One Hundred Consecutive Neutropenic Febrile Episodes Demonstrate That CXCR3 Ligands Have Predictive Value in Discriminating the Severity of Infection in Children with Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions
2.3. Laboratory Measurements
2.4. Statistical Analysis
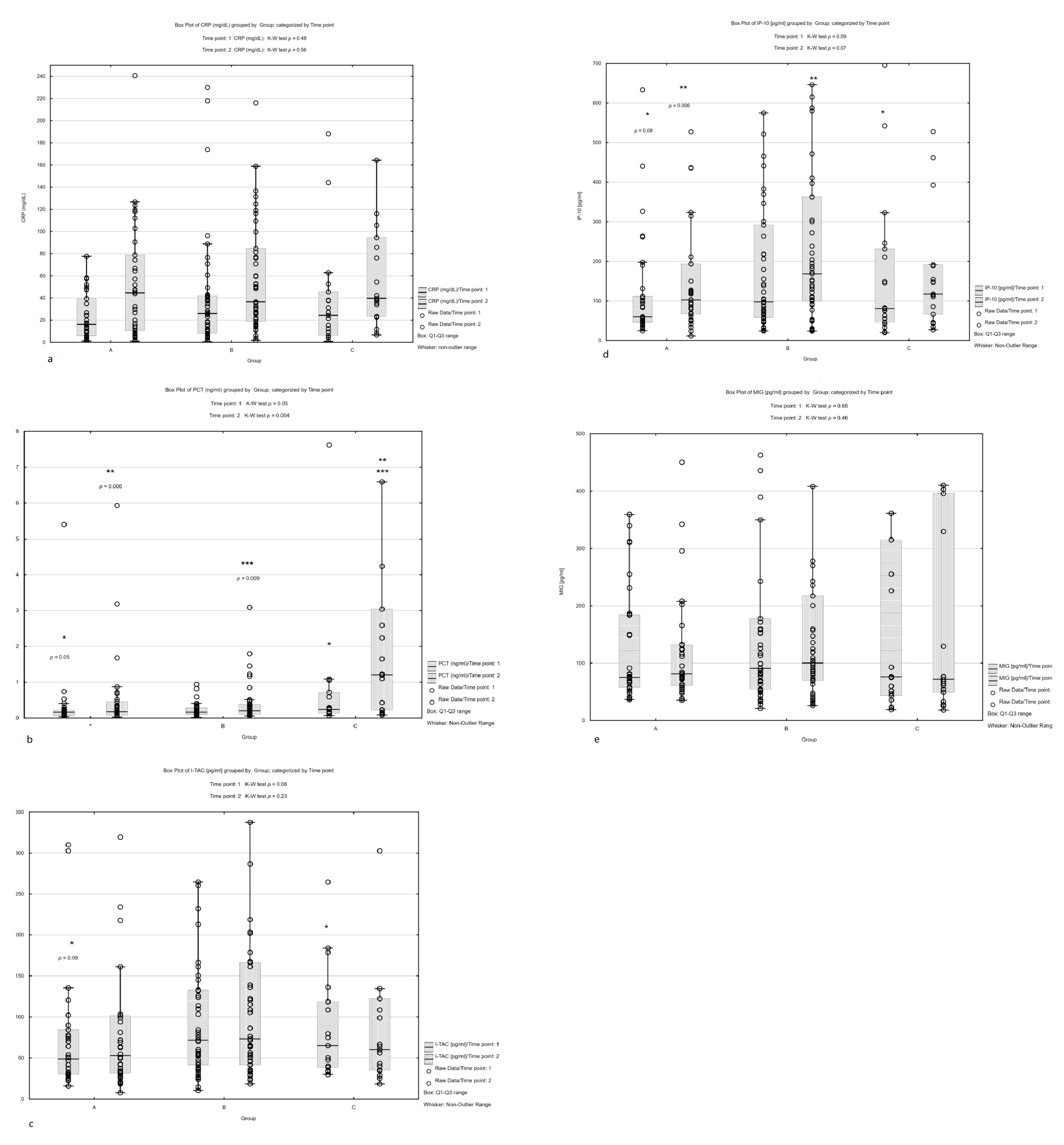
3. Results
3.1. Patient Characteristics
3.2. Biomarker Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, L.; Paula, R.; Brian, F.; Sarah, A.; Rpland, A.A.; Melissa, B.; Fabianne, C.; Andreas, H.G.; Gabrielle, M.H.; María, E.S.P.; et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef] [Green Version]
- Kebudi, R.; Kizilocak, H. Febrile Neutropenia in Children with Cancer: Approach to Diagnosis and Treatment. Curr. Pediatr. Rev. 2018, 14, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.E.; Neugebauer, S.; Engelmann, F.; Hagel, S.; Ludewig, K.; Rosée, P.L.; Sayer, H.G.; Hochhaus, A.; Lilienfeld-Toal, M.; Bretschneider, T.; et al. Biomarker candidates for the detection of an infectious etiology of febrile neutropenia. Infection 2016, 44, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mimaroğlu, E.; Çıtak, E.Ç.; Kuyucu, N.; Eskendari, G. The diagnostic and prognostic value of angiopoietins compared with C-reactive protein and procalcitonin in children with febrile neutropenia. Turk. J. Pediatr. 2017, 59, 418–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, T.; Phillips, R.S. Updated systematic review and meta-analysis of the predictive value of serum biomarkers in the assessment and management of fever during neutropenia in children with cancer. Pediatr. Blood Cancer 2019, 66, e27887. [Google Scholar] [CrossRef]
- van der Galiën, H.T.; Loeffen, E.A.H.; Miedema, K.G.E.; Tissing, W.J.E. Predictive value of PCT and IL-6 for bacterial infection in children with cancer and febrile neutropenia. Support. Care Cancer 2018, 26, 3819–3826. [Google Scholar] [CrossRef] [Green Version]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Lee, K.; Chung, W.; Jung, Y.; Kim, Y.; Park, J.; Sheen, S.; Park, K. CXCR3 ligands as clinical markers for pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2015, 19, 191–199. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Van den Steen, P.E.; Liekens, S.; Van Damme, J.; Struyf, S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015, 26, 311–327. [Google Scholar] [CrossRef]
- Cavalcanti, N.V.; Torres, L.C.; da Matta, M.C.; Lindoso, C.D.; ACarvalho, L.N.; Duarte MC, M.B.; Correia, J.B. Chemokine Patterns in Children with Acute Bacterial Infections. Scand. J. Immunol. 2016, 84, 338–343. [Google Scholar] [CrossRef]
- Ng, P.C.; Li, K.; Chui, K.M.; Leung, T.F.; Wong, R.P.; Chu, W.C.; Wong, E.; Fok, T.F. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr. Res. 2007, 61, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, S.S.; Tezer, H.; Polat, M.; Yayla, B.C.C.; Demirdağ, T.B.; Okur, A.; Fettah, A.; Yüksek, S.K.; Tapisiz, A.; Kaya, Z.; et al. Risk factors for bacteremia in children with febrile neutropenia. Turkish J. Med. Sci. 2019, 49, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Wu, J.Y.; Chen, C.K.; Huang, S.L.; Hsu, S.C.; Lee, M.; Tse, G.; Chang, S.S.; Lee, C.C. Does procalcitonin, C-reactive protein, or interleukin-6 test have a role in the diagnosis of severe infection in patients with febrile neutropenia? A systematic review and meta-analysis. Support. Care Cancer 2015, 23, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, G.M.; Phillips, R.S.; Lehrnbecher, T.; Thursky, K.A.; Sung, L.; Ammann, R.A. Core outcomes and definitions for pediatric fever and neutropenia research: A consensus statement from an international panel. Pediatr. Blood Cancer 2015, 62, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, S.; Zhang, J.; Ren, J.; Chen, M.; Lin, K.; Zhu, H.; Zheng, R.; Zheng, Z.; Chen, Z.; et al. Procalcitonin as a marker of Gram-negative bloodstream infections in hematological patients with febrile neutropenia. Leuk. Lymphoma 2019, 60, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.P.; Brijwal, M.; Seth, R.; Gupta, A.K.; Jethani, J.; Kapil, A.; Jat, K.R.; Choudhary, A.; Kabra, S.K.; Dwivedi, S.N.; et al. Prevalence and clinical outcome of respiratory viral infections among children with cancer and febrile neutropenia. Pediatr. Hematol. Oncol. 2019, 36, 330–343. [Google Scholar] [CrossRef]
- Sbrana, A.; Torchio, M.; Comolli, G.; Antonuzzo, A.; Danova, M. Use of procalcitonin in clinical oncology: A literature review. N. Microbiol. 2016, 39, 174–180. [Google Scholar]
- Lappalainen, M.; Jokkala, J.; Juutilainen, A.; Hämäläinen, S.; Koivula, I.; Jantunen, E.; Hanhihneva, K.; Pulkki, K. Novel biomarker candidates for febrile neutropenia in hematological patients using nontargeted metabolomics. Dis. Markers 2018, 2018, 6964529. [Google Scholar] [CrossRef] [Green Version]
- Hangai, S.; Nannya, Y.; Kurokawa, M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk. Lymphoma 2015, 56, 910–914. [Google Scholar] [CrossRef]
- Singh, N.; Sundar, S. Inflammatory chemokines and their receptors in human visceral leishmaniasis: Gene expression profile in peripheral blood, splenic cellular sources and their impact on trafficking of inflammatory cells. Mol. Immunol. 2017, 85, 111–119. [Google Scholar] [CrossRef]
- Sun, H.; Fan, J.; Shang, X.; Tuohetaerbaike, B.; Li, Y.; Lv, J.; Wang, Y.; Wang, L.; Wang, J.; Ma, X. Study on the relationship between CXCR3 and its ligands and tubal tuberculosis. Life Sci. 2021, 272, 119047. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, Z.; Wu, T.; Ma, M.; Zhang, Z.; Chu, Z.; Hu, Q.; Ding, H.; Han, X.; Xu, J.; et al. The combination of CXCL9, CXCL10 and CXCL11 levels during primary HIV infection predicts HIV disease progression. J. Transl. Med. 2019, 17, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameda, M.; Otsuka, M.; Chiba, H.; Kuronuma, K.; Hasegawa, T.; Takahashi, H.; Takahashi, H. CXCL9, CXCL10, and CXCL11; biomarkers of pulmonary inflammation associated with autoimmunity in patients with collagen vascular diseases–associated interstitial lung disease and interstitial pneumonia with autoimmune features. PLoS ONE 2020, 15, e0241719. [Google Scholar] [CrossRef] [PubMed]


| All Episodes | Group A | Group B | Group C | p Value | |
|---|---|---|---|---|---|
| (n = 100) | (n = 34) | (n = 47) | (n = 19) | ||
| Sex | 0.49 * | ||||
| n (% of female) | 48 (48%) | 19 (56%) | 20 (43%) | 9 (47%) | |
| n (% of male) | 52 (52%) | 15 (44%) | 27 (57%) | 10 (53%) | |
| Age (years) | 9.8 | 9.8 | 10.5 | 7.53 | 0.48 ** |
| Age (median [Q1–Q3]) | (4.9–13.6) | (6.1–13.5) | (4.8–14) | (4.5–13.5) | |
| Underlying disease: | 0.05 * | ||||
| Leucaemias and Lymphomas | 66 | 16 | 36 | 14 | |
| 58 | 15 | 31 | 12 | |
| 8 | 1 | 5 | 2 | |
| Brain tumors | 8 | 4 | 1 | 3 | |
| Solid tumors | 26 | 14 | 10 | 2 | |
| Disease status: | 0.66 * | ||||
| Remission | 85 | 30 | 40 | 15 | |
| Relapse/Progression of malignancy | 15 | 4 | 7 | 4 | |
| Neutrophil count, mean ± SD (×103/uL) | 0.065 | 0.03 | 0.08 | 0.1 | 0.12 ** |
| (median [Q1–Q3]) | (0.02–0.17) | (0.02–0.1) | (0.03–0.23) | (0.02–0.13) | |
| Duration of neutropenia (days) | 4 (2–6.5) | 4 (2–6) | 3 (4–13) | 7 (2–6) | 0.17 ** |
| Fever at home | 52 | 15 | 30 | 7 | 0.07 * |
| Inpatient fever | 48 | 19 | 17 | 12 | |
| Type of infection | FUO | MDI/CDI: | MDI/CDI: | ||
| Gastroenteritis—22 | Bacteriemia—11 | ||||
| Mucositis—8 | Sepsis—4 | ||||
| Pneumonia—7 | Thyphilitis—2 | ||||
| Urinary tract infection—6 | Multiorgan failure—2 | ||||
| Soft tissue | |||||
| Infection—4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.; Bobeff, K.; Walenciak, J.; Kołodrubiec, J.; Wyka, K.; Młynarski, W.; Trelińska, J. One Hundred Consecutive Neutropenic Febrile Episodes Demonstrate That CXCR3 Ligands Have Predictive Value in Discriminating the Severity of Infection in Children with Cancer. Children 2023, 10, 39. https://doi.org/10.3390/children10010039
Nowak M, Bobeff K, Walenciak J, Kołodrubiec J, Wyka K, Młynarski W, Trelińska J. One Hundred Consecutive Neutropenic Febrile Episodes Demonstrate That CXCR3 Ligands Have Predictive Value in Discriminating the Severity of Infection in Children with Cancer. Children. 2023; 10(1):39. https://doi.org/10.3390/children10010039
Chicago/Turabian StyleNowak, Małgorzata, Katarzyna Bobeff, Justyna Walenciak, Julia Kołodrubiec, Krystyna Wyka, Wojciech Młynarski, and Joanna Trelińska. 2023. "One Hundred Consecutive Neutropenic Febrile Episodes Demonstrate That CXCR3 Ligands Have Predictive Value in Discriminating the Severity of Infection in Children with Cancer" Children 10, no. 1: 39. https://doi.org/10.3390/children10010039






