRef]
- Clark, J.; Lanzani, G. Organic photonics for communications. Nat. Photon. 2010, 4, 438–446. [Google Scholar] [CrossRef]
- Cáceres, D.; Cebrián, C.; Rodríguez, A.R.; Carrillo, J.R.; Díaz-Ortiz, A.; Prieto, P.; Aparicio, F.; García, F.; Sánchez, L. Optical waveguides from 4-aryl-4H-1,2,4-triazole-based supramolecular structures. Chem. Commun. 2013, 49, 621–623. [Google Scholar] [CrossRef] [Green Version]
- Pastor, M.J.; Torres, I.; Cebrián, C.; Carrillo, J.R.; Díaz-Ortiz, A.; Matesanz, E.; Buendía, J.; García, F.; Barberá, J.; Prieto, P.; et al. 4-Aryl-3,5-bis(arylethynyl)aryl-4H-1,2,4-triazoles: Multitasking Skeleton as a Self-Assembling Unit. Chem. Eur. J. 2015, 21, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Wettach, H.; Pasker, F.; Höger, S. 2-Aryl-2H-benzotriazoles as Building Blocks for New Low-Bandgap Poly(arylene−ethynylene)s. Macromolecules 2008, 41, 9513–9515. [Google Scholar] [CrossRef]
- Torres, I.; Carrillo, J.R.; Díaz-Ortiz, A.; Martín, R.; Gómez, M.V.; Stegemann, L.; Strassert, C.A.; Orduna, J.; Buendía, J.; Greciano, E.E.; et al. Self-assembly of T-shape 2H-benzo[d][1,2,3]-triazoles. Optical waveguide and photophysical properties. RSC Adv. 2016, 6, 36544–36553. [Google Scholar] [CrossRef]
- Meier, H. Conjugated Oligomers with Terminal Donor–Acceptor Substitution. Angew. Chem. Int. Ed. 2005, 44, 2482–2506. [Google Scholar] [CrossRef]
- Mulliken, R.S.; Person, W.B. Donor-Acceptor Complexes. Annu. Rev. Phys. Chem. 1962, 13, 107–126. [Google Scholar] [CrossRef]
- Foster, R. Organic Charge-Transfer Complexes; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Foster, R. Electron donor-acceptor complexes. J. Phys. Chem. 1980, 84, 2135–2141. [Google Scholar] [CrossRef]
- Wang, J.L.; Xiao, Q.; Pei, J. Benzothiadiazole-Based D−π-A−π-D Organic Dyes with Tunable Band Gap: Synthesis and Photophysical Properties. J. Org. Lett. 2010, 12, 4164–4167. [Google Scholar] [CrossRef] [PubMed]
- Homnick, P.J.; Tinkham, J.S.; Devaughn, R.; Lahti, P.M. Engineering Frontier Energy Levels in Donor–Acceptor Fluoren-9-ylidene Malononitriles versus Fluorenones. J. Phys. Chem. A 2014, 118, 475–486. [Google Scholar] [CrossRef]
- Lu, X.; Fan, S.; Wu, J.; Jia, X.; Wang, Z.S.; Zhou, G. Controlling the Charge Transfer in D–A–D Chromophores Based on Pyrazine Derivatives. J. Org. Chem. 2014, 79, 6480–6489. [Google Scholar] [CrossRef] [PubMed]
- Turro, N.J.; Ramamurthy, V.; Scaianon, J.C. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, USA, 2010. [Google Scholar]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules. Photochem. Photobiol. 2012, 88, 1033. [Google Scholar] [CrossRef]
- Varughese, S. Non-covalent routes to tune the optical properties of molecular materials. J. Mater. Chem. C 2014, 2, 3499–3516. [Google Scholar] [CrossRef]
- Wang, X.A.; Zhou, Y.; Lei, T.; Hu, N.; Chen, E.Q.; Pei, J. Structural−Property Relationship in Pyrazino[2,3-g]quinoxaline Derivatives: Morphology, Photophysical, and Waveguide Properties. Chem. Mater. 2010, 22, 3735–3745. [Google Scholar] [CrossRef]
- Ando, S.; Murakami, R.; Nishida, J.I.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. n-Type Organic Field-Effect Transistors with Very High Electron Mobility Based on Thiazole Oligomers with Trifluoromethylphenyl Groups. J. Am. Chem. Soc. 2005, 127, 14996–14997. [Google Scholar] [CrossRef]
- Deng, P.; Yan, Y.; Wang, S.D.; Zhang, Q. Naphthoylene(trifluoromethylbenzimidazole)-dicarboxylic acid imides for high-performance n-type organic field-effect transistors. Chem. Commun. 2012, 48, 2591–2593. [Google Scholar] [CrossRef]
- Sonar, P.; Ng, G.M.; Lin, T.T.; Dodabalapur, A.; Chen, Z.K. Solution processable low bandgap diketopyrrolopyrrole (DPP) based derivatives: Novel acceptors for organic solar cells. J. Mater. Chem. 2010, 20, 3626–3636. [Google Scholar] [CrossRef]
- Chung, J.W.; Youç, Y.; Huh, H.S.; An, B.K.; Yoon, S.J.; Kim, S.H.; Lee, S.W.; Park, S.Y. Shear- and UV-Induced Fluorescence Switching in Stilbenic π-Dimer Crystals Powered by Reversible [2 + 2] Cycloaddition. J. Am. Chem. Soc. 2009, 131, 8163–8172. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Díaz-Ortiz, A.; Sánchez, L.; Orduña, J.; Blesa, M.J.; Carrillo, J.R.; Prieto, P. Tunable emission in aggregated T-Shaped 2H-Benzo[d][1,2,3]triazoles with waveguide behaviour. Dyes Pigm. 2017, 142, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Grigorenko, A.N.; Polini, M.; Novoselov, K.S. Graphene plasmonics. Nat. Photon. 2012, 6, 749–758. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Mueller, T.; Xia, F.; Avouris, P. Graphene photodetectors for high-speed optical communications. AC Nat. Photon. 2010, 4, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Wi, D.; Tian, J.; Li, L.; Yang, R. Plasmon induced transparency and refractive index sensing in a new type of graphene-based plasmonic waveguide. Opt. Commun. 2018, 412, 41–48. [Google Scholar]
- Liuc, M.; Yin, X.; Zhang, X. Double-Layer Graphene Optical Modulator. Nano Lett. 2012, 12, 1482–1485. [Google Scholar]
- Koester, S.J.; Li, M. High-speed waveguide-coupled graphene-on-graphene optical modulators. Appl. Phys. Lett. 2012, 100, 171107. [Google Scholar] [CrossRef]
- León, V.; Quintana, M.; Herrero, M.A.; Fierro, J.L.G.; de la Hoz, A.; Prato, M.; Vázquez, E. Few-layer graphenes from ball-milling of graphite with melamine. Chem. Commun. 2011, 47, 10936–10938. [Google Scholar] [CrossRef]
- León, V.; Rodriguez, A.M.; Prieto, P.; Prato, M.; Vazquez, E. Exfoliation of Graphite with Triazine Derivatives under Ball-Milling Conditions: Preparation of Few-Layer Graphene via Selective Noncovalent Interactions. ACS Nano 2014, 8, 563–571. [Google Scholar] [CrossRef]
- Gonzalez-Domínguez, J.M.; León, V.; Lucío, M.I.; Prato, M.; Vázquez, E. Production of ready-to-use few-layer graphene in aqueous suspensions. Nat. Protoc. 2018, 13, 495–506. [Google Scholar] [CrossRef]
- Torres, I.; Gonzalez-Domínguez, J.M.; Díaz-Ortiz, A.; Romero-Nieto, C.; Rominguer, F.; Vázquez, E.; Carrillo, J.R.; Prieto, P. Modulation of waveguide behaviour of an ICT 2H-Benzo[d][1,2,3]Triazole derivative with graphene. Org. Electron. 2019, 68, 1–8. [Google Scholar] [CrossRef]
- Wakayama, Y.; Hayakawa, R.; Seo, H.-S. Recent progress in photoactive organic field-effect transistors. Sci. Technol. Adv. Mater. 2014, 15, 024202. [Google Scholar] [CrossRef] [Green Version]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Size, S.M.; Lee, M.K. Semiconductor Devices: Physics and Technology, 3rd ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Ponce, R.; Facchetti, A.; Marks, T.J. High-k Organic, Inorganic, and Hybrid Dielectrics for Low-Voltage Organic Field-Effect Transistors. Chem. Rev. 2010, 110, 205–239. [Google Scholar]
- Torres-Moya, I.; Arrechea-Marcos, I.; Tardío, C.; Carrillo, J.R.; Díaz-Ortiz, A.; López Navarrete, J.T.; Ruiz Delgado, M.C.; Prieto, P.; Ponce, R. D–A–D 2H-benzo[d][1,2,3]triazole derivatives as p-type semiconductors in organic field-effect transistors. RSC Adv. 2018, 8, 21879–21888. [Google Scholar] [CrossRef] [Green Version]
- Strickler, J.H.; Webb, W.W. CAN-AM Easten ’90; Antos, R.L., Krisiloff, A.J., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; pp. 107–118. [Google Scholar]
- Strickler, J.H.; Webb, W.W. Three-dimensional optical data storage in refractive media by two-photon point excitation. Opt. Lett. 1991, 16, 1780–1782. [Google Scholar]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongin, O.; Porrès, L.; Charlot, M.; Katan, C.; Blanchard-Desce, M. Synthesis, Fluorescence, and Two-Photon Absorption of a Series of Elongated Rodlike and Banana-Shaped Quadrupolar Fluorophores: A Comprehensive Study of Structure–Property Relationships. Chem. Eur. J. 2007, 13, 1481–1498. [Google Scholar] [CrossRef]
- Chung, S.J.; Zheng, S.; Odani, T.; Beverina, L.; Fu, J.; Padilha, L.A.; Biesso, A.; Hales, J.M.; Zhan, X.; Schmidt, K.; et al. Extended Squaraine Dyes with Large Two-Photon Absorption Cross-Sections. J. Am. Chem. Soc. 2006, 128, 14444–14445. [Google Scholar] [CrossRef]
- Albota, M.; Hess, S.E.; Webb, W.W.; Xu, C.; Beljonne, D.; Brédas, J.; Fu, J.Y.; Heikal, A.A.; Rumi, M.; Wu, X.L.; et al. Design of Organic Molecules with Large Two-Photon Absorption Cross Sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beverina, L.; Fu, J.; Leclercq, A.; Zojer, E.; Pacher, P.; Barlow, S.; Van Stryland, E.W.; Hagan, D.J.; Brédas, J.L.; Marder, S.R. Two-Photon Absorption at Telecommunications Wavelengths in a Dipolar Chromophore with a Pyrrole Auxiliary Donor and Thiazole Auxiliary Acceptor. J. Am. Chem. Soc. 2005, 127, 7282–7283. [Google Scholar] [CrossRef] [PubMed]
- Varnavski, O.; Yan, X.; Mongin, O.; Blanchard-Desce, M.; Goodson, T. Strongly Interacting Organic Conjugated Dendrimers with Enhanced Two-Photon Absorption. J. Phys. Chem. C 2007, 111, 149–162. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. Engl. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.I.; Matsumoto, T.; Ishi-I, T.; Thiemann, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Yamashita, Y.; Mataka, S. Strongly red-fluorescent novel donor–π-bridge–acceptor–π-bridge–donor (D–π–A–π–D) type 2,1,3-benzothiadiazoles with enhanced two-photon absorption cross-sections. Chem. Commun. 2004, 10, 2342–2343. [Google Scholar] [CrossRef]
- Kato, S.I.; Matsumoto, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Ishi-i, T.; Mataka, S. Novel 2,1,3-Benzothiadiazole-Based Red-Fluorescent Dyes with Enhanced Two-Photon Absorption Cross-Sections. Chem. Eur. J. 2006, 12, 2303–2317. [Google Scholar] [CrossRef]
- Yang, Z.D.; Feng, J.K.; Ren, A.M. Theoretical study of one-photon and two-photon absorption properties for 2,1,3-benzothiadiazole-based red-fluorescent dyes. J. Mol. Struct. Theochem 2008, 848, 24–33. [Google Scholar] [CrossRef]
- Martín, R.; Prieto, P.; Carrillo, J.R.; Torres, I.; Strassert, C.A.; Soloviova, K.; Rodríguez, M.A.; Sánchez, L.; Díaz-Ortiz, A. Colored optical waveguides in self-assembled thiadiazole-based materials. Dyes Pigm. 2018, 151, 327–334. [Google Scholar] [CrossRef]
- Martín, R.; Prieto, P.; Carrillo, J.R.; Rodríguez, A.M.; de Cózar Boj, A.P.G.; Díaz-García, M.A.; Ramírez, M.G. Design, synthesis and amplified spontaneous emission of 1,2,5-benzothiadiazole derivatives. J. Mater. Chem. C 2019, 7, 9996–10007. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Benitez-Martín, C.; Donoso, B.; Tardio, C.; Martín, R.; Carrillo, J.R.; Díaz-Ortiz, A.; Nájera, F.; Prieto, P.; Pérez-Inestrosa, E. Extended Alkenyl and Alkynyl Benzotriazoles with Enhanced Two-Photon Absorption Properties as a Promising Alternative to Benzothiadiazoles. Chem. Eur. J. 2019, 25, 15572–15579. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sundarajan, P.R. Low molecular weight organogels based on long-chain carbamates. Langmuir 2005, 21, 3802–3807. [Google Scholar] [CrossRef]
- Rao, M.R.; Sun, S. Supramolecular Assemblies of Amide-Derived Organogels Featuring Rigid π-Conjugated Phenylethynyl Frameworks. Langmuir 2013, 29, 15146–15158. [Google Scholar] [CrossRef]
- Dou, C.D.; Chem, D.; Iqbal, J.; Yuan, Y.; Zhang, H.Y. Multistimuli-Responsive Benzothiadiazole-Cored Phenylene Vinylene derivative with nanoassembly properties. Langmuir 2011, 27, 6323–6329. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Yoon, S.-J.; Lim, S.-J.; An, B.-K.; Park, S.Y. Dual-Mode Switching in Highly Fluorescent Organogels: Binary Logic Gates with Optical/Thermal Inputs. Angew. Chem. Int. Ed. 2009, 48, 7030–7034. [Google Scholar] [CrossRef]
- Prathap, A.; Sureshan, K.M. A mannitol based phase selective supergelator offers a simple, viable and greener method to combat marine oil spills. Chem. Commun. 2012, 48, 5250–5252. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, S.J.; Croisetu, C.M.; Stuart, M.C.A.; Feringa, B.L. Reversible gel–sol photoswitching with an overcrowded alkene-based bis-urea supergelator. Chem. Sci. 2016, 7, 4341–4346. [Google Scholar] [CrossRef] [Green Version]
- Van Gorp, J.J.; Vekemans, J.A.J.N.; Meijer, E.W. C3-Symmetrical Supramolecular Architectures: Fibers and Organic Gels from Discotic Trisamides and Trisureas. J. Am. Chem. Soc. 2002, 124, 14759–14769. [Google Scholar] [CrossRef] [PubMed]
- Ya Malkin, A.; Isayev, A.I. Rheology: Concepts, Methods and Applications, 3rd ed.Chemtech Publishing: Toronto, ON, Canada, 2016. [Google Scholar]
- Matsuo, K.; Matsuoka, M. Solid-State Polymorphic Transition of Theophylline Anhydrate and Humidity Effect. Cryst. Growth Des. 2007, 7, 411–415. [Google Scholar] [CrossRef]
- Seton, L.; Khamar, D.; Bradshaw, I.J.; Hutcheon, G.A. Solid State Forms of Theophylline: Presenting a NewAnhydrous Polymorph. Cryst. Growth Des. 2010, 10, 3879–3886. [Google Scholar] [CrossRef]
- Patel, M.K.; Tailor, S.M.; Patel, U.H. DFT study and Hirshfeld surface analysis of third polymorph of sulfamerazine. AIP Conf. Proc. 2016, 1728, 020257. [Google Scholar]
- Munroe, A.; Rasmuson, A.C.; Modnett, B.K.; Croker, D.M. Relative Stabilities of the Five Polymorphs of Sulfathiazole. Cryst. Growth Des. 2012, 12, 2825–2835. [Google Scholar] [CrossRef]
- Anwar, J.; Tarling, S.E.; Barnes, P. Polymorphism of Sulfathiazole. J. Pharm. Sci. 1989, 78, 337–342. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Saikia, B.; Prieto, P.; Carrillo, J.R.; Steed, B.J.W. High thermal stability, pH responsive organogels of 2H-benzo[d]1,2,3-triazole derivatives as pharmaceutical crystallization media. Cryst. Eng. Comm. 2019, 21, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Chen, F.; Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano. Lett. 2004, 4, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Zhan, R.; Liu, B. Benzothiadiazole-containing conjugated polyelectrolytes for biological sensing and imaging. Macromol. Chem. Phys. 2015, 216, 131–144. [Google Scholar] [CrossRef]
- Dias, F.B.; King, S.; Monkman, A.P.; Perepichka, I.I.; Kryuchkov, M.A.; Perepichka, I.F.; Bryce, M.R. Dipolar stabilization of emissive singlet charge transfer excited states in polyfluorene copolymers. J. Phys. Chem. B 2008, 112, 6557–6566. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sun, H.; Yang, H.; Liu, S.; Jenkins, G.; Feng, W.; Li, F.; Zhao, Q.; Liu, B.; Huang, W. Cationic polyfluorenes with phosphorescent iridium(III) complexes for time-resolved luminescent biosensing and fluorescence lifetime imaging. Adv. Funct. Mater. 2013, 23, 3268–3276. [Google Scholar] [CrossRef]
- Liu, B.; Bazan, G.C. Synthesis of cationic conjugated polymers for use in label-free DNA microarrays. Nat. Protoc. 2006, 1, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Mallavia, R.; Martinez-Peréz, D.; Chmelka, B.F.; Bazan, G.C. Blue fluorescent films based on poly-2,7-fluorene-phenylene derivatives. Bol. Soc. Esp. Ceram. Vidr. 2004, 43, 327–330. [Google Scholar] [CrossRef]
- Molina, R.; Gómez-Ruiz, S.; Montilla, F.; Salinas-Castillo, A.; Fernández-Arroyo, S.; Del Mar Ramos, M.; Micol, V.; Mallavia, R. Progress in the synthesis of poly(2,7-fluorene-alt-1,4-phenylene), PFP, via suzuki coupling. Macromolecules 2009, 42, 5471–5477. [Google Scholar] [CrossRef]
- Kahveci, Z.; Vázquez-Guilló, R.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. New Red-Emitting Conjugated Polyelectrolyte: Stabilization by Interaction with Biomolecules and Potential Use as Drug Carriers and Bioimaging Probes. ACS Appl. Mater. Interfaces 2016, 8, 1958–1969. [Google Scholar] [CrossRef]
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly(methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 17205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, R.; Martinez-Tomé, M.J.; Kahveci, Z.; Torres-Moya, I.; Falco, A.; Mallavia, R.; Reyes, C. Synthesis and Characterization of a Novel Green Cationic Polyfluorene and Its Potential Use as a Fluorescent Membrane Probe. Polymers 2018, 10, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]

|
Semiconductor (HMDS 90 °C) |
HOMO * (eV) |
LUMO * (eV) | μh (cm2 V−1 s−1) | VT (V) | ION/IOFF |
|---|---|---|---|---|---|
| 5b | −5.29 | −2.65 | 2.69 × 10−4 | −57 | 2 × 103 |
| 5c | −5.07 | −2.68 | 3.31 × 10−5 | −40 | 1 × 102 |
| 5e | −5.10 | −2.83 | 1.80 × 10−4 | −69 | 8 × 103 |
| 5f | −5.41 | −2.64 | 3.76 × 10−5 | −13 | 4 × 102 |
| 5h | −4.81 | −2.42 | 1.21 × 10−4 | −38 | 2 × 102 |
| 5i | −5.30 | −2.72 | 2.02 × 10−5 | −58 | 3 × 102 |
| 5j | −4.73 | −2.84 | 2.04 × 10−4 | −40 | 5 × 102 |
| Compound | Benzotriazole Band (cm−1) | C≡C Triple Bond Stretching (cm–1) |
|---|---|---|
| 5a | 1474 (1462) | 2202 (2300) |
| 5b | 1474 (1460) | 2197 (2287) |
| 5c | 1476 (1460) | 2207 (2277) |
| 5d | 1473 (1459) | 2187 (2272) |
| 5e | 1472 (1456) | 2193 (2259) |
| 5f | 1472 (1461) | 2200 (2295) |
| 5g | 1472 (1461) | 2204 (2291) |
| 5h | 1473 (1461) | 2198 (2289) |
| 5i | 1475 (1461) | 2193 (2296) |
| 5j | 1474 (1461) | 2210 (2300) |
| Compound | σ[GM] | Compound | σ[GM] | Compound | σ[GM] |
|---|---|---|---|---|---|
| 5k | 9 | 6a | 39 | 7a | 29 |
| 5f | 33 | 6b | 125 | 7b | 129 |
| 5a | 104 | 6c | 131 | 7c | 88 |
| 5h | 1510 | 6d | 790 | 7d | 545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Moya, I.; Carrillo, J.R.; Díaz-Ortiz, Á.; Prieto, P. New Organic Materials Based on Multitask 2H-benzo[d]1,2,3-triazole Moiety. Chemosensors 2021, 9, 267. https://doi.org/10.3390/chemosensors9090267
Torres-Moya I, Carrillo JR, Díaz-Ortiz Á, Prieto P. New Organic Materials Based on Multitask 2H-benzo[d]1,2,3-triazole Moiety. Chemosensors. 2021; 9(9):267. https://doi.org/10.3390/chemosensors9090267
Chicago/Turabian StyleTorres-Moya, Iván, José Ramón Carrillo, Ángel Díaz-Ortiz, and Pilar Prieto. 2021. "New Organic Materials Based on Multitask 2H-benzo[d]1,2,3-triazole Moiety" Chemosensors 9, no. 9: 267. https://doi.org/10.3390/chemosensors9090267