Febrile Urinary Tract Infections in Children: The Role of High Mobility Group Box-1
Abstract
:1. Introduction
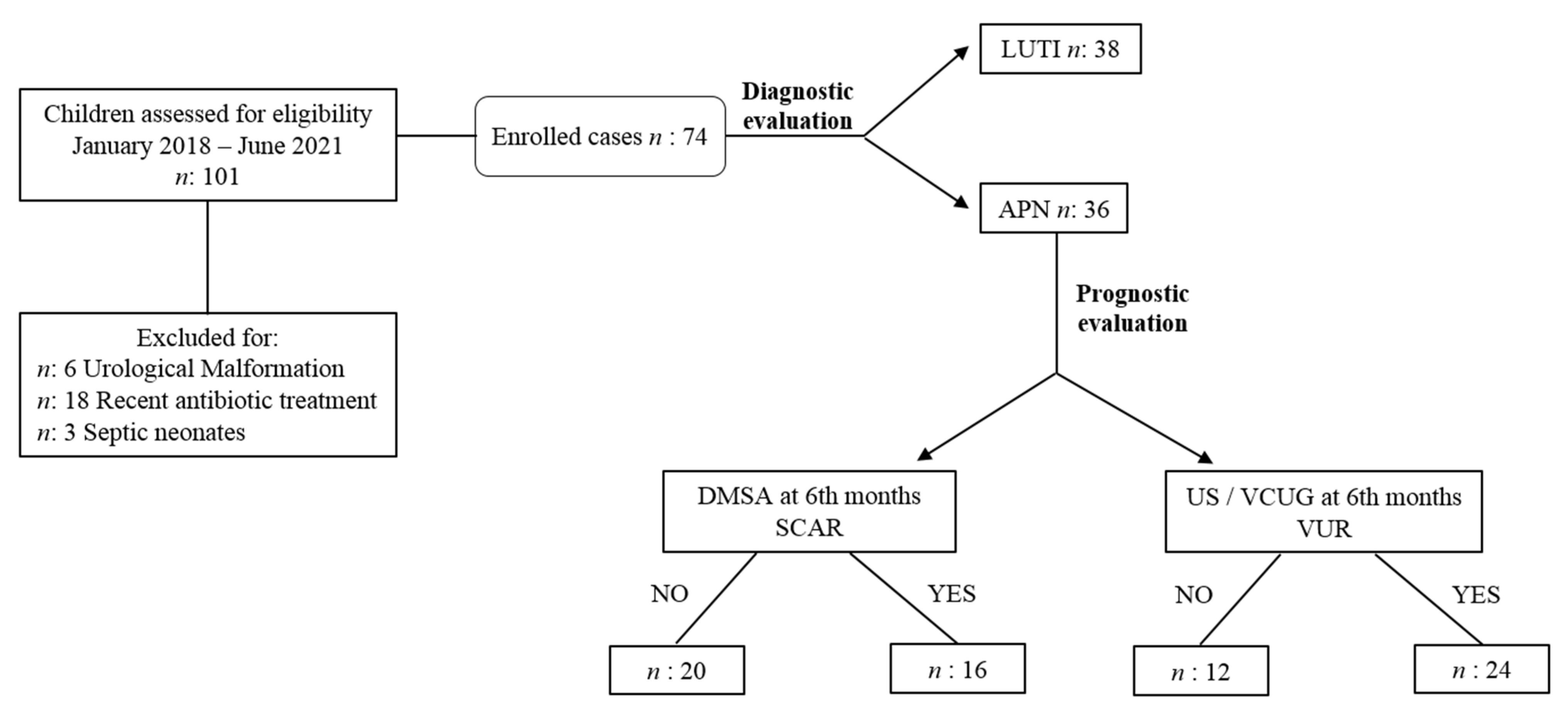
2. Patients and Methods
2.1. Study Design
2.2. Diagnostic Tests
2.3. HMGB1 Analysis
2.4. Follow-Up Period
2.5. Statistical Analyses
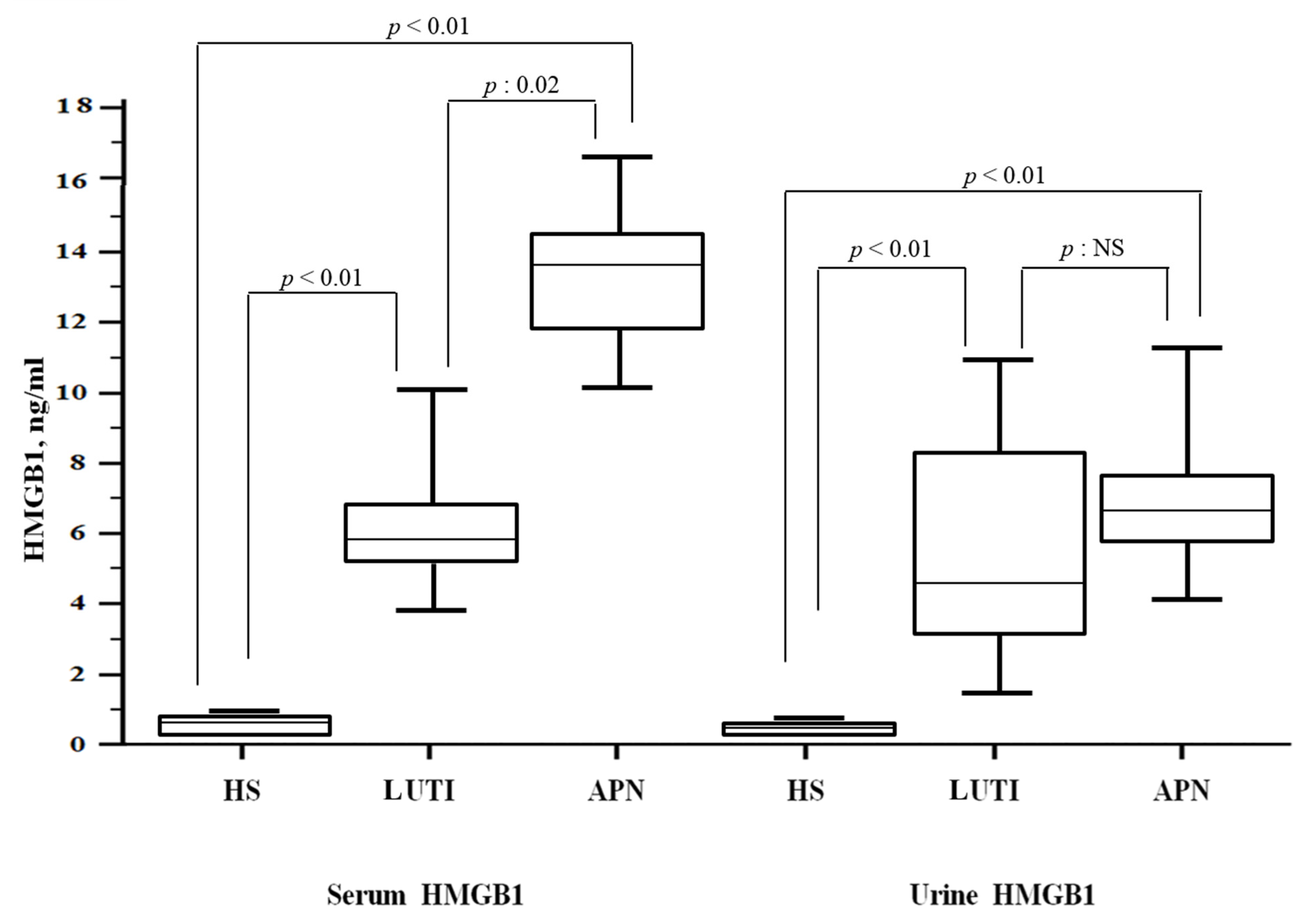
3. Results
3.1. Patients’ Baseline Characteristics
3.2. HMGB1 Levels and Correlations
3.3. ROC Curves
3.4. Follow-Up Period
3.5. Univariate/Multiple Cox Regression Analysis and Persistent Renal Scar
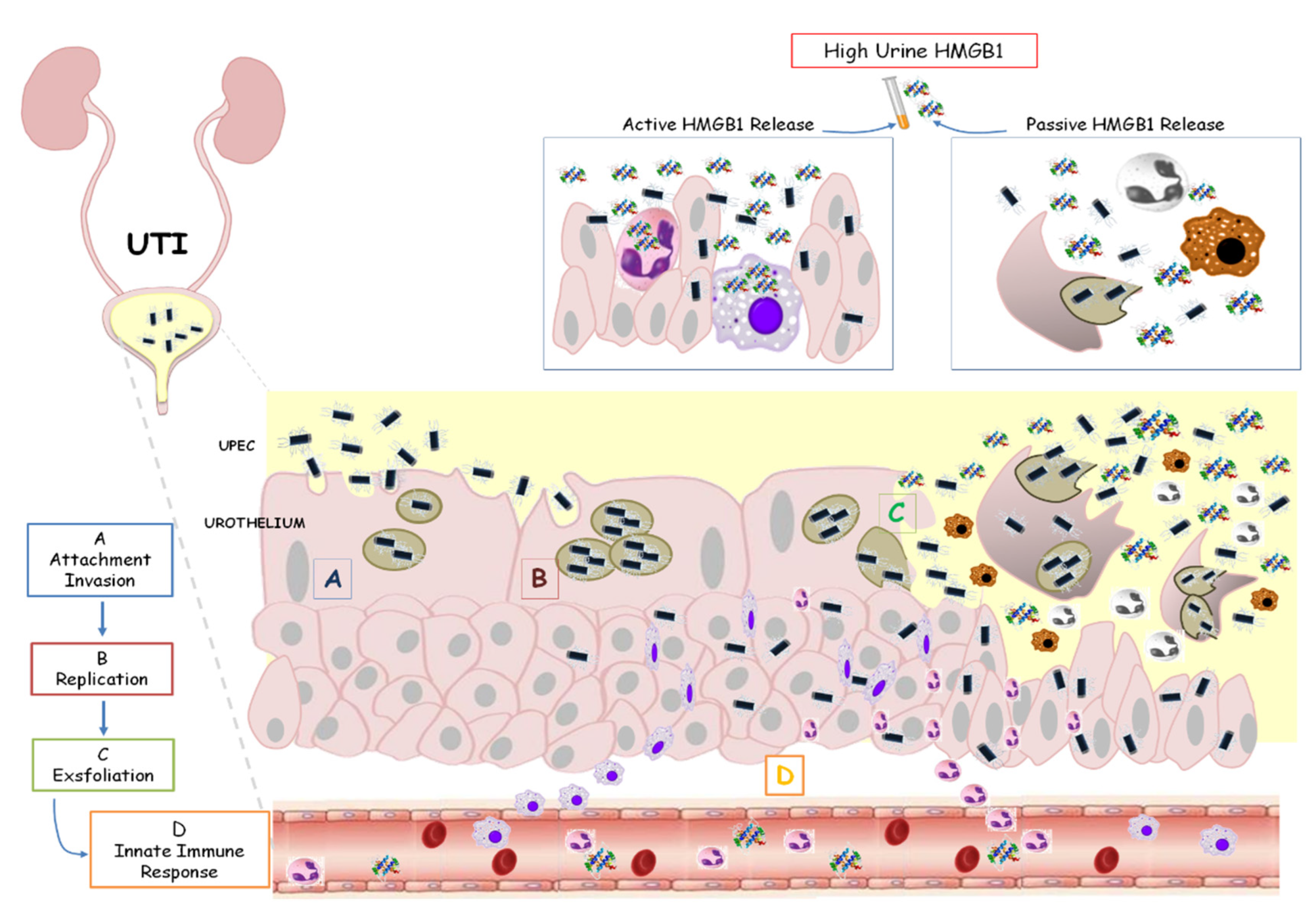
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoberman, A.; Charron, M.; Hickey, R.W.; Baskin, M. Imaging studies after a first febrile urinary tract infection in young children. N. Engl. J. Med. 2003, 348, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Margalit, R.; Golan, E.; Twig, G.; Leiba, A.; Tzur, D.; Afek, A.; Skorecki, K.; Vivante, A. History of childhood kidney disease and risk of adult end-stage renal disease. N. Engl. J. Med. 2018, 378, 428–438. [Google Scholar] [CrossRef]
- Pleniceanu, O.; Twig, G.; Tzur, D.; Sherman, G.; Afek, A.; Erlich, T.; Keinan-Boker, L.; Skorecki, K.; Vivante ACalderon-Margalit, R. Acute pyelonephritis in children and the risk of end-stage kidney disease. J. Nephrol. 2021, 34, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Toffolo, A.; Ammenti, A.; Montini, G. Long-term clinical consequences of urinary tract infections during childhood: A review. Acta Paediatr. 2012, 101, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Wennerström, M.; Hansson, S.; Hedner, T.; Himmelmann, A.; Jodal, U. Ambulatory blood pressure 16–26 years after the first urinary tract infection in childhood. J. Hypertens. 2000, 18, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, T.K.; Chesney, R.W.; Greenfield, S.P.; Hoberman, A.; Keren, R.; Mathews, R.; Gravens-Mueller, L.; Ivanova, A.; Carpenter, M.A.; Moxey-Mims, M.; et al. Renal Scarring in the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Scola, C.; Hewitt, I.; Pasini, A.; Pugliese, F.; Montini, G. Postnatal management of congenital bilateral renal hypodysplasia. J. Matern. Fetal Neonatal Med. 2010, 23, 97–100. [Google Scholar] [CrossRef]
- Bae, H.J.; Park, Y.H.; Cho, J.H.; Jang, K.M. Comparison of 99mTc-DMSA renal scan and power Doppler ultrasonography for the detection of acute pyelonephritis and vesicoureteral reflux. Child. Kidney Dis. 2018, 22, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Mohkam, M. Novel Urinary Biomarkers for Diagnosis of Acute Pyelonephritis in Children. Iran. J. Kidney Dis. 2020, 14, 1–7. [Google Scholar]
- Zhang, H.; Yang, J.; Lin, L.; Huo, B.; Dai, H.; He, Y. Diagnostic value of serum procalcitonin for acute pyelonephritis in infants and children with urinary tract infections: An updated meta-analysis. World J. Urol. 2016, 34, 431–441. [Google Scholar] [CrossRef]
- Fjaertoft, G.; Foucard, T.; Xu, S.; Venge, P. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: A study of the kinetics. Acta Pediatr. 2005, 94, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ichino, M.; Kuroyanagi, Y.; Kusaka, M.; Mori, T.; Ishikawa, K.; Shiroki, R.; Kurahashi, H.; Hoshinaga, K. Increased urinary neutrophil gelatinase-associated lipocalin levels in a rat model of upper urinary tract infection. J. Urol. 2009, 181, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, S.; Kimata, T.; Akagawa, Y.; Akagawa, S.; Kino, J.; Tsuji, S.; Kaneko, K. Reduced urinary excretion of neutrophil gelatinase-associated lipocalin as a risk factor for recurrence of febrile urinary tract infection in children. Pediatr. Nephrol. 2021, 36, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Caccamo, C.; Salis, P.; Chirico, V.; Buemi, A.; Cernaro, V.; Noto, A.; Pettinato, G.; Santoro, D.; Bertani, T.; et al. Delayed graft function and chronic allograft nephropathy: Diagnostic and prognostic role of neutrophil gelatinase-associated lipocalin. Biomarkers 2016, 21, 371–378. [Google Scholar] [CrossRef]
- Meena, J.; Kumar, J.; Thomas, C.C.; Dawman, L.; Tiewsoh, K.; Yadav, M.; Mathew, G. Diagnostic accuracy of renal angina index alone or in combination with biomarkers for predicting acute kidney injury in children. Pediatr. Nephrol. 2022, 37, 1263–1275. [Google Scholar] [CrossRef]
- Mora-Bau, G.; Platt, A.M.; van Rooijen, N.; Randolph, G.J.; Albert, M.L.; Ingersoll, M.A. Macrophages subvert adaptive immunity to urinary tract infection. PLoS Pathog. 2015, 11, e1005044. [Google Scholar] [CrossRef]
- Schiwon, M.; Weisheit, C.; Franken, L.; Gutweiler, S.; Dixit, A.; Meyer-Schwesinger, C.; Pohl, J.M.; Maurice, N.J.; Thiebes, S.; Lorenz, K.; et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 2014, 156, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Agrawal, D.K. Therapeutic Potential of Targeting the HMGB1/RAGE Axis in Inflammatory Diseases. Molecules 2022, 27, 7311. [Google Scholar] [CrossRef]
- Ludes, P.O.; De Roquetaillade, C.; Chousterman, B.G.; Pottecher, J.; Mebazaa, A. Role of Damage-Associated Molecular Patterns in Septic Acute Kidney Injury, From Injury to Recovery. Front. Immunol. 2021, 12, 606622. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y. The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases. Cells 2021, 10, 1044. [Google Scholar] [CrossRef]
- Huebener, P.; Pradere, J.P.; Hernandez, C.; Gwak, G.Y.; Caviglia, J.M.; Mu, X.; Loike, J.D.; Schwabe, R.F. The HMGB1/RAGE Axis Triggers Neutrophil-Mediated In-jury Amplification Following Necrosis. J. Clin. Investig. 2019, 130, 1802. [Google Scholar] [CrossRef] [PubMed]
- Gaboriaud, C.; Lorvellec, M.; Rossi, V.; Dumestre-Pérard, C.; Thielens, N.M. Com-plement System and Alarmin HMGB1 Crosstalk: For Better or Worse. Front. Im-Munol. 2022, 13, 869720. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Augi, T.; Williamson, K.M.; Onishi, K.; Hogan, M.V.; Neal, M.D.; Wang, J.H. Platelet HMGB1 in Platelet-Rich Plasma (PRP) promotes tendon wound healing. PLoS ONE 2021, 16, e0251166. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Ideguchi, H.; Aoyagi, H.; Yoshihara-Hirata, C.; Hirai, A.; Suzuki-Kyoshima, R.; Zhang, Y.; Wake, H.; Nishibori, M.; Yamamoto, T.; et al. High Mobility Group Box 1 Expression in Oral Inflammation and Regeneration. Front. Immun. 2020, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chu, C.; Li, Y.; Li, G.; Lei, X.; Zhou, W.; Chen, Z. High expression of HMGB1 in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 2018, 18, 439. [Google Scholar] [CrossRef]
- Zheng, W.; Shi, H.; Chen, Y.; Xu, Z.; Chen, J.; Jin, L. Alteration of serum high-mobility group protein 1 (HMGB1) levels in children with enterovirus 71-induced hand, foot, and mouth disease. Medicine 2017, 96, e6764. [Google Scholar] [CrossRef]
- Chimenz, R.; Chirico, V.; Basile, P.; Carcione, A.; Conti, G.; Monardo, P.; Lacquaniti, A. HMGB-1 and TGFβ-1 highlight immuno-inflammatory and fibrotic processes before proteinuria onset in pediatric patients with Alport syndrome. J. Nephrol. 2021, 34, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Chimenz, R.; Lacquaniti, A.; Colavita, L.; Chirico, V.; Fede, C.; Buemi, M.; Fede, C. High mobility group box 1 and tumor growth factor β: Useful biomarkers in pediatric patients receiving peritoneal dialysis. Ren. Fail. 2016, 38, 1370–1376. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.J.; Work, D.F. Measurement and estimation of GFR in children and adolescents. Clin. J. Am. Soc. Nephrol. 2009, 4, 1832–1843. [Google Scholar] [CrossRef] [Green Version]
- Alberici, I.; La Manna, A.; Pennesi, M.; Starc, M.; Scozzola, F.; Nicolini, G.; Toffolo, A.; Marra, G.; Chimenz, R.; Sica, F.; et al. First urinary tract infections in children: The role of the risk factors proposed by the Italian recommendations. Acta Paediatr. 2019, 108, 544–550. [Google Scholar] [CrossRef]
- Stokland, E.; Hellström, M.; Jacobsson, B.; Jodal, U.; Sixt, R. Renal damage one year after first urinary tract infection: Role of dimercaptosuccinic acid scintigraphy. J. Pediatr. 1996, 129, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Ammenti, A.; Cataldi, L.; Chimenz, R.; Fanos, V.; La Manna, A.; Marra, G.; Materassi, M.; Pecile, P.; Pennesi, M.; Pisanello, L.; et al. Febrile urinary tract infections in young children: Recommendations for the diagnosis, treatment, and follow-up. Acta Paediatr. 2012, 101, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ammenti, A.; Alberici, I.; Brugnara, M.; Chimenz, R.; Guarino, S.; La Manna, A.; La Scola, C.; Maringhini, S.; Marra, G.; Materassi, M.; et al. Updated Italian recommendations for the diagnosis, treatment, and follow-up of the first febrile urinary tract infection in young children. Acta Paediatr. 2020, 109, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arapović, A.; Punda, A.; Brdar, D.; Čapkun, V.; Bajo, D.; Veljačić, D.; Punda, H.; Simičić-Majce, A.; Saraga-Babić, M.; Vukojević, K.; et al. Types of Parenchymal Changes Diagnosed on DMSA Scans of Kidneys Affected by Different Grades of Vesicoureteral Reflux. Int. Med. J. Exp. Clin. Res. 2021, 27, e929617. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, K.J.; Osio, V.A.; Leeflang, M.M.; Shaikh, N. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst. Rev. 2020, 9, CD009185. [Google Scholar]
- Krzemień, G.; Szmigielska, A.; Turczyn, A.; Pańczyk-Tomaszewska, M. Urine interleukin-6, interleukin-8 and transforming growth factor β1 in infants with urinary tract infection and asymptomatic bacteriuria. Cent. Eur. J. Immunol. 2016, 41, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Montini, G.; Tullus, K.; Hewitt, I. Febrile urinary tract infections in children. N. Engl. J. Med. 2011, 365, 239–250. [Google Scholar] [CrossRef]
- Ragnarsdóttir, B.; Svanborg, C. Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: Host-pathogen interaction in urinary tract infections. Pediatr. Nephrol. 2012, 27, 2017–2029. [Google Scholar] [CrossRef]
- Yadav, M.; Zhang, J.; Fischer, H.; Huang, W.; Lutay, N.; Cirl, C.; Lum, J.; Miethke, T.; Svanborg, C. Inhibition of TIR domain signaling by TcpC: MyD88-dependent and independent effects on Escherichia coli virulence. PLoS Pathog. 2010, 6, e1001120. [Google Scholar] [CrossRef]
- Wei, Y.; Li, K.; Wang, N.; Cai, G.D.; Zhang, T.; Lin, Y.; Gui, B.S.; Liu, E.Q.; Li, Z.F.; Zhou, W. Activation of endogenous anti-inflammatory mediator cyclic AMP attenuates acute pyelonephritis in mice induced by uropathogenic Escherichia coli. Am. J. Pathol. 2015, 185, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Tang, D.; Dai, Y.; Diao, H. Establishment of microRNA, transcript and protein regulatory networks in Alport syndrome induced pluripotent stem cells. Mol. Med. Rep. 2019, 19, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, E.; Edefonti, A.; Puteo, F.; Chimenz, R.; Gianoglio, B.; Lavoratti, G.; Leozappa, G.; Maringhini, S.; Mencarelli, F.; Pecoraro, C.; et al. Encapsulating peritoneal sclerosis in pediatric peritoneal dialysis patients: The experience of the Italian Registry of Pediatric Chronic Dialysis. Nephrol. Dial. Transplant. 2013, 28, 1603–1609. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, L.; Tang, J.; Guo, X.; Dong, K.; Chen, S.Y. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am. J. Physiol. Ren. Physiol. 2015, 308, F69–F75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arena, S.; Chimenz, R.; Antonelli, E.; Peri, F.M.; Romeo, P.; Impellizzeri, P.; Romeo, C. A long-term follow-up in conservative management of unilateral ureteropelvic junction obstruction with poor drainage and good renal function. Eur. J. Pediatr. 2018, 177, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Haridas, B.; Jackson, A.R.; Cortado, H.; Mayne, N.; Kohnken, R.; Bolon, B.; McHugh, K.M.; Schwaderer, A.L.; Spencer, J.D.; et al. Inflammation drives renal scarring in experimental pyelonephritis. Am. J. Physiol. Ren. Physiol. 2017, 312, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Roberts, K.B.; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef] [Green Version]
- De Palma, D. Radionuclide Tools in Clinical Management of Febrile UTI in Children. Semin. Nucl. Med. 2020, 50, 50–55. [Google Scholar] [CrossRef]
- Westwood, M.E.; Whiting, P.F.; Cooper, J.; Watt, I.S.; Kleijnen, J. Further investigation of con-firmed urinary tract infection (UTI) in children under five years: A systematic review. BMC Pediatr. 2005, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Herz, D.; Merguerian, P.; McQuiston, L.; Danielson, C.; Gheen, M.; Brenfleck, L. 5-Year Prospective Results of Dimercapto-Succinic Acid Imaging in Children with Febrile Urinary Tract Infection: Proof That the Top-Down Approach Works. J. Urol. 2010, 184, 1703–1709. [Google Scholar] [CrossRef]
- Garin, E.H.; Olavarria, F.; Araya, C. Diagnostic significance of clinical and laboratory findings to localize site of UTI. Pediatr. Nephrol. 2007, 22, 1002–1006. [Google Scholar] [CrossRef]
- Chen, M.G.; Yang, Y.; Yang, Q.; Zhuang, J.Q.; Ye, X.H.; Zheng, W.J. New strategy of color and power doppler sonography combined with DMSA in the assessment of acute pyelonephritis in in-fants. BMC Nephrol. 2021, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, D.G.; Park, S.J.; Pai, K.S. Discordant findings on dimercaptosuccinic acid scin-tigraphy in children with multi-detector row computed tomography-proven acute pyelonephritis. Korean J. Pediatr. 2011, 54, 212–218. [Google Scholar] [CrossRef] [PubMed]


| Parameter | All Patients (n: 74) | APN Group (n: 36) | LUTI Group (n: 38) |
|---|---|---|---|
| Median age, years | 3 (1–6.5) | 2 (1–5) | 7 (4–10.5) |
| M/F | 36/38 | 21/15 | 15/23 |
| BMI, Kg/m2 | 18.2 ± 3.7 | 16.6 ± 2.1 | 18.6 ± 2.7 |
| WBC count (×103) | 12.3 ± 5.1 | 16.7 ± 3.1 | 8.1 ± 2.2 |
| hsCRP, mg/dL | 6.6 (2–16.7) | 18.5 (12–28) | 2.1 (1.3–4) |
| sCreatinine, mg/dL | 0.6 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.1 |
| eGFR, mL/min | 106.7 ± 12.4 | 103.2 ± 9.1 | 105.7 ± 5.6 |
| sHMGB1, ng/mL | 6.3 (5.1–14.4) | 13.3 (11.8–14.3) | 5.9 (5.2–6.8) |
| uHMGB1, ng/mL | 6.1 (2.9–8.5) | 6.2 (5.7–7.4) | 4.3 (3–8.2) |
| Positive 1st DMSA n | 36 | 36 | 0 |
| Univariate Correlation Coefficient | p | Multivariate Correlation Coefficient (β) | p | |
|---|---|---|---|---|
| (log) hsCRP | 0.41 | 0.0004 | 0.47 | 0.02 |
| (log) uHMGB1 | 0.27 | 0.10 | ||
| (log) Age | 0.12 | 0.16 | ||
| BMI | −0.21 | 0.10 | ||
| Hemoglobin | 0.16 | 0.21 | ||
| Platelet Count | 0.33 | 0.008 | 0.10 | 0.14 |
| Creatinine | −0.23 | 0.09 | ||
| WBC count | 0.36 | 0.002 | 0.39 | 0.08 |
| Follow-Up Period (Sixth Month) | ||
|---|---|---|
| APN Group (n: 36) | Persistent Scars n: 16 (44%) | No Scars n: 20 (56%) |
| I-II grade Hydronephrosis, n | 1 | 9 |
| III-IV grade Hydronephrosis, n | 13 | 1 |
| VUR, n | 14 | 10 |
| I-II grade VUR, n | 1 | 9 |
| III-IV grade VUR, n | 13 | 1 |
| hsCRP, mg/dL | 23.7 (17.3–27.4) | 13.5 (9.7–21.2) |
| WBC count (×103) | 17.7 ± 2.7 | 13.8 ± 2.2 |
| sHMGB1 | 13.9 (12.2–16.8) | 10.7 (8.9–11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimenz, R.; Chirico, V.; Cuppari, C.; Sallemi, A.; Cardile, D.; Baldari, S.; Ascenti, G.; Monardo, P.; Lacquaniti, A. Febrile Urinary Tract Infections in Children: The Role of High Mobility Group Box-1. Children 2023, 10, 47. https://doi.org/10.3390/children10010047
Chimenz R, Chirico V, Cuppari C, Sallemi A, Cardile D, Baldari S, Ascenti G, Monardo P, Lacquaniti A. Febrile Urinary Tract Infections in Children: The Role of High Mobility Group Box-1. Children. 2023; 10(1):47. https://doi.org/10.3390/children10010047
Chicago/Turabian StyleChimenz, Roberto, Valeria Chirico, Caterina Cuppari, Alessia Sallemi, Davide Cardile, Sergio Baldari, Giorgio Ascenti, Paolo Monardo, and Antonio Lacquaniti. 2023. "Febrile Urinary Tract Infections in Children: The Role of High Mobility Group Box-1" Children 10, no. 1: 47. https://doi.org/10.3390/children10010047





