The Relationship between Eosinophil Density in the Colonic Mucosa and Eosinophil Blood Count in Children: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Molecular Aspects of Eosinophilia
4.2. Challenges in the Clinical Interpretation of Colonic Eosinophilia
4.3. Association of Mucosal Eosinophilia with Inflammation, but Not Serum IgE
4.4. Results Reflect Specific Eosinophil Regulation in Younger Children
4.5. Specific Aspects and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chusid, M.J. Eosinophils: Friends or Foes? J. Allergy Clin. Immunol. Pract. 2018, 6, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P.; Rosenberg, H.F.; Walsh, G.M. Molecular Biology of Eosinophils: Introduction. Methods Mol. Biol. 2021, 2241, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, F.; Weller, P.F. Practical approach to the patient with hypereosinophilia. J. Allergy Clin. Immunol. 2010, 126, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBrosse, C.W.; Case, J.W.; Putnam, P.E.; Collins, M.H.; Rothenberg, M.E. Quantity and Distribution of Eosinophils in the Gastrointestinal Tract of Children. Pediatr. Dev. Pathol. 2006, 9, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kovalszki, A.; Weller, P.F. Eosinophilia. Prim. Care 2016, 43, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Gebreselassie, N.G.; Gagliardo, L.F.; Ruyechan, M.C.; Lee, N.A.; Lee, J.J.; Appleton, J.A. Eosinophil-derived IL-10 supports chronic nematode infection. J. Immunol. 2014, 193, 4178–4187. [Google Scholar] [CrossRef] [Green Version]
- Rauscher, C.; Freeman, A. Drug-induced eosinophilia. Allergy Asthma Proc. 2018, 39, 252–256. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Marasco, G.; Eusebi, L.H.; Salfi, N.; Bazzoli, F.; Zagari, R.M. Eosinophilic colitis: A clinical review. Dig. Liver Dis. 2019, 51, 769–773. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Storr, M.A.; Shaffer, E.A. Eosinophilic colitis: Epidemiology, clinical features, and current management. Ther. Adv. Gastroenterol. 2011, 4, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Koutri, E.; Papadopoulou, A. Eosinophilic Gastrointestinal Diseases in Childhood. Ann. Nutr. Metab. 2018, 73, 18–28. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Koletzko, S.; Heuschkel, R.; Dias, J.A.; Allen, K.J.; Murch, S.H.; Chong, S.; Gottrand, F.; Husby, S.; Lionetti, P.; et al. Management guidelines of eosinophilic esophagitis in childhood. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 107–118. [Google Scholar] [CrossRef]
- Ullmann, N.; Bossley, C.J.; Fleming, L.; Silvestri, M.; Bush, A.; Saglani, S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy 2013, 68, 402–406. [Google Scholar] [CrossRef]
- Bedolla-Barajas, M.; Raúl Ortiz-Peregrina, J.; Daniel Hernández-Colín, D.; Morales-Romero, J.; Ramses Bedolla-Pulido, T.; Larenas-Linnemann, D. The characterization of asthma with blood eosinophilia in adults in Latin America. J. Asthma 2019, 56, 1138–1146. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Schatz, M.; Li, Q.; Chen, W.; Khatry, D.B.; Gossage, D.; Tran, T.N. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 741–750. [Google Scholar] [CrossRef]
- Baxi, S.; Gupta, S.K.; Swigonski, N.; Fitzgerald, J.F. Clinical presentation of patients with eosinophilic inflammation of the esophagus. Gastrointest. Endosc. 2006, 64, 473–478. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Blanchard, C.; Kirby, C.; Buckmeier, B.K.; Cohen, M.B.; Heubi, J.E.; Putnam, P.E.; Rothenberg, M.E. Potential of Blood Eosinophils, Eosinophil-Derived Neurotoxin, and Eotaxin-3 as Biomarkers of Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2006, 4, 1328–1336. [Google Scholar] [CrossRef]
- Straumann, A.; Conus, S.; Degen, L.; Felder, S.; Kummer, M.; Engel, H.; Bussmann, C.; Beglinger, C.; Schoepfer, A.; Simon, H. Budesonide Is Effective in Adolescent and Adult Patients with Active Eosinophilic Esophagitis. Gastroenterology 2010, 139, 1526–1537.e1. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Gómez-Torrijos, E.; de-la Santa-Belda, E.; López-Viedma, B.; Martín-Dávila, F.; Pilkington-Woll, J.P.; Donado-Palencia, P.; Sánchez-Miranda, P.; Olmedo-Camacho, J. Effectiveness of serological markers of eosinophil activity in monitoring eosinophilic esophagitis. Rev. Esp. Enferm. Dig. 2013, 105, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Haasnoot, M.L.; Mookhoek, A.; Duijvestein, M.; D’Haens, G.R.A.M.; Bredenoord, A.J. Prognostic Value of Colonic Tissue and Blood Eosinophils in Ulcerative Colitis. Inflamm. Bowel. Dis. 2022, izac044. [Google Scholar] [CrossRef]
- Morgenstern, S.; Brook, E.; Rinawi, F.; Shamir, R.; Assa, A. Tissue and peripheral eosinophilia as predictors for disease outcome in children with ulcerative colitis. Dig. Liver Dis. 2017, 49, 170–174. [Google Scholar] [CrossRef]
- Barrie, A.; Mourabet, M.E.; Weyant, K.; Clarke, K.; Gajendran, M.; Rivers, C.; Park, S.Y.; Hartman, D.; Saul, M.; Regueiro, M.; et al. Recurrent Blood Eosinophilia in Ulcerative Colitis Is Associated with Severe Disease and Primary Sclerosing Cholangitis. Dig. Dis. Sci. 2013, 58, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Zilbauer, M.; Heuschkel, R.; Phillips, A.; Salvestrini, C.; Torrente, F.; Bates, A.W. Defining Eosinophilic Colitis in Children: Insights From a Retrospective Case Series. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Bandhauer, M.E.; Bunker, A.M.; Roberts, W.L.; Hill, H.R. New childhood and adult reference intervals for total IgE. J. Allergy Clin. Immunol. 2014, 133, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.M.; Potter, M.D.; Talley, N.J. Eosinophilic colitis and colonic eosinophilia. Curr. Opin. Gastroenterol. 2019, 35, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Blumberg, R.S.; Macdonald, T.T. Society for Mucosal Immunology (Menomonee Falls, Wisconsin). In Principles of Mucosal Immunology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Matucci, A.; Vultaggio, A.; Maggi, E.; Kasujee, I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 2018, 19, 113. [Google Scholar] [CrossRef] [Green Version]
- Diny, N.L.; Schonfeldova, B.; Shapiro, M.; Winder, M.L.; Varsani-Brown, S.; Stockinger, B. The aryl hydrocarbon receptor contributes to tissue adaptation of intestinal eosinophils in mice. J. Exp. Med. 2022, 219, e20210970. [Google Scholar] [CrossRef]
- Su, K.-W.; Shreffler, W.G.; Yuan, Q. Gastrointestinal immunopathology of food protein-induced enterocolitis syndrome and other non-immunoglobulin E-mediated food allergic diseases. Ann. Allergy Asthma Immunol. 2021, 126, 516–523. [Google Scholar] [CrossRef]
- Chua, H.-H.; Chou, H.-C.; Tung, Y.-L.; Chiang, B.-L.; Liao, C.-C.; Liu, H.-H.; Ni, Y.-H. Intestinal Dysbiosis Featuring Abundance of Ruminococcus gnavus Associates With Allergic Diseases in Infants. Gastroenterology 2018, 154, 154–167. [Google Scholar] [CrossRef]
- Harris, J.K.; Fang, R.; Wagner, B.D.; Choe, H.N.; Kelly, C.J.; Schroeder, S.; Moore, W.; Stevens, M.J.; Yeckes, A.; Amsden, K.; et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE 2015, 10, e0128346. [Google Scholar] [CrossRef]
- Sohn, K.-H.; Baek, M.-G.; Choi, S.-M.; Bae, B.; Kim, R.Y.; Kim, Y.-C.; Kim, H.-Y.; Yi, H.; Kang, H.-R. Alteration of Lung and Gut Microbiota in IL-13-Transgenic Mice Simulating Chronic Asthma. J. Microbiol. Biotechnol. 2020, 30, 1819–1826. [Google Scholar] [CrossRef]
- Chen, M.; Shepard, K.; Yang, M.; Raut, P.; Pazwash, H.; Holweg, C.T.J.; Choo, E. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin. Exp. Allergy 2021, 51, 546–555. [Google Scholar] [CrossRef]
- Chapman, K.R.; Albers, F.C.; Chipps, B.; Muñoz, X.; Devouassoux, G.; Bergna, M.; Galkin, D.; Azmi, J.; Mouneimne, D.; Price, R.G.; et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy 2019, 74, 1716–1726. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, S.; Liu, P.; Mu, Z.; Zhang, J. Clinical relevance of eosinophils, basophils, serum total IgE level, allergen-specific IgE, and clinical features in atopic dermatitis. J. Clin. Lab. Anal. 2020, 34, e23214. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, C.; Bessissow, T.; Desmet, A.-S.; Vanheel, H.; Tack, J.; Vanden Berghe, P. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am. J. Gastroenterol. 2015, 110, 1205–1215. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many Patients with Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated with Immunoglobulin E. Gastroenterology 2019, 157, 109–118.e5. [Google Scholar] [CrossRef] [Green Version]
- Wauters, L.; Ceulemans, M.; Frings, D.; Lambaerts, M.; Accarie, A.; Toth, J.; Mols, R.; Augustijns, P.; De Hertogh, G.; Van Oudenhove, L.; et al. Proton Pump Inhibitors Reduce Duodenal Eosinophilia, Mast Cells, and Permeability in Patients with Functional Dyspepsia. Gastroenterology 2021, 160, 1521–1531.e9. [Google Scholar] [CrossRef]
- Uppal, V.; Kreiger, P.; Kutsch, E. Eosinophilic Gastroenteritis and Colitis: A Comprehensive Review. Clin Rev Allergy Immunol 2016, 50, 175–188. [Google Scholar] [CrossRef]
- Talley, N.J.; Shorter, R.G.; Phillips, S.F.; Zinsmeister, A.R. Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990, 31, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Grzybowska-Chlebowczyk, U.; Horowska-Ziaja, S.; Kajor, M.; Więcek, S.; Chlebowczyk, W.; Woś, H. Eosinophilic colitis in children. Adv. Dermatol. Allergol. 2017, 34, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, R.A.; Prindiville, T.P.; Pecha, R.E.; Ruebner, B.H. Unusual presentations of eosinophilic gastroenteritis: Case series and review of literature. World J. Gastroenterol. 2009, 15, 2156–2161. [Google Scholar] [CrossRef]
- Ong, G.-Y.; Hsu, C.-C.; Changchien, C.-S.; Lu, S.-N.; Huang, S.-C. Eosinophilic gastroenteritis involving the distal small intestine and proximal colon. Chang Gung. Med. J. 2002, 25, 56–61. [Google Scholar] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, A.W.H. Diagnosing Eosinophilic Colitis: Histopathological Pattern or Nosological Entity? Scientifica 2012, 2012, e682576. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.; Tél, B.; Farkas, N.; Garami, A.; Vincze, Á.; Bajor, J.; Sarlós, P.; Márta, K.; Eros, A.; Mikó, A.; et al. Eosinophil Counts in the Small Intestine and Colon of Children Without Apparent Gastrointestinal Disease: A Meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 6–12. [Google Scholar] [CrossRef]
- Licari, A.; Votto, M.; D’Auria, E.; Castagnoli, R.; Caimmi, S.M.E.; Marseglia, G.L. Eosinophilic Gastrointestinal Diseases in Children: A Practical Review. Curr. Pediatr. Rev. 2020, 16, 106–114. [Google Scholar] [CrossRef]
- Ko, H.M.; Morotti, R.A.; Yershov, O.; Chehade, M. Eosinophilic Gastritis in Children: Clinicopathological Correlation, Disease Course, and Response to Therapy. Am. J. Gastroenterol. 2014, 109, 1277–1285. [Google Scholar] [CrossRef]
- Straumann, A.; Aceves, S.S.; Blanchard, C.; Collins, M.H.; Furuta, G.T.; Hirano, I.; Schoepfer, A.M.; Simon, D.; Simon, H.-U. Pediatric and adult eosinophilic esophagitis: Similarities and differences. Allergy 2012, 67, 477–490. [Google Scholar] [CrossRef]
- Lingblom, C.; Käppi, T.; Bergquist, H.; Bove, M.; Arkel, R.; Saalman, R.; Wennerås, C. Differences in eosinophil molecular profiles between children and adults with eosinophilic esophagitis. Allergy 2017, 72, 1406–1414. [Google Scholar] [CrossRef]
- Uzunismail, H.; Hatemi, I.; Doğusoy, G.; Akin, O. Dense eosinophilic infiltration of the mucosa preceding ulcerative colitis and mimicking eosinophilic colitis: Report of two cases. Turk. J. Gastroenterol. 2006, 17, 53–57. [Google Scholar]
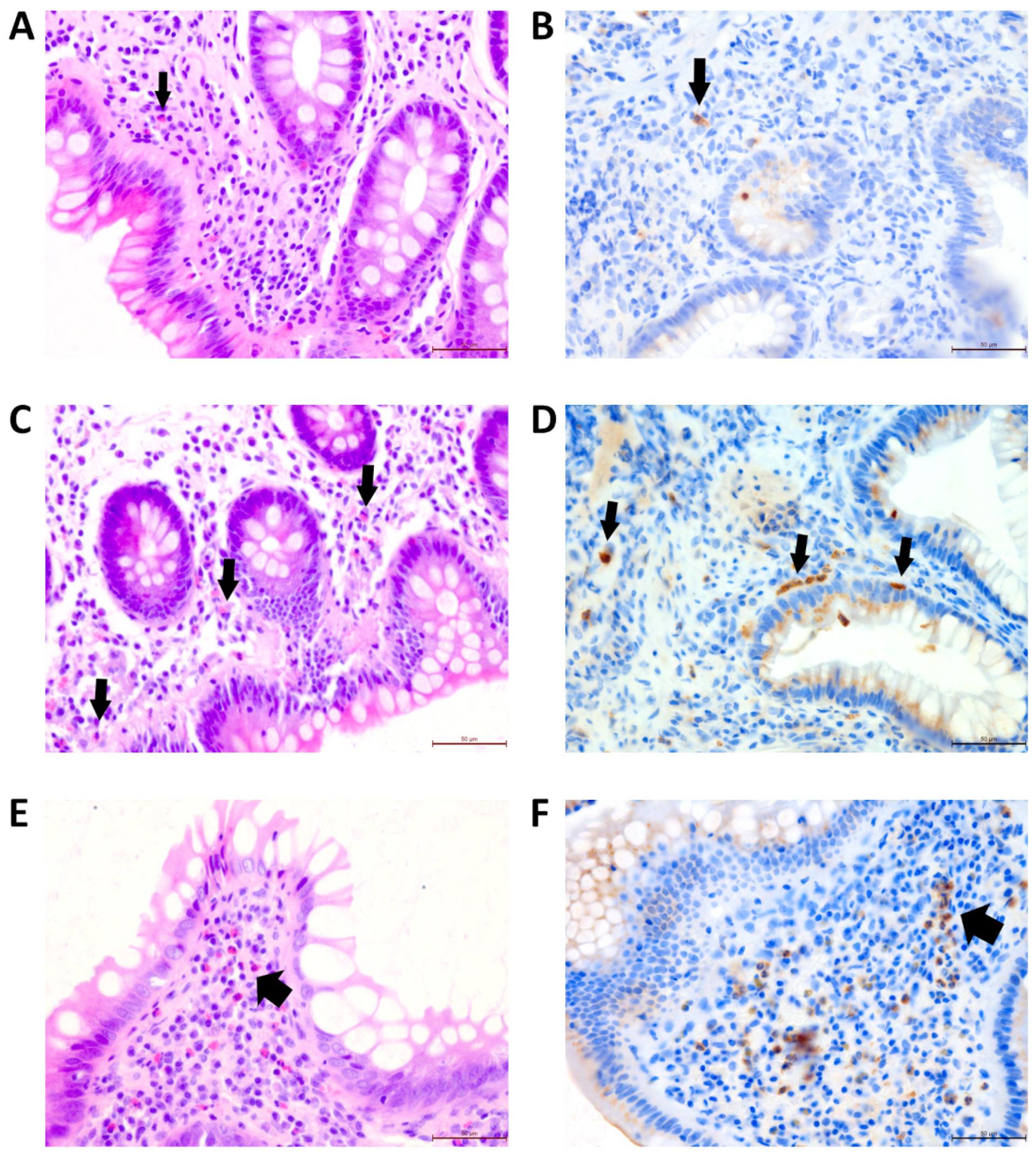

| Characteristic | UC | CD | Other |
|---|---|---|---|
| n | 34 | 16 | 59 |
| Gender—female, % | 64.7% | 37.5% | 40.7% |
| Age, years | 11.7 (8.2–15.5) | 13.7 (10.8–16.4) | 12.1 (8.0–15.5) |
| Weight, kg | 43.7 (23.4–53.5) | 44.0 (27.3–61.1) | 39.0 (28.0–62.0) |
| Height, cm | 149.5 (126.0–162.0) | 161.5 (144–165.5) | 166.9 (131.0–151.0) |
| CRP, mg/dL | 0.13 (0.005–0.99) | 1.1 (0.2–2.9) | 0 (0–0.08) |
| WBC, G/L | 8.3 (6.3–11.3) | 8.5 (7.0–9.4) | 6.8 (5.5–8.4) |
| Eosinophils, G/L | 0.18 (0.09–0.40) | 0.14 (0.12–0.27) | 0.17 (0.10–0.24) |
| Eosinophils, % | 2.2% (1.2–3.9%) | 2.2% (1.6–3.4%) | 2.6% (1.6–3.5%) |
| IgE, kU/L | 23 (12–87) | 81 (56–284) | 55 (11–152) |
| Colonic mucosal eosinophil density, per HPF | 32 (25–39) | 19 (10–24) | 18 (7–24) |
| Assessed Correlation, in All Subjects | r | 95% CI | p |
| colonic mucosal eosinophil density vs. blood eosinophil count | 0.295 | 0.108–0.462 | 0.0018 * |
| colonic mucosal eosinophil density vs. blood eosinophil count if CRP < 0.5 mg/dL | 0.245 | 0.022–0.445 | 0.027 * |
| colonic mucosal eosinophil density vs. blood eosinophil count if CRP > 0.5 mg/dL | 0.529 | 0.167–0.766 | 0.0054 * |
| colonic mucosal eosinophil density vs. relative eosinophil count in the blood | 0.175 | −0.019–0.356 | 0.069 |
| colonic mucosal eosinophil density vs. total WBC count | 0.262 | 0.072–0.433 | 0.0059 * |
| colonic mucosal eosinophil density vs. CRP | 0.182 | −0.013–0.364 | 0.060 |
| colonic mucosal eosinophil density vs. total IgE levels | −0.024 | −0.254–0.208 | 0.833 |
| * if p < 0.05 | |||
| Assessed Correlation, in Adolescents > 12.4 Years | r | 95% CI | p |
| colonic mucosal eosinophil density vs. blood eosinophil count in subjects > 12.4 years | 0.448 | 0.197–0.644 | 0.00068 * |
| colonic mucosal eosinophil density vs. relative eosinophil count in the blood in subjects > 12.4 years | 0.432 | 0.177–0.632 | 0.0011 * |
| colonic mucosal eosinophil density vs. total WBC count in subjects > 12.4 years | 0.300 | 0.026–0.531 | 0.028 * |
| * if p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brylak, J.; Nowak, J.K.; Szczepanik, M.; Holubiec, M.; Kurzawa, P.; Walkowiak, J. The Relationship between Eosinophil Density in the Colonic Mucosa and Eosinophil Blood Count in Children: A Cross-Sectional Study. Children 2023, 10, 6. https://doi.org/10.3390/children10010006
Brylak J, Nowak JK, Szczepanik M, Holubiec M, Kurzawa P, Walkowiak J. The Relationship between Eosinophil Density in the Colonic Mucosa and Eosinophil Blood Count in Children: A Cross-Sectional Study. Children. 2023; 10(1):6. https://doi.org/10.3390/children10010006
Chicago/Turabian StyleBrylak, Jan, Jan K. Nowak, Mariusz Szczepanik, Magdalena Holubiec, Pawel Kurzawa, and Jaroslaw Walkowiak. 2023. "The Relationship between Eosinophil Density in the Colonic Mucosa and Eosinophil Blood Count in Children: A Cross-Sectional Study" Children 10, no. 1: 6. https://doi.org/10.3390/children10010006
APA StyleBrylak, J., Nowak, J. K., Szczepanik, M., Holubiec, M., Kurzawa, P., & Walkowiak, J. (2023). The Relationship between Eosinophil Density in the Colonic Mucosa and Eosinophil Blood Count in Children: A Cross-Sectional Study. Children, 10(1), 6. https://doi.org/10.3390/children10010006





