Atmospheric Plasma Lingual Frenectomy Followed by Post Operative Tongue Exercises: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Measurements
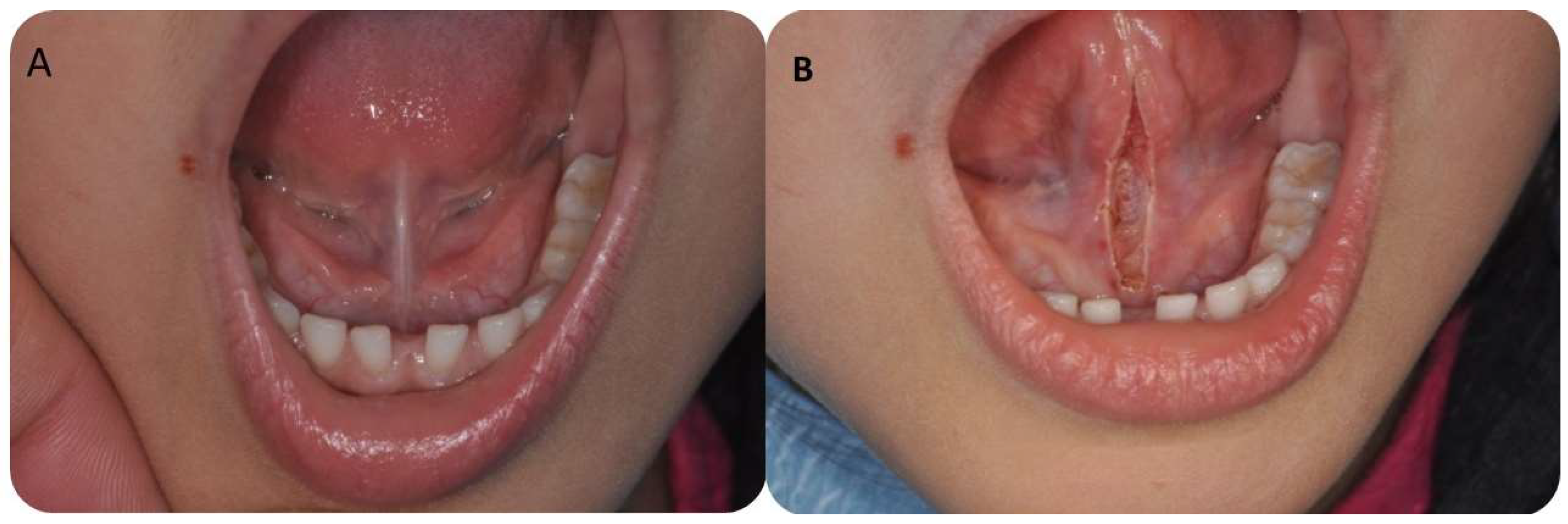
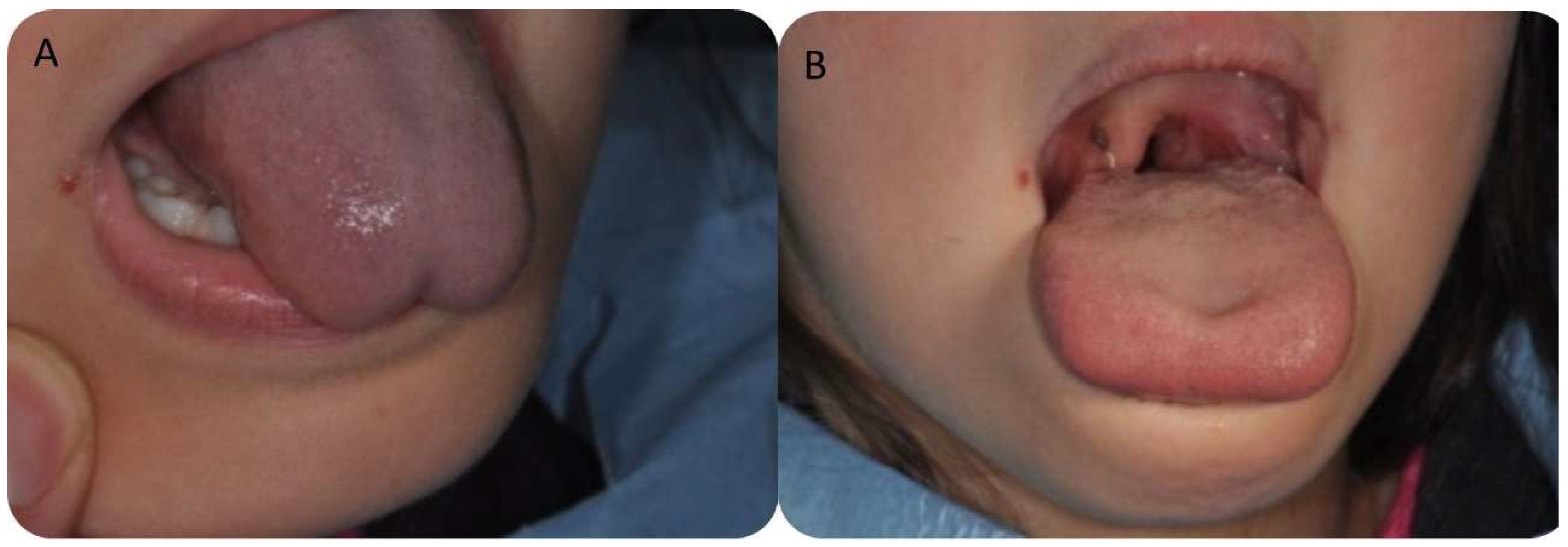
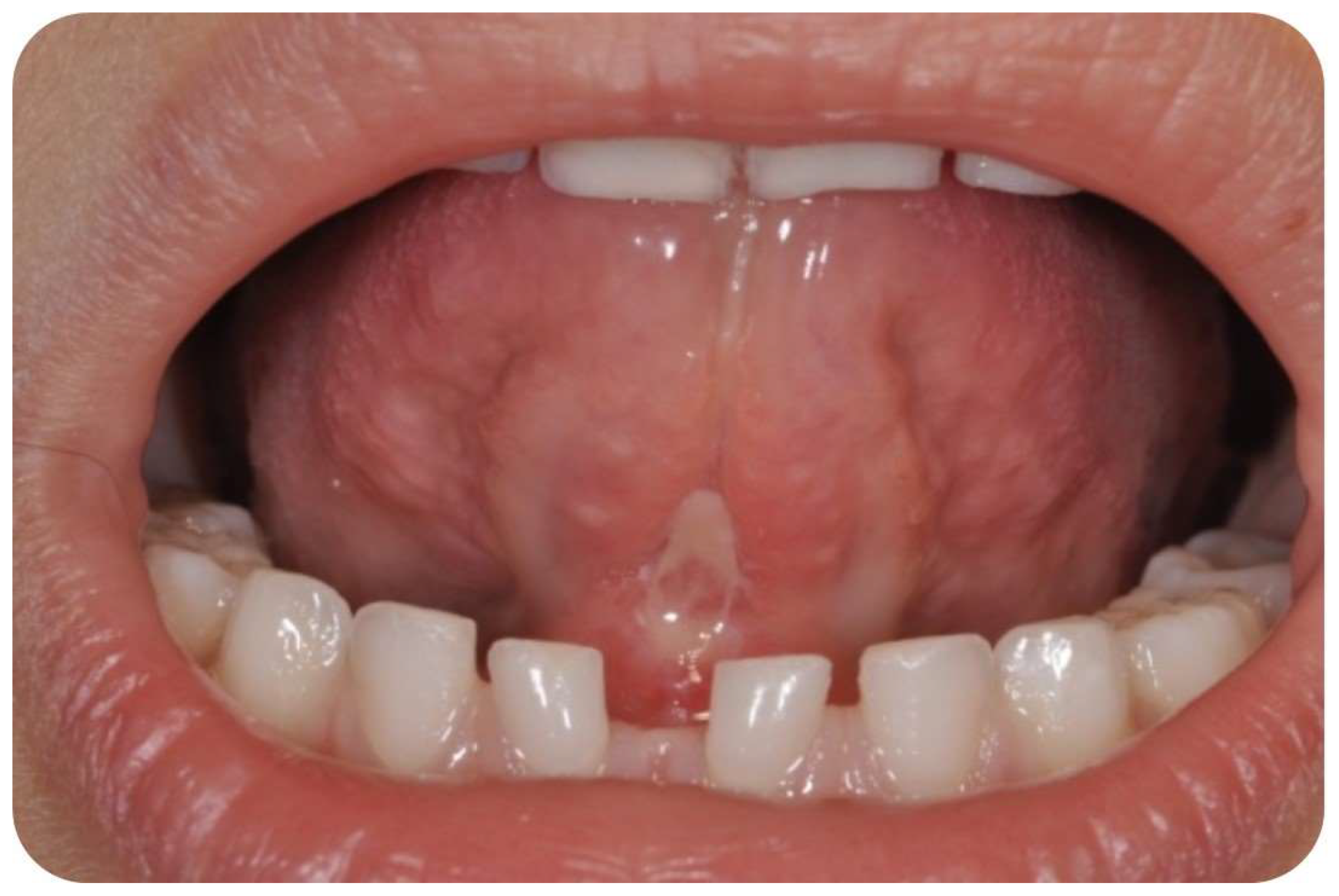
2.3. Surgical Procedure
- -
- 1st week before intervention: lingual exercises (one sequence 15 times/day)
- -
- 24 h after surgery: lingual exercises (two sequence 15 times/day)
- -
- 48 h after surgery: lingual exercises (three sequence 15 times/day)
- -
- 72 h after surgery: execution control
- -
- next 15 days: lingual exercises (three sequence 15 times/day)
- -
- 15 days after: execution control
- -
- next 30 days: lingual exercises (one sequence 15 times/day)
- -
- 45 days after: execution control
2.4. Statistical Methodologies
3. Results
3.1. Data Evidence
- 0 < rxy < 0.3 weak correlation (positive/negative)
- 0.3 < rxy < 0.7 moderate correlation (positive/negative)
- 0.7 < rxy < 1 strong correlation (positive/negative)
3.2. Clinical Measurements
3.3. Postoperative Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Last’s Anatomy—12th Edition. Available online: https://www.elsevier.com/books/last’s-anatomy/978-0-7020-3395-7 (accessed on 22 September 2022).
- McIntyre, E. Supporting Sucking Skills in Breastfeeding Infants. Matern. Child Nutr. 2008, 4, 233. [Google Scholar] [CrossRef]
- Benoiton, L.; Morgan, M.; Baguley, K. Management of Posterior Ankyloglossia and Upper Lip Ties in a Tertiary Otolaryngology Outpatient Clinic. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Kotlow, L.A. Ankyloglossia (Tongue-Tie): A Diagnostic and Treatment Quandary. Quintessence Int. 1999, 30, 259–262. [Google Scholar] [PubMed]
- Visconti, A.; Hayes, E.; Ealy, K.; Scarborough, D.R. A Systematic Review: The Effects of Frenotomy on Breastfeeding and Speech in Children with Ankyloglossia. Int. J. Speech Lang. Pathol. 2021, 23, 349–358. [Google Scholar] [CrossRef] [PubMed]
- O’Callahan, C.; Macary, S.; Clemente, S. The Effects of Office-Based Frenotomy for Anterior and Posterior Ankyloglossia on Breastfeeding. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 827–832. [Google Scholar] [CrossRef]
- Pransky, S.M.; Lago, D.; Hong, P. Breastfeeding Difficulties and Oral Cavity Anomalies: The Influence of Posterior Ankyloglossia and Upper-Lip Ties. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1714–1717. [Google Scholar] [CrossRef]
- Ghaheri, B.A.; Cole, M.; Fausel, S.C.; Chuop, M.; Mace, J.C. Breastfeeding Improvement Following Tongue-Tie and Lip-Tie Release: A Prospective Cohort Study. Laryngoscope 2017, 127, 1217–1223. [Google Scholar] [CrossRef]
- Joseph, K.S.; Kinniburgh, B.; Metcalfe, A.; Razaz, N.; Sabr, Y.; Lisonkova, S. Temporal Trends in Ankyloglossia and Frenotomy in British Columbia, Canada, 2004–2013: A Population-Based Study. CMAJ Open 2016, 4, E33–E40. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.; Links, A.; Boss, E.; Tunkel, D. Ankyloglossia and Lingual Frenotomy: National Trends in Inpatient Diagnosis and Management in the United States, 1997–2012. Otolaryngol. Head Neck Surg. 2017, 156, 735–740. [Google Scholar] [CrossRef]
- Kapoor, V.; Douglas, P.S.; Hill, P.S.; Walsh, L.J.; Tennant, M. Frenotomy for Tongue-Tie in Australian Children, 2006–2016: An Increasing Problem. Med. J. Aust. 2018, 208, 88–89. [Google Scholar] [CrossRef]
- Hill, R.R.; Richard, M.A.; Pados, B.F. Breastfeeding Symptoms with Tongue- and Lip-Tie. MCN Am. J. Matern. Child Nurs. 2023, 48, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.L.; Lai, C.T.; Geddes, D.T. Case Report of Nipple Shield Trauma Associated with Breastfeeding an Infant with High Intra-Oral Vacuum. BMC Pregnancy Childbirth 2015, 15, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, A.C.; Bai, P.M. Lingual Frenulum and Malocclusion: An Overlooked Tissue or a Minor Issue. Indian J. Dent. Res. 2015, 26, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Sepet, E.; Yildiz, C.; Erdem, A.P.; Ikikarakayali, G.; Gorken, F.N.; Kuru, S. Relationship between Mandibular Incisor Irregularity and Type of Occlusion in Ankyloglossia. Oral Health Prev. Dent. 2015, 13, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Carinci, F.; Candotto, V.; Lorusso, F. Eradication of Benign Skin Lesions of the Face by Voltaic Arc Dermabrasion (Atmospheric Plasma): Postoperative Pain Assessment by Thermal Infrared Imaging. Aesthetic Plast. Surg. 2020, 44, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Amuso, D.; Scogna, G.; Amore, R.; Lorusso, F. Static Crow’s Feet Treated with Voltaic Arc Dermabrasion (Atmospheric Plasma): Post-Operative Pain Assessment by Thermal Infrared Imaging. J. Clin. Med. 2021, 10, 3074. [Google Scholar] [CrossRef]
- Thammajak, P.; Louwakul, P.; Srisuwan, T. Effects of Cold Atmospheric Pressure Plasma Jet on Human Apical Papilla Cell Proliferation, Mineralization, and Attachment. Clin. Oral Investig. 2021, 25, 3275–3283. [Google Scholar] [CrossRef]
- Yoon, A.; Zaghi, S.; Weitzman, R.; Ha, S.; Law, C.S.; Guilleminault, C.; Liu, S.Y.C. Toward a Functional Definition of Ankyloglossia: Validating Current Grading Scales for Lingual Frenulum Length and Tongue Mobility in 1052 Subjects. Sleep Breath. 2017, 21, 767–775. [Google Scholar] [CrossRef]
- Ferrés-Amat, E.; Pastor-Vera, T.; Ferrés-Amat, E.; Mareque-Bueno, J.; Prats-Armengol, J.; Ferrés-Padró, E. Multidisciplinary Management of Ankyloglossia in Childhood. Treatment of 101 Cases. A Protocol. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e39–e47. [Google Scholar] [CrossRef]
- Solis-Pazmino, P.; Kim, G.S.; Lincango-Naranjo, E.; Prokop, L.; Ponce, O.J.; Truong, M.T. Major Complications after Tongue-Tie Release: A Case Report and Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110356. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Festa, F.; Candotto, V.; Amore, R.; Lorusso, F. Periauricular Wrinkles Removed with Voltaic Arc Dermabrasion (Atmospheric Plasma Technique). J. Cosmet. Dermatol. 2020, 19, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Mortellaro, C.; Mavriqi, L.; Di Cerbo, A. Evaluation Effectiveness of the Voltaic Arc Dermabrasion in Perioral Rhytides Eradication. J. Craniofac. Surg. 2016, 27, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Migliario, M.; Greco Lucchina, A.; Rocchetti, V.; Renò, F. Laser Surgical Approach to Impacted Maxillary Incisors: Case Series and Brief Review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9691–9696. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Navarro, R.S.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Horliana, A.C.R.T.; Silva, T.; Santos, E.M.; Sobral, A.P.T.; Júnior, A.B.; Nammour, S.; et al. Comparison of the Effects of High-Power Diode Laser and Electrocautery for Lingual Frenectomy in Infants: A Blinded Randomized Controlled Clinical Trial. J. Clin. Med. 2022, 11, 3783. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.; Geddes, D.T.; Amirapu, S.; Mirjalili, S.A. Understanding the Lingual Frenulum: Histological Structure, Tissue Composition, and Implications for Tongue Tie Surgery. Int. J. Otolaryngol. 2020, 2020, 1820978. [Google Scholar] [CrossRef]
- Taheri, A.; Mansoori, P.; Sandoval, L.F.; Feldman, S.R.; Pearce, D.; Williford, P.M. Electrosurgery: Part I. Basics and Principles. J. Am. Acad. Dermatol. 2014, 70, 591.e1–591.e14. [Google Scholar] [CrossRef]


| Kotlow | MOTTIP | MIO | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEFORE | T0 | T1 | T2 | T3 | BEFORE | T0 | T1 | T2 | T3 | BEFORE | T0 | T1 | T2 | T3 | |
| Mean | 17.2 | 27.1 | 25.8 | 25.1 | 24.9 | 17.7 | 23.0 | 21.5 | 20.9 | 22.3 | 30.0 | 42.1 | 41.0 | 39.9 | 40.4 |
| Std. Deviation | 2.10 | 4.10 | 2.48 | 1.99 | 2.55 | 4.63 | 3.63 | 5.96 | 4.21 | 2.53 | 5.50 | 5.00 | 5.14 | 3.42 | 3.35 |
| Lower 95% CI | 16.4 | 25.6 | 24.8 | 24.3 | 23.9 | 15.9 | 21.6 | 19.3 | 19.4 | 21.3 | 27.9 | 40.2 | 39.0 | 38.6 | 39.1 |
| Upper 95% CI | 18.0 | 28.7 | 26.7 | 25.8 | 25.8 | 19.4 | 24.3 | 23.7 | 22.5 | 23.2 | 32.0 | 43.9 | 42.9 | 41.1 | 41.6 |
| Kotlow | MOTTIP | MIO | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEFORE | T0 | T1 | T2 | T3 | BEFORE | T0 | T1 | T2 | T3 | BEFORE | T0 | T1 | T2 | T3 | ||
| Kotlow | BEFORE | - | - | - | - | - | 0.054 | −0.045 | −0.203 | −0.055 | −0.011 | −0.345 | 0.245 | 0.051 | −0.182 | 0.106 |
| T0 | - | - | - | - | - | −0.2779 | 0.109 | 0.333 | −0.007 | −0.176 | 0.042 | −0.065 | 0.032 | 0.23 | 0.099 | |
| T1 | - | - | - | - | - | 0.159 | 0.048 | −0.329 | 0.013 | 0.125 | −0.094 | 0.129 | 0.149 | −0.06 | −0.222 | |
| T2 | - | - | - | - | - | −0.143 | −0.036 | −0.074 | 0.076 | −0.381 | 0.157 | 0.034 | 0.335 | 0.102 | −0.565 | |
| T3 | - | - | - | - | - | −0.31 | 0.387 | −0.122 | 0.219 | −0.321 | 0.198 | −0.363 | −0.176 | 0.248 | 0.289 | |
| MOTTIP | BEFORE | 0.053 | −0.278 | 0.159 | −0.143 | −0.31 | - | - | - | - | - | −0.164 | 0.0511 | 0.123 | −0.211 | 0.113 |
| T0 | −0.045 | 0.101 | 0.048 | −0.036 | 0.387 | - | - | - | - | - | −0.013 | −0.229 | 0.052 | −0.123 | 0.036 | |
| T1 | −0.203 | 0.333 | −0.329 | −0.074 | −0.122 | - | - | - | - | - | −0.191 | 0.099 | −0.074 | 0.345 | 0.071 | |
| T2 | −0.055 | −0.007 | 0.013 | 0.076 | 0.219 | - | - | - | - | - | −0.044 | −0.167 | −0.104 | −0.019 | 0.203 | |
| T3 | −0.011 | −0.176 | 0.125 | −0.381 | −0.322 | - | - | - | - | - | −0.196 | 0.360131 | −0.311 | −0.301 | 0.037 | |
| MIO | BEFORE | −0.345 | 0.042 | −0.095 | 0.157 | 0.199 | −0.164 | −0.013 | −0.191 | −0.044 | −0.196 | - | - | - | - | - |
| T0 | 0.244 | −0.064 | 0.129 | 0.034 | −0.362 | 0.051 | −0.229 | 0.098 | −0.167 | 0.36 | - | - | - | - | - | |
| T1 | 0.051 | 0.031 | 0.149 | 0.335 | −0.175 | 0.123 | 0.051 | −0.073 | −0.104 | −0.31 | - | - | - | - | - | |
| T2 | −0.182 | 0.23 | −0.06 | 0.103 | 0.248 | −0.211 | −0.123 | 0.345 | −0.019 | −0.301 | - | - | - | - | - | |
| T3 | 0.107 | 0.099 | −0.222 | −0.566 | 0.289 | 0.113 | 0.036 | 0.071 | 0.203 | 0.037 | - | - | - | - | - | |
| Kotlow ANOVA Tukey’s Multiple Comparisons | Mean Diff, | 95.00% CI | Adjusted p Value | |
|---|---|---|---|---|
| BASELINE vs. T0 | −9.965 | −11.93 to −8.004 | <0.0001 | A–B |
| BASELINE vs. T1 | −8.589 | −10.55 to −6.628 | <0.0001 | A–C |
| BASELINE vs. T2 | −7.875 | −9.836 to −5.913 | <0.0001 | A–D |
| BASELINE vs. T3 | −7.68 | −9.641 to −5.719 | <0.0001 | A–E |
| T0 vs. T1 | 1.376 | −0.5854 to 3.337 | 0.3022 | B–C |
| T0 vs. T2 | 2.091 | 0.1294 to 4.052 | 0.0304 | B–D |
| T0 vs. T3 | 2.285 | 0.3239 to 4.246 | 0.0136 | B–E |
| T1 vs. T2 | 0.7148 | −1.246 to 2.676 | 0.8519 | C–D |
| T1 vs. T3 | 0.9093 | −1.052 to 2.870 | 0.7034 | C–E |
| T2 vs. T3 | 0.1945 | −1.767 to 2.156 | 0.9988 | D–E |
| MOTTIP Tukey’s multiple comparisons test | Mean Diff, | 95.00% CI of diff, | Adjusted p Value | |
| BASELINE vs. T0 | −5.306 | −8.401 to −2.210 | <0.0001 | A–B |
| BASELINE vs. T1 | −3.824 | −6.920 to −0.7288 | 0.0073 | A–C |
| BASELINE vs. T2 | −3.261 | −6.357 to −0.1655 | 0.0335 | A–D |
| BASELINE vs. T3 | −4.609 | −7.704 to −1.513 | 0.0006 | A–E |
| T0 vs. T1 | 1.481 | −1.614 to 4.577 | 0.6782 | B–C |
| T0 vs. T2 | 2.044 | −1.051 to 5.140 | 0.3636 | B–D |
| T0 vs. T3 | 0.6966 | −2.399 to 3.792 | 0.9714 | B–E |
| T1 vs. T2 | 0.5633 | −2.532 to 3.659 | 0.987 | C–D |
| T1 vs. T3 | −0.7845 | −3.880 to 2.311 | 0.9562 | C–E |
| T2 vs. T3 | −1.348 | −4.443 to 1.748 | 0.7498 | D–E |
| MIO Tukey’s multiple comparisons test | Mean Diff, | 95,00% CI of diff, | Adjusted p Value | |
| BASELINE vs. T0 | −12.12 | −15.38 to −8.858 | <0.0001 | A–B |
| BASELINE vs. T1 | −11.01 | −14.27 to −7.745 | <0.0001 | A–C |
| BASELINE vs. T2 | −9.899 | −13.16 to −6.638 | <0.0001 | A–D |
| BASELINE vs. T3 | −10.43 | −13.69 to −7.171 | <0.0001 | A–E |
| T0 vs. T1 | 1.113 | −2.148 to 4.374 | 0.8796 | B–C |
| T0 vs. T2 | 2.221 | −1.040 to 5.482 | 0.3322 | B–D |
| T0 vs. T3 | 1.687 | −1.574 to 4.948 | 0.6101 | B–E |
| T1 vs. T2 | 1.108 | −2.154 to 4.369 | 0.8815 | C–D |
| T1 vs. T3 | 0.5738 | −2.687 to 3.835 | 0.9885 | C–E |
| T2 vs. T3 | −0.5338 | −3.795 to 2.727 | 0.9913 | D–E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Di Giulio, R.; Gehrke, S.A.; Tagariello, G.; Romano, F.; Lorusso, F. Atmospheric Plasma Lingual Frenectomy Followed by Post Operative Tongue Exercises: A Case Series. Children 2023, 10, 105. https://doi.org/10.3390/children10010105
Scarano A, Di Giulio R, Gehrke SA, Tagariello G, Romano F, Lorusso F. Atmospheric Plasma Lingual Frenectomy Followed by Post Operative Tongue Exercises: A Case Series. Children. 2023; 10(1):105. https://doi.org/10.3390/children10010105
Chicago/Turabian StyleScarano, Antonio, Rosanna Di Giulio, Sergio Alexandre Gehrke, Gianluca Tagariello, Francesco Romano, and Felice Lorusso. 2023. "Atmospheric Plasma Lingual Frenectomy Followed by Post Operative Tongue Exercises: A Case Series" Children 10, no. 1: 105. https://doi.org/10.3390/children10010105
APA StyleScarano, A., Di Giulio, R., Gehrke, S. A., Tagariello, G., Romano, F., & Lorusso, F. (2023). Atmospheric Plasma Lingual Frenectomy Followed by Post Operative Tongue Exercises: A Case Series. Children, 10(1), 105. https://doi.org/10.3390/children10010105











