Postprandial Abdominal Pain Caused by Gastroptosis—A Case Report
Abstract
:1. Introduction
2. Case Presentation
2.1. Family History
2.2. Medical History
2.3. Symptoms
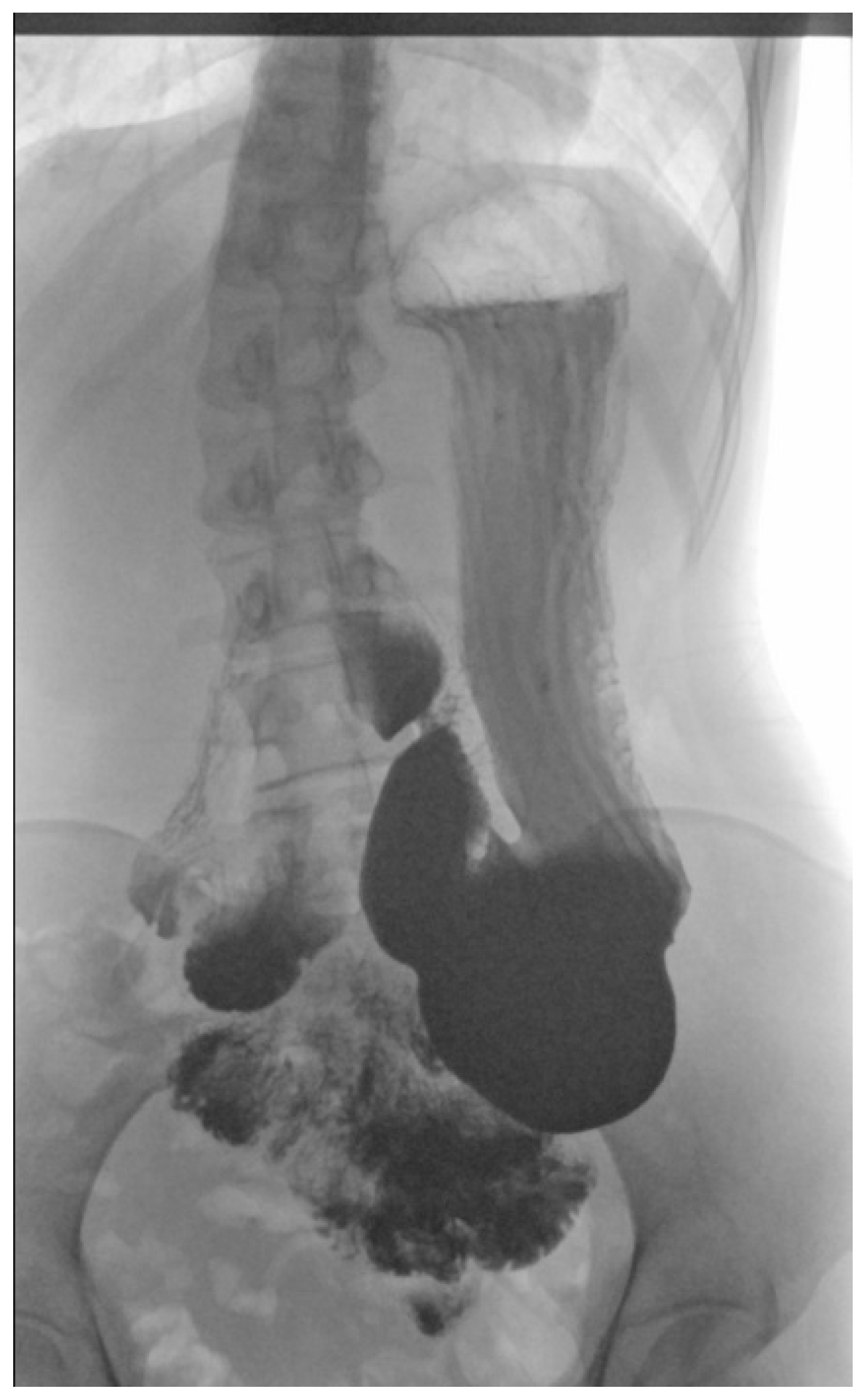
2.4. Physical Examination, Laboratory Tests’ Results and Imaging Studies
2.5. Diagnosis and Treatment
2.6. Follow-Up
3. Discussion
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beilin, D.S. When has visceroptosis clinical significance? Radiology 1930, 15, 223–226. [Google Scholar] [CrossRef]
- Sarangapani, A.; Rasane, S.; Kohli, V.D.; Chandy, G.M. Glenard’s disease. Arch. Med. Health Sci. 2016, 4, 153–154. [Google Scholar] [CrossRef]
- American Gastroenterological Association. American Gastroenterological Association Medical Position Statement: Nausea and Vomiting. Gastroenterology 2001, 120, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Van Welie, A.J.M.; Klein, W.M.; Draaisma, J.M.T. The clinical or radiographic diagnosis of gastroptosis: Still relevant? Gastro Open J. 2017, 2, 14–19. [Google Scholar] [CrossRef]
- Aaron, C.D. Gastroptosis. JAMA 1897, XXIX, 224. [Google Scholar] [CrossRef] [Green Version]
- Bestari, M.B.; Chandra, M.; Joewono, I.R.; Girawan, D.; Andhika, R.; Wahyudi, Y.; Abdurachman, S.A. Gastroptosis due to Gastric Outlet Obstruction Secondary to Duodenal Tumor: Glenard’s Disease Revisited. Karger. Case Rep. Gastroenterol. 2022, 16, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Raphael, Y.; Sukov, R.; Liu, X. Ehlers-Danlos syndrome type III (EDS) and visceroptosis: Getting to the bottom of this diagnosis: 469. Off. J. Am. Coll. Gastroenterol. 2018, 113, S270–S271. [Google Scholar] [CrossRef]
- Kusano, M.; Moki, F.; Hosaka, H.; Shimoyama, Y.; Kawamura, O.; Nagoshi, A.; Maeda, M.; Kuribayashi, S.; Zai, H.; Mizuide, M.; et al. Gastroptosis is associated with less dyspepsia, rather than a cause of dyspepsia, in Japanese persons. Intern. Med. 2011, 50, 667–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christianakis, E.; Bouchra, K.; Koliatou, A.; Paschalidis, N.; Filippou, D. Gastroparesis associated with gastroptosis presenting as a lower abdominal bulking mass in a child: A case report. Cases J. 2009, 2, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, S.N. Gastroptosis: A cause of postprandial abdominal bloating. Can. J. Gastroenterol. Hepatol. 1991, 5, 3. [Google Scholar] [CrossRef]
- Natsis, K.; Apostolidis, S.; Papadopoulou, A.L.; Vlasis, K.; Totlis, T.; Skandalakis, P. Gastric femoral hernia in a male cadaver with gastroptosis: Case report and review of the literature. Hernia 2008, 12, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.; Sood Manu, R. Motility disorders of the gastrointestinal tract. Indian J. Pediatr. 2006, 73, 927–930. [Google Scholar]
- Vandenplas, Y.; Hauser, B.; Salvatore, S. Current pharmacological treatment of gastroparesis. Expert Opin. Pharmacother. 2004, 5, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, L.; Flores, A.; Putnam, P.E.; Hyman, P.E.; Di Lorenzo, C. Postviral gastroparesis: Presentation, treatment, and outcome. J. Pediatr. 1997, 131, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakamura, T.; Tucker, H.E. Segmental resection for gastroptosis. J. Am. Osteopath Assoc. 1959, 58, 412–414. [Google Scholar] [PubMed]
- Perez, M.E.; Youssef, N.N. Dyspepsia in childhood and adolescence: Insights and treatment considerations. Curr. Gastroenterol. Rep. 2007, 9, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hall-Edwards, J.F. The diagnosis and treatment of gastroptosis. Br. Med. J. 1921, 1, 698–700. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszewska, A.; Jarzumbek, A.; Saran, A.; Gierak-Firszt, S.; Kwiecien, J. Postprandial Abdominal Pain Caused by Gastroptosis—A Case Report. Children 2023, 10, 116. https://doi.org/10.3390/children10010116
Staszewska A, Jarzumbek A, Saran A, Gierak-Firszt S, Kwiecien J. Postprandial Abdominal Pain Caused by Gastroptosis—A Case Report. Children. 2023; 10(1):116. https://doi.org/10.3390/children10010116
Chicago/Turabian StyleStaszewska, Anna, Anna Jarzumbek, Anna Saran, Sylwia Gierak-Firszt, and Jaroslaw Kwiecien. 2023. "Postprandial Abdominal Pain Caused by Gastroptosis—A Case Report" Children 10, no. 1: 116. https://doi.org/10.3390/children10010116
APA StyleStaszewska, A., Jarzumbek, A., Saran, A., Gierak-Firszt, S., & Kwiecien, J. (2023). Postprandial Abdominal Pain Caused by Gastroptosis—A Case Report. Children, 10(1), 116. https://doi.org/10.3390/children10010116





