Diagnosing Acute Cellular Rejection after Paediatric Liver Transplantation—Is There Room for Interleukin Profiles?
Abstract
1. Introduction
2. Materials and Methods
2.1. ChilSfree Study Design
2.2. Patients
2.3. Immunosuppression
2.4. Liver Biopsies
2.5. Clinical Chemistry
2.6. Soluble Immune Markers
2.7. Statistics
2.8. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigo, E.; Lopez-Hoyos, M.; Corral, M.; Fabrega, E.; Fernandez-Fresnedo, G.; San, S.D.; Pinera, C.; Arias, M. ImmuKnow as a diagnostic tool for predicting infection and acute rejection in adult liver transplant recipients: A systematic review and meta-analysis. Liver Transpl. 2012, 18, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Germani, G.; Rodriguez-Castro, K.; Russo, F.P.; Senzolo, M.; Zanetto, A.; Ferrarese, A.; Burra, P. Markers of acute rejection and graft acceptance in liver transplantation. World J. Gastroenterol. 2015, 21, 1061–1068. [Google Scholar] [CrossRef]
- Brunet, M.; Millan, L.O.; Lopez-Hoyos, M. T-Cell Cytokines as Predictive Markers of the Risk of Allograft Rejection. Ther. Drug Monit. 2016, 38 (Suppl. 1), S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Millan, O.; Rafael-Valdivia, L.; Torrademe, E.; Lopez, A.; Fortuna, V.; Sanchez-Cabus, S.; Lopez-Pua, Y.; Rimola, A.; Brunet, M. Intracellular IFN-gamma and IL-2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine 2013, 61, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Minguela, A.; Torio, A.; Marin, L.; Sanchez-Bueno, F.; Garcia-Alonso, A.M.; Ontanon, J.; Parrilla, P.; Alvarez-Lopez, M.R. Implication of Th1, Th2, and Th3 cytokines in liver graft acceptance. Transplant. Proc. 1999, 31, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Gras, J.; Wieers, G.; Vaerman, J.L.; Truong, D.Q.; Sokal, E.; Otte, J.B.; Delepaut, B.; Cornet, A.; De Ville De, G.J.; Latinne, D.; et al. Early immunological monitoring after pediatric liver transplantation: Cytokine immune deviation and graft acceptance in 40 recipients. Liver Transpl. 2007, 13, 426–433. [Google Scholar] [CrossRef]
- Millan, O.; Benitez, C.; Guillen, D.; Lopez, A.; Rimola, A.; Sanchez-Fueyo, A.; Brunet, M. Biomarkers of immunoregulatory status in stable liver transplant recipients undergoing weaning of immunosuppressive therapy. Clin. Immunol. 2010, 137, 337–346. [Google Scholar] [CrossRef]
- Ganschow, R.; Broering, D.C.; Nolkemper, D.; Albani, J.; Kemper, M.J.; Rogiers, X.; Burdelski, M. Th2 cytokine profile in infants predisposes to improved graft acceptance after liver transplantation. Transplantation 2001, 72, 929–934. [Google Scholar] [CrossRef]
- Millan, O.; Rafael-Valdivia, L.; San, S.D.; Boix, F.; Castro-Panete, M.J.; Lopez-Hoyos, M.; Muro, M.; Valero-Hervas, D.; Rimola, A.; Navasa, M.; et al. Should IFN-gamma, IL-17 and IL-2 be considered predictive biomarkers of acute rejection in liver and kidney transplant? Results of a multicentric study. Clin. Immunol. 2014, 154, 141–154. [Google Scholar] [CrossRef]
- Boix, F.; Mrowiec, A.; Muro, M. Cytokine Expression Profile as Predictive Surrogate Biomarkers for Clinical Events in the Field of Solid Organ Transplantation. Curr. Protein. Pept. Sci. 2017, 18, 240–249. [Google Scholar] [CrossRef]
- Truong, D.Q.; Darwish, A.A.; Gras, J.; Wieers, G.; Cornet, A.; Robert, A.; Mourad, M.; Malaise, J.; De Ville De, G.J.; Reding, R.; et al. Immunological monitoring after organ transplantation: Potential role of soluble CD30 blood level measurement. Transpl. Immunol. 2007, 17, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.S.; Kim, J.W.; Chung, H.S.; Park, C.S.; Lee, J.; Choi, J.H.; Hong, S.H. The impact of serum cytokines in the development of early allograft dysfunction in living donor liver transplantation. Medicine 2018, 97, e0400. [Google Scholar] [CrossRef]
- Conti, F.; Frappier, J.; Dharancy, S.; Chereau, C.; Houssin, D.; Weill, B.; Calmus, Y. Interleukin-15 production during liver allograft rejection in humans. Transplantation 2003, 76, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Iwaki, Y.; Demetris, A.J.; Starzl, T.E. Evaluation of sequential serum interleukin-6 levels in liver allograft recipients. Transplantation 1994, 57, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- San Segundo, D.; Ruiz, P.; Irure, J.; Arias-Loste, M.T.; Cuadrado, A.; Puente, A.; Casafont, F.; Lopez-Hoyos, M.; Crespo, J.; Fabrega, E. Serum Levels of Interleukin-34 During Acute Rejection in Liver Transplantation. Transplant. Proc. 2016, 48, 2977–2979. [Google Scholar] [CrossRef]
- Fabrega, E.; Lopez-Hoyos, M.; San Segundo, D.; Casafont, F.; Pons-Romero, F. Changes in the serum levels of interleukin-17/interleukin-23 during acute rejection in liver transplantation. Liver Transpl. 2009, 15, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Ganschow, R.; Baade, B.; Hellwege, H.H.; Broering, D.C.; Rogiers, X.; Burdelski, M. Interleukin-1 receptor antagonist in ascites indicates acute graft rejection after pediatric liver transplantation. Pediatr. Transplant. 2000, 4, 289–292. [Google Scholar] [CrossRef]
- Goldschmidt, I.; Karch, A.; Mikolajczyk, R.; Mutschler, F.; Junge, N.; Pfister, E.D.; Mohring, T.; d’Antiga, L.; McKiernan, P.; Kelly, D.; et al. Immune monitoring after pediatric liver transplantation—The prospective ChilSFree cohort study. BMC Gastroenterol. 2018, 18, 63. [Google Scholar] [CrossRef]
- Mohring, T.; Karch, A.; Falk, C.S.; Laue, T.; D’Antiga, L.; Debray, D.; Hierro, L.; Kelly, D.; McLin, V.; McKiernan, P.; et al. Immune Status in Children Before Liver Transplantation-A Cross-Sectional Analysis within the ChilsSFree Multicentre Cohort Study. Front. Immunol. 2019, 10, 52. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis for the behavioural sciences. In Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Mathy, N.L.; Scheuer, W.; Lanzendorfer, M.; Honold, K.; Ambrosius, D.; Norley, S.; Kurth, R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000, 100, 63–69. [Google Scholar] [CrossRef]
- Kimura, N.; Itoh, S.; Nakae, S.; Axtell, R.C.; Velotta, J.B.; Bos, E.J.; Merk, D.R.; Gong, Y.; Okamura, H.; Nagamine, C.M.; et al. Interleukin-16 deficiency suppresses the development of chronic rejection in murine cardiac transplantation model. J. Heart Lung Transplant. 2011, 30, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Obara, H.; Takayanagi, A.; Tanabe, M.; Kawachi, S.; Itano, O.; Shinoda, M.; Kitago, M.; Hibi, T.; Chiba, T.; et al. Suppressive effects of interleukin-18 on liver function in rat liver allografts. J. Surg. Res. 2012, 176, 293–300. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsumura, F.; Liang, J.; Akizuki, E.; Matsuda, T.; Okabe, K.; Ohshiro, H.; Ishihara, K.; Yamada, S.; Mori, K.; et al. Reduced interleukin-12, interleukin-18, and interferon-gamma production with prolonged rat hepatic allograft survival after donor-specific blood transfusion. Dig. Dis. Sci. 2000, 45, 2429–2435. [Google Scholar] [CrossRef]
- Yagi, T.; Iwagaki, H.; Urushihara, N.; Kobashi, K.; Nakao, A.; Matsukawa, H.; Sadamori, H.; Inagaki, M.; Tanaka, N. Participation of IL-18 in human cholestatic cirrhosis and acute rejection: Analysis in living donor liver transplantation. Transplant. Proc. 2001, 33, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Ronca, V.; Wootton, G.; Milani, C.; Cain, O. The Immunological Basis of Liver Allograft Rejection. Front. Immunol. 2020, 11, 2155. [Google Scholar] [CrossRef]
- Dudek, K.; Kornasiewicz, O.; Koziak, K.; Kotulski, M.; Kalinowski, P.; Zieniewicz, K.; Krawczyk, M. Clinical significance of lymphocytes hepatocyte growth factor mRNA expression in patients after liver transplantation. Transplant. Proc. 2007, 39, 2788–2792. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Oukka, M.; Kuchroo, V.K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007, 8, 345–350. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Calarota, S.A.; Vidali, F.; Macdonald, T.T.; Corazza, G.R. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011, 22, 19–33. [Google Scholar] [CrossRef]
- Han, E.S.; Na, G.H.; Choi, H.J.; You, Y.K.; Kim, D.G. Effectiveness of Perioperative Immunologic Markers Monitoring for Predicting Early Acute Cellular Rejection After Living Donor Liver Transplantation. Transplant. Proc. 2019, 51, 2648–2654. [Google Scholar] [CrossRef]

| n (%)/Median (Range) | ||
|---|---|---|
| Sex | Boys | 31 (62%) |
| Girls | 19 (38%) | |
| Age at transplant | 1.8 years (0.5–17.5) | |
| Time lapse transplant to biopsy | 18 days (7–427) | |
| Primary disease | Biliary atresia | 30 (60%) |
| Acute liver failure | 1 (2%) | |
| Metabolic liver disease | 2 (4%) | |
| Sclerosing cholangitis | 1 (2%) | |
| Autoimmune hepatitis | 1 (2%) | |
| PFIC | 3 (6%) | |
| Alagille syndrome | 3 (6%) | |
| Hepatoblastoma | 1 (2%) | |
| Other | 8 (16%) | |
| Living related liver transplantation | 3 (6%) | |
| ABO-incompatible transplantation | 3 (6%) | |
| Liver biopsies | Acute cellular rejection | 36 (67.9%) |
| No rejection | 17 (32.1%) | |
| RAI scores | Median 6 (2–9) | |
| 2 | 2 (5.7%) | |
| 4 | 3 (8.6%) | |
| 5 | 7 (20.0%) | |
| 6 | 13 (37.1%) | |
| 7 | 4 (11.4%) | |
| 8 | 5 (14.3%) | |
| 9 | 1 (2.9%) | |
| Immunosuppression at time of biopsy | ||
| Tacrolimus monotherapy | 35/ 53 (66.0%) | |
| Tacrolimus + steroids * | 10/53 (18.9%) | |
| Tacrolimus + MMF | 4/53(7.5%) | |
| Tacrolimus + MMF + steroids | 2/53(3.8%) | |
| Cyclosporine + steroids | 2/53 (3.8%) | |
| All 1 | No Rejection | Rejection | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| AST (times ULN) | 2.1 ± 1.9 | 1.8 ± 2.3 | 2.2 ± 1.7 | 0.43 |
| ALT (times ULN) | 3.4 ± 2.8 | 2.5 ± 2.4 | 3.8 ± 2.9 | 0.11 |
| GGT (times ULN) | 7.7 ± 12.3 | 5.2 ±5.5 | 8.8 ± 14.3 | 0.34 |
| Bilirubin (times ULN) | 2.8 ± 3.5 | 1.0 ± 1.2 | 3.6 ± 3.9 | <0.01 |
| CRP (times ULN) | 6.6 ± 8.7 | 3.7 ± 5.6 | 7.5 ± 9.3 | 0.23 |
| All | No Rejection | Rejection | p 1 | |
|---|---|---|---|---|
| AST | 27/51 (52.9%) | 5/16 (31.3%) | 22/35 (62.9%) | 0.04 |
| ALT | 38/52 (73.1%) | 8/16 (50.0%) | 30/36 (83.3%) | 0.01 |
| GGT | 43/52 (82.7%) | 10/16 (62.5%) | 33/36 (91.7%) | 0.03 |
| Bilirubin | 20/52 (38.5%) | 3/16 (18.8%) | 17/36 (47.2%) | 0.048 |
| CRP | 29/42 (69.0%) | 5/11 (45.5%) | 24/31 (77.4%) | 0.049 |
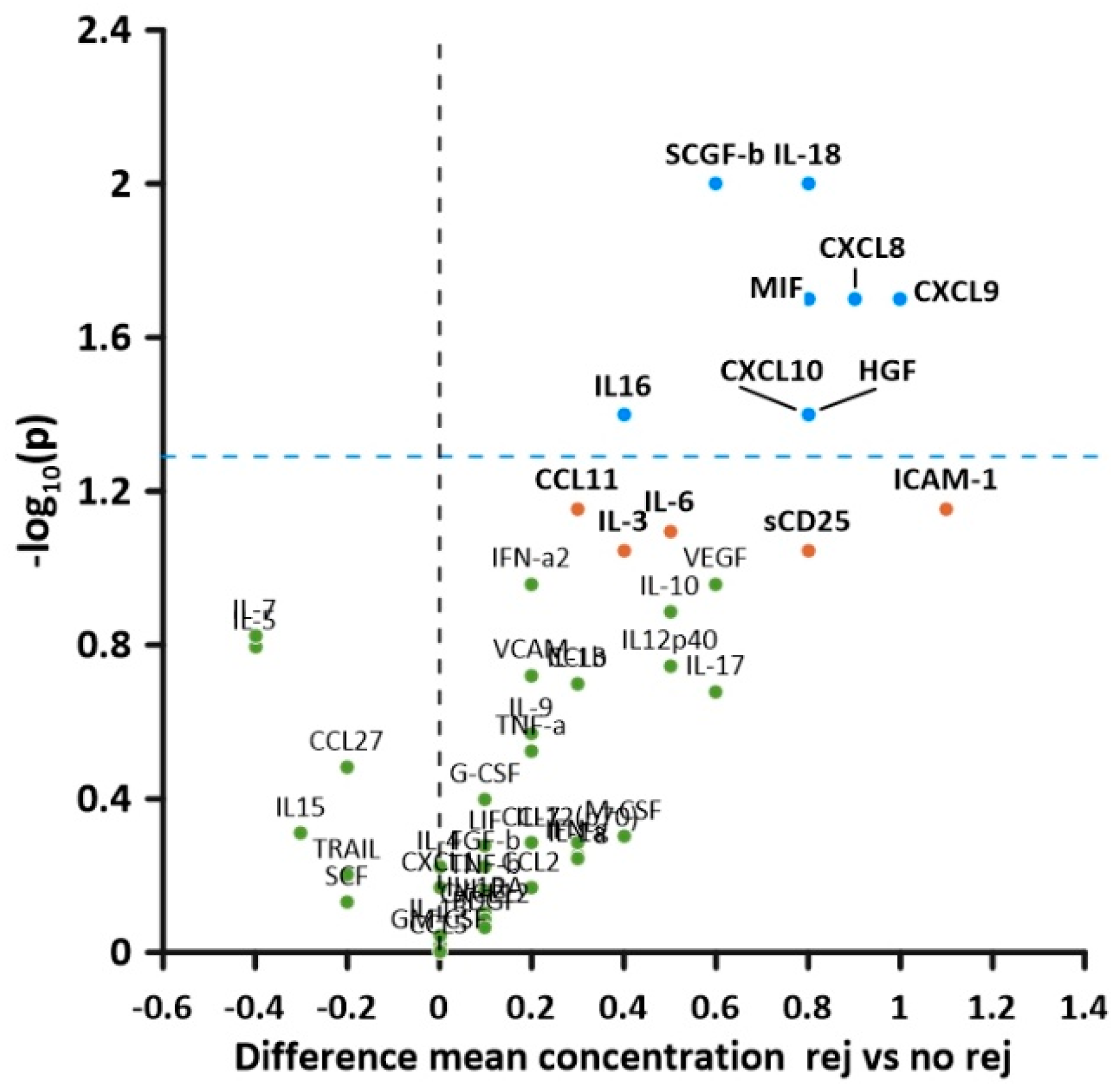
| Rejection Ln (conc) (pg/mL) mean ± SD | No Rejection Ln (conc) (pg/mL) mean ± SD | T (df) | p | Effect Size Cohen’s d | |
|---|---|---|---|---|---|
| CXCL8 | 3.1 ± 1.4 | 2.2 ± 0.8 | 2.4 (50) | 0.02 | 1.2 |
| CXCL9 | 7.0 ± 1.0 | 6.0 ± 1.8 | 2.4 (22) | 0.02 | 1.3 |
| CXCL10 | 6.4 ± 1.2 | 5.6 ± 1.1 | 2.2 (50) | 0.04 | 1.2 |
| IL-16 | 6.3 ± 0.8 | 5.9 ± 0.7 | 2.1 (50) | 0.04 | 1.1 |
| IL-18 | 5.0 ± 1.1 | 4.2 ± 1.2 | 2.6 (51) | 0.01 | 1.1 |
| CCL4 | 4.1 ± 0.6 | 3.8 ± 0.4 | 2.2 (50) | 0.03 | 0.5 |
| MIF | 8.2 ± 1.2 | 7.4 ± 1.0 | 2.4 (51) | 0.02 | 1.1 |
| SCGF-b | 10.0 ± 0.7 | 9.4 ± 1.0 | 2.5 (51) | 0.01 | 0.8 |
| HGF | 6.4 ± 1.3 | 5.6 ± 1.1 | 2.2 (51) | 0.04 | 0.52 |
| sCD25 | 6.3 ± 1.4 | 5.5 ± 1.5 | 1.7 (51) | 0.09 | 1.4 |
| ICAM-1 | 10.9 ± 0.5 | 9.8 ± 2.3 | 1.9 (16.8) | 0.07 | 1.4 |
| IL-6 | 2.5 ± 1.1 | 2.0 ± 1.0 | 1.8 (50) | 0.08 | 1.1 |
| CCL11 | 3.7 ± 0.7 | 3.4 ± 0.6 | 1.8 (50) | 0.07 | 0.7 |
| IL-3 | 5.9 ± 0.7 | 5.5 ± 0.8 | 1.7 (51) | 0.09 | 0.8 |
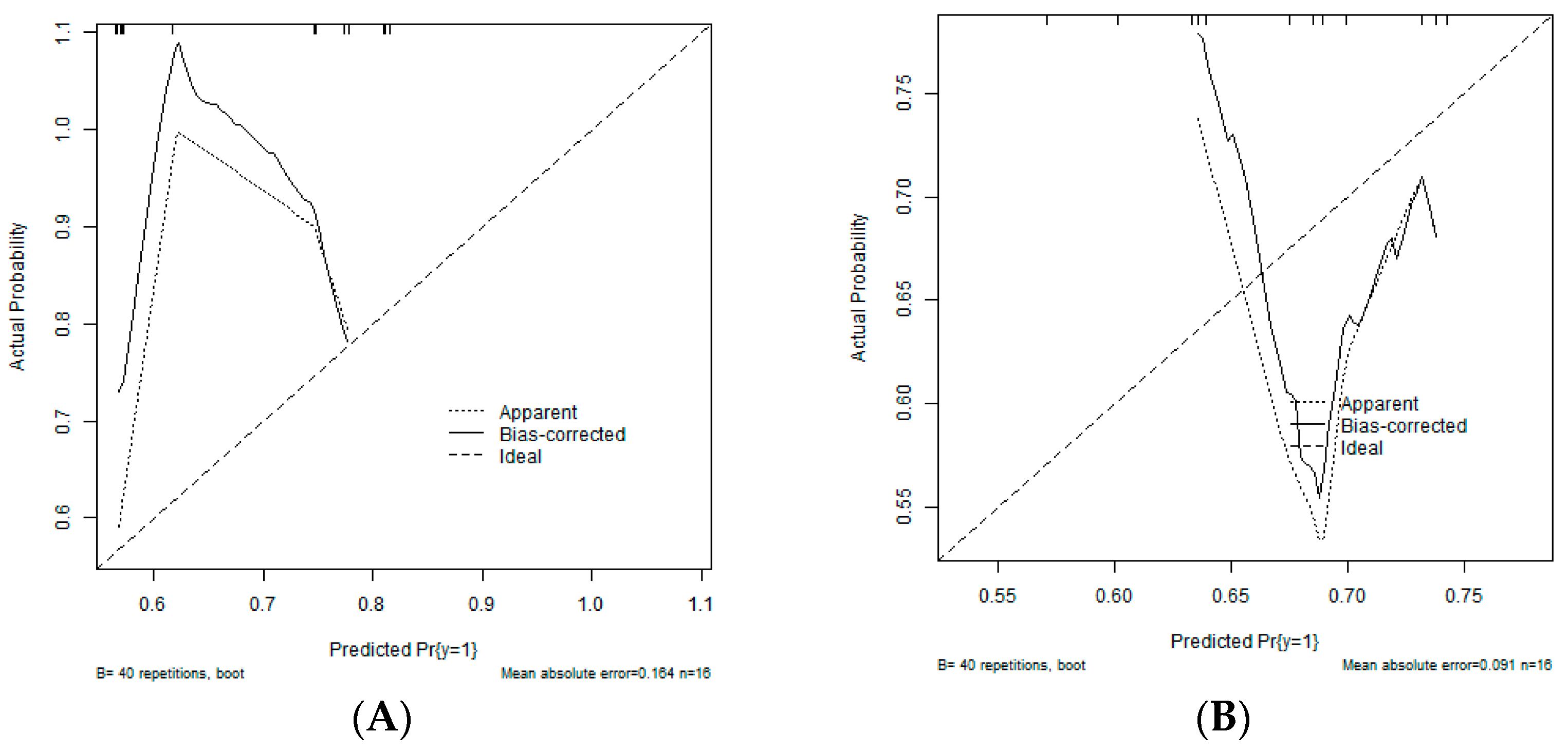
| Variable | Coefficient | Variable | Coefficient |
|---|---|---|---|
| (Intercept) | −0.9172 | CCL11_log | 0.0511 |
| Sex: female | 0.0774 | CCL27_log | −0.0059 |
| Diagnosis: acute liver failure | −0.2218 | CXCL1_log | 0.0214 |
| IL_1b_log | 0.0094 | CXCL8_log | 0.0356 |
| IL_3_log | 0.0135 | CXCL9_log | 0.0254 |
| IL_7_log | −0.0215 | SCGF_b_log | 0.0113 |
| IL_16_log | 0.0326 | MIF_log | 0.0219 |
| IL_18_log | 0.0318 | sCD25_log | 0.0007 |
| IFN_a2_log | 0.0405 | ICAM_1_log | 0.011 |
| LIF_log | 0.002 | CCL4_log | 0.0223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldschmidt, I.; Chichelnitskiy, E.; Rübsamen, N.; Jaeger, V.K.; Karch, A.; D’Antiga, L.; Di Giorgio, A.; Nicastro, E.; Kelly, D.A.; McLin, V.; et al. Diagnosing Acute Cellular Rejection after Paediatric Liver Transplantation—Is There Room for Interleukin Profiles? Children 2023, 10, 128. https://doi.org/10.3390/children10010128
Goldschmidt I, Chichelnitskiy E, Rübsamen N, Jaeger VK, Karch A, D’Antiga L, Di Giorgio A, Nicastro E, Kelly DA, McLin V, et al. Diagnosing Acute Cellular Rejection after Paediatric Liver Transplantation—Is There Room for Interleukin Profiles? Children. 2023; 10(1):128. https://doi.org/10.3390/children10010128
Chicago/Turabian StyleGoldschmidt, Imeke, Evgeny Chichelnitskiy, Nicole Rübsamen, Veronika K. Jaeger, André Karch, Lorenzo D’Antiga, Angelo Di Giorgio, Emanuele Nicastro, Deirdre A. Kelly, Valerie McLin, and et al. 2023. "Diagnosing Acute Cellular Rejection after Paediatric Liver Transplantation—Is There Room for Interleukin Profiles?" Children 10, no. 1: 128. https://doi.org/10.3390/children10010128
APA StyleGoldschmidt, I., Chichelnitskiy, E., Rübsamen, N., Jaeger, V. K., Karch, A., D’Antiga, L., Di Giorgio, A., Nicastro, E., Kelly, D. A., McLin, V., Korff, S., Debray, D., Girard, M., Hierro, L., Klaudel-Dreszler, M., Markiewicz-Kijewska, M., Falk, C., & Baumann, U. (2023). Diagnosing Acute Cellular Rejection after Paediatric Liver Transplantation—Is There Room for Interleukin Profiles? Children, 10(1), 128. https://doi.org/10.3390/children10010128






