1. Introduction
Asthma is a chronic obstructive inflammatory airway disease that often develops early in life [
1,
2], and affects around 10–15% of school children [
3,
4]. Childhood asthma is complex and heterogeneous, characterized by reversible airflow obstruction and inflammation triggered by allergens, environmental pollutants and infections [
3,
5]. The treatment often consist of inhaled bronchodilators for symptom relief, anti-inflammatory drugs such as corticosteroids [
3,
6] and in serious disease the addition of immunomodulatory medication for control [
7,
8]. The aetiology is multifactorial and partly still unknown [
9,
10], while it is likely that the disease origins from early life [
11,
12]. Atopic dermatitis (AD) in early infancy is a risk factor for, and often precedes asthma as well as other allergic diseases [
13,
14]. Collectively, the progression from one allergic disease to another is often referred to as the atopic march, indicating a link between the allergic diseases [
15,
16].
Epithelial barrier dysfunction may be involved in allergic disease development, including the airways and the skin [
17,
18]. The skin acts as a natural protective barrier from allergens and other environmental exposures [
19,
20]. In AD, an inflammatory condition characterized by eczematous itchy skin lesions [
15,
21], reduced skin barrier function and dysregulation of the immune system play an important role [
22,
23]. Increased transepidermal water loss (TEWL), a non-invasive measurement of skin barrier function, has been associated with development of allergic sensitization [
24,
25] and AD [
26,
27], and has been shown to precede the clinical manifestations of AD [
26,
28]. Reduced skin barrier function may also play a role in the development and progression of asthma [
29,
30]. By the activation of T cells due to cutaneous allergens on a permeable skin barrier [
28], accelerated by loss-of-function mutations in the Filaggrin (
FLG) gene [
29], prenatal and early postnatal exposures may have a lifelong effect on respiratory health [
3].
FLG mutations are linked to higher TEWL [
31], eczema [
31,
32], AD [
33,
34], allergic sensitization and asthma severity [
29,
35,
36].
Lower lung function, determined by tidal breath flow-volume (TFV) loops, is associated with higher risk of developing asthma in childhood [
37], and reduced lung function from birth to adolescence has been observed in children with allergic asthma and AD [
38]. Compared to healthy children, infants and children with obstructive airway diseases generally reach peak tidal expiratory flow (
tPTEF) earlier in the expiratory phase with a subsequent lower ratio of time to peak tidal expiratory flow to expiratory time (
tPTEF/
tE) [
39,
40,
41].
Though reduced skin barrier function is strongly associated with AD, and AD as well as lower infant lung function are known risk factors for asthma, the association between the skin barrier- and lung function in early life is largely unexplored. We hypothesized that a reduced skin barrier function could be a common origin of AD and lower lung function in early life. Our primary aim was to explore if high TEWL, and secondarily manifestations of eczema or FLG mutations were associated with lower lung function in healthy three-month-old infants.
2. Materials and Methods
2.1. Study Design and Study Population
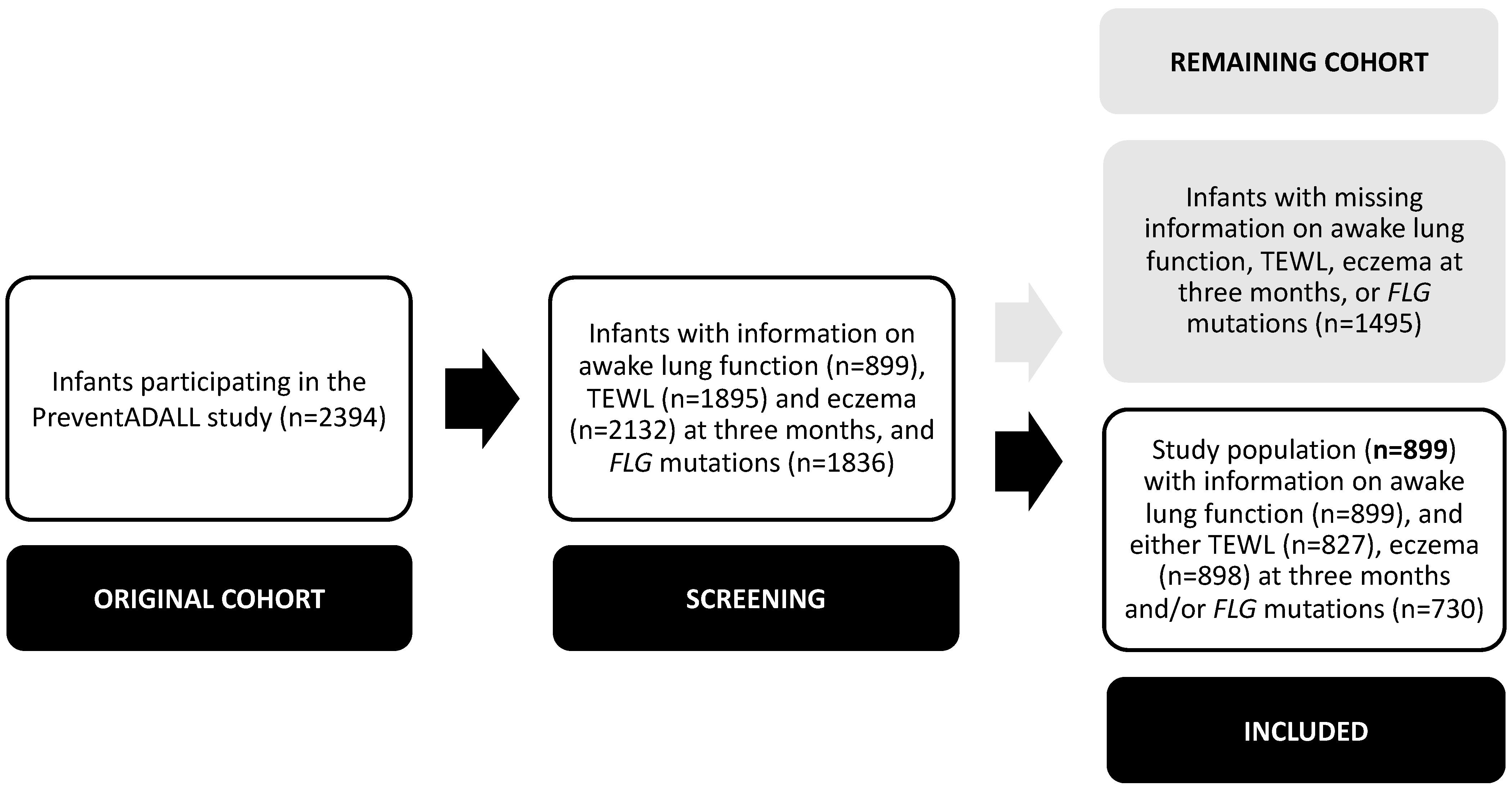
The present study is a prospective observational study, using information from the Preventing Atopic Dermatitis and ALLergies in children (PreventADALL) cohort. The study population consisted of 899 participating infants with available TFV loop measurements in the awake state, and information on either TEWL, eczema at three months of age and/or
FLG mutations. The PreventADALL study, consisting of 2394 mother-child pairs actively participating, is a Scandinavian multicentre population-based prospective birth cohort study with two factorial designed randomized controlled interventions; skin emollients from two weeks to nine months and food introduction from three months of age [
42]. Pregnant women were recruited between 2014 and 2016 during the routine ultrasound examination at 18 weeks gestational age (GA) in Norway and Sweden. The participating infants, born without serious illnesses at GA ≥35 weeks, were enrolled during the first days of life.
Informed written consent was collected from the mothers at enrolment, and from both parents at infant inclusion. The PreventADALL study, including the present sub study, was approved by the Regional Committee for Medical and Health Research Ethics in South-Eastern Norway (2014/518) and in Sweden (2014/2242-31/4), as well as registered at
clinicaltrials.gov (NCT02449850). The data is stored at the project database in “Service for Sensitive Data” (TSD) at Oslo University, in compliance with General Data Protection Regulation (GDPR) legislation.
2.2. Data Collection
2.2.1. Three-Month Clinical Investigation
Lung function by TFV loops was measured in calm awake infants positioned in a semi-recumbent position. The TFV loop parameters were sampled and recorded using the employed Exhalyzer
® D (Eco Medics AG, Duernten, Switzerland), with a soft-rimmed air-inflated face mask covering the mouth and nose. All measurements were manually scrutinized with focus on loop shape and reproducibility by one of three trained investigators in Oslo (Norway) and in Stockholm (Sweden). Technically unacceptable loops were manually removed before approval. Further information about the standard operating procedure for TFV loop measurements and manual loop selection is described elsewhere [
43].
TEWL (g/m
2/h) was measured on the left lateral upper arm with an open chamber DermaLab USB (Cortex, Hadsund, Denmark), at room temperature between 20 °C to 25 °C, in line with international recommendations. All ranges of humidity from 6.40% to 72.9%, with a mean of 30.9%, were accepted in line with previous findings [
44]. After 15 min of acclimatization, with the infants wearing only diapers, three consecutive measurements were performed in calm infants, distanced from direct sunlight, while windows and doors were kept closed.
Clinical skin examinations were performed by trained study personnel, educated at workshops to minimize inter-observer variability. Parents were advised not to bathe the infants or use any skin emollients for at least 24 h prior to the visits. Observation of eczematous skin lesions suggestive of AD were verified by a medical doctor, clinically excluding common differential diagnoses to AD [
45].
2.2.2. FLG Mutations
DNA was isolated from blood and genotyped for the most common
FLG mutations in Europeans; R501X, 2282del4 and R2447X, using the TaqMan-based allelic discrimination assay, as previously described by Hoyer et al. [
32]. Allele-specific Taqman MGB (minor groove binder) probes were labelled with the fluorescent dyes FAM and VIC, respectively. Polymerase chain reactions (PCR) were carried out in 384-well plates with use of genomic DNA, a reaction mix containing the specific TaqMan assay solution and 1X TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Amplification was done following the Taqman Universal PCR protocol. Allelic discrimination was performed with the QuantStudion 6 and 7 Flex (QS6 and 7 FLX).
2.2.3. Birth and Background Characteristics
Background data were collected from electronic questionnaires answered at approximately 18 and 34 weeks of pregnancy and three months postpartum. Birth data, including anthropometrics and health were recorded from electronic medical records. At the three-month clinical investigation, infant weight and length were measured according to a standard operating procedure.
2.3. Definitions
2.3.1. Primary Outcome
Lower
tPTEF/
tE: a
tPTEF/
tE <0.25, previously associated with airway obstruction and asthma [
37,
46,
47,
48].
2.3.2. Secondary Outcome
Lower
tPTEF: a
tPTEF <0.17 s (below the 25th percentile) was chosen, as lower values have previously found in infants and children with airway obstruction and asthma [
39,
40,
41].
2.3.3. Sensitivity Analysis
The continuous tPTEF/tE and tPTEF.
2.3.4. Exposures
High TEWL: a mean TEWL value >8.83 g/m
2/h (above the 75th percentile), in line with previous studies [
24,
44].
Eczema: Defined as clinically observed eczematous skin lesions with the exclusion of common differential diagnosis to AD such as infantile seborrheic dermatitis and irritative contact dermatitis [
45]. As few three-month-old infants fulfil the strict diagnostic criteria for AD by the United Kingdom Working Party (UKWP) [
49], eczema was used as a proxy.
FLG mutations: Being carrier of any of the three mutations R501X, 2282del4 and R2447X of the
FLG gene, hypothesized to contribute to asthma [
15].
2.4. Statistical Analyses
Comparisons were performed using parametric or non-parametric tests; independent t-tests for continuous variables presented with means, standard deviations (SD) and minimum-maximum (min-max), and Chi2 tests for categorical variables presented with numbers (n) and percentages (%). The TFV loop parameters are presented with mean (SD; min-max) for continuous variables. Associations between high TEWL, eczema, FLG mutations and lower tPTEF/tE and tPTEF were examined using univariate and multivariate logistic regression models, presented with odds ratios (OR) and 95% confidence intervals (CI). In a sensitivity analysis, the univariate and multivariate linear associations between high TEWL, eczema and FLG mutations, and the continuous tPTEF/tE and tPTEF were explored, presented with ß coefficients (95% CI). Analyses for possible interactions between the exposures (high TEWL, eczema or FLG mutations) and the skin intervention, and the primary and secondary outcomes (lower tPTEF/tE and tPTEF) were conducted. The statistical significance for all tests was set to 0.05. Statistical analyses were conducted using IBM SPSS Statistics 26 software.
3. Results
3.1. Study Population
The study population consisted of 899 infants participating in the PreventADALL study with available TFV loop measurements in the awake state, and information on either TEWL, eczema at three months of age and/or
FLG mutations. The remaining 1495 infants with missing information on either TFV loop measurements in the awake state, TEWL, eczema at three months of age and/or FLG mutations were excluded (
Figure 1).
The 899 included infants were born at a mean ± SD GA of 40.1 ± 1.32 weeks and 439 (48.8%) were girls. Compared to the remaining cohort not included in this study, the included infants more often had parents with higher socioeconomic status, a lower mean TEWL and higher frequency of eczema, though similar rates of
FLG mutation carriers. At three months of age, the mean TEWL among 827/899 (92.0%) included infants was 7.94 ± 5.66 g/m
2/h, eczema was present in 135/898 (15.0%) and
FLG mutations were identified in 73/730 (10.0%) infants (
Table 1), evenly distributed between boys and girls (data not shown). The included infants had a mean ± SD
tPTEF/
tE of 0.39 ± 0.08. The infants with a lower
tPTEF/
tE (n = 48) had a mean
tPTEF/
tE of 0.23 ± 0.02 and a mean
tPTEF of 0.16 ± 0.04. The infants with a lower
tPTEF (n = 223) had a mean
tPTEF/
tE of 0.34 ± 0.08 and a mean
tPTEF of 0.15 ± 0.01. The TFV loop parameters are further described in
Supplementary Table S1.
3.2. Primary Analysis: High TEWL, Eczema, FLG Mutations and Lower tPTEF/tE
A high TEWL was not significantly associated with a lower
tPTEF/
tE in the multivariate analyses; adjusted OR (95% CI) 1.21 (0.58, 2.53). Likewise, the presence of eczema or
FLG mutations were not associated with a lower
tPTEF/
tE; adjusted OR (95% CI) 1.09 (0.44, 2.70) and 0.93 (0.27, 3.18), respectively (
Table 2,
Table 2.1,
Figure 2a). The relationship between mean TEWL and the continuous
tPTEF/
tE at three months of age is displayed in a scatter plot, see
Supplementary Figure S1a.
3.3. Secondary Analysis: High TEWL, Eczema, FLG Mutations and Lower tPTEF
A high TEWL was significantly associated with a lower
tPTEF in the multivariate analysis; adjusted OR (95% CI) 1.61 (1.08, 2.42). Neither the presence of eczema, nor
FLG mutations were associated with a lower
tPTEF; adjusted OR (95% CI) 1.19 (0.74, 1.92) and 1.09 (0.57, 2.06), respectively (
Table 2,
Table 2.2,
Figure 2b). The relationship between mean TEWL and the continuous
tPTEF at three months of age is displayed in a scatterplot, see
Supplementary Figure S1b.
3.4. Sensitivity Analysis: High TEWL, Eczema, FLG Mutations and Continuous tPTEF/tE and tPTEF as Well as Interaction Effects
Neither a high TEWL, nor eczema or
FLG mutations were associated with the continuous
tPTEF/
tE in the multivariate analyses (
Supplementary Table S2.1). While a high TEWL was negatively associated with the continuous
tPTEF in the multivariate analysis, the presence of eczema or
FLG mutations were not (
Supplementary Table S2.2).
No significant interaction effects of high TEWL, eczema or
FLG mutations and the skin intervention on lower
tPTEF/
tE or
tPTEF were found (
Supplementary Table S3.1,2).
4. Discussion
In this population-based birth cohort study of 899 three-month-old healthy infants, no associations between high TEWL, eczema nor FLG mutations, and a lower tPTEF/tE were observed. While a high TEWL was inversely associated with tPTEF, the presence of eczema or FLG mutations were not. The significant associations indicate a possible link between the skin barrier- and lung function in early infancy.
4.1. TEWL and Lung Function
This study provides novel insight into a potential link between the skin barrier function and infant lung function. While a high TEWL was not associated with the ratio
tPTEF/
tE at three months of age, a significant negative association was observed between high TEWL and lower
tPTEF as well as the continuous
tPTEF. To our knowledge, this is the first study to explore the association between the skin barrier function and lung function within a large population of healthy infants. In relation to asthma in adolescents and adults, a previous study of 95 individuals found no differences in TEWL between allergic asthmatics and healthy controls [
50]. Infants and children with obstructive airway diseases generally reach
tPTEF earlier in the expiratory phase with a subsequent lower
tPTEF/
tE ratio, compared to healthy children [
39,
40,
41], providing an important rationale for including
tPTEF as an outcome in this study. Although reduced skin barrier function was not associated with lower lung function measured by
tPTEF/
tE, the inverse association with
tPTEF, where high TEWL increased the risk of having shorter time before reaching peak expiratory flow, suggests that lung function and skin barrier function may share a common developmental origin, be manifestations of barrier dysfunction observed in two different organ systems, or infer a causal link between the two. However, as no crude associations to
tPTEF were seen, further longitudinal studies are needed to exclude the possibility that the significant associations represented chance findings. Whether high TEWL in infancy increases the risk of childhood asthma needs to be investigated in future studies.
4.2. Eczema and Lung Function
No evidence of any associations between eczema and lower infant lung function was found in this study. As AD is related to asthma development [
15], we had hypothesized that early life eczema might also be related to altered lung function. However, though based on older children, our results correspond well with Hu et al.’s findings of no differences in lung function by spirometry and distinctive eczema phenotypes among 4227 school children [
51]. Likewise, in 135 children from the Norwegian Environment and Childhood Asthma (ECA) study, lung function development by TFV loops from birth to two years appeared independent of AD [
52]. Since few infants fulfil the major requirement of itch at three months of age, the appropriateness of the commonly used UKWP criteria for AD in early infancy has previously been questioned [
49]. As only 1.60% of the infants fulfilled the criteria of the UKWP for AD [
53] in this study, clinically observed eczema (15.0%) by medical doctors was used as a proxy. Though a link between AD severity and asthma previously have been suggested [
54], the severity of eczema was not evaluated in this study. In a previous paper by Lødrup Carlsen et al., reduced lung function from birth to adolescence was retrospectively observed in children with allergic asthma and AD [
38].
4.3. FLG Mutations and Lung Function
In our study, no associations between
FLG mutations and lower infant lung function were observed. Among markers associated with allergic diseases, mutations in the
FLG gene, a protein essential for the skin barrier function [
29,
30], are hypothesized to contribute to asthma [
15]. To our knowledge, there are no previously published reports on
FLG mutations and infant lung function, and thus, our finding of no association is novel. In relation to symptoms of airway obstruction, the GO-CHILD birth cohort of 2312 British infants discovered an association between
FLG mutations and wheezing at six months of age [
55]. Interestingly, the risk of developing asthma in
FLG mutation carriers appears to be limited to children with a previous history of AD [
30,
33].
4.4. Strengths and Limitations
There are several strengths to this study. First, infants were enrolled antenatally, and potential risk factors for adjustment in analyses were recorded prior to the clinical investigation from which the present results are presented. The study comprises lung function measurements in almost 900 healthy three-month-old infants from a general population, with TEWL measurements available in 92.0%. The TFV measurements were performed in early infancy, limiting the time for postnatal events to adversely impact lung function development. Both the study personnel measuring TEWL and medical doctors assessing eczema were educated at joint workshops, to increase the inter-observer reliability in both Norway and Sweden. As reported by Hoyer et al., the 9.00% prevalence of
FLG mutation carriers in the original PreventADALL cohort was comparable to other European studies [
32], corresponding with the rates (10.0%) in our study population. The thorough quality assessment of TFV loop measurements performed by the investigators further strengthen our findings [
43]. While a
tPTEF/
tE below 0.25 in infancy has been related to future obstructive lung diseases [
37,
46,
47,
48], we are not aware of any defined cut-off values for
tPTEF in infancy. Infants with obstructive airway diseases generally reach
tPTEF faster in the expiratory phase compared to healthy children [
39,
40,
41], therefore, as we were interested in the lower range of
tPTEF, a
tPTEF below the 25th percentile (<0.17 s) was selected as secondary outcome. Furthermore, the continuous
tPTEF was used in our sensitivity analysis. However, our observations are limited to three-month-old infants which may be a limitation of the study. Repeated observations in the same infants later in infancy might potentially yield more conclusive results.
In addition to higher frequency of eczema and lower mean TEWL, socioeconomical differences between the study population and the excluded infants were found. Some of the differences seen may be explained by the exclusion of infants living in the less densely populated county Østfold (Norway), where lung function was not measured. At inclusion, the approximately 340 infants from Østfold more often lived in rural environments, had younger parents with higher frequencies of previous pregnancies and lower parental education and income, compared to the infants from the capital cities, Oslo (Norway) and Stockholm (Sweden) [
42].
Possible modifications of the skin intervention on the associations between TEWL, eczema or
FLG mutations and lung function, were assessed in interaction analyses. As we found no significant interaction effects between the skin intervention and the exposures on the outcomes, we included all infants in our analyses, regardless of intervention group allocation. Based on the existing literature, parental asthma [
56], parental education level [
56], nicotine exposure (snus/smoking) in pregnancy [
57], sex [
45,
58], GA at birth [
45,
59], weight at three months of age [
11,
60] and the skin intervention were all treated as possible confounders in multivariate regression models. At the time exposures and outcomes were measured the food intervention had not yet started, hence we adjusted for the skin intervention, only. By isolating the effect of potential effect modifiers from the TFV loop parameters, adjusting for relevant confounders, we were also able to detract the possibility that effect-modifying factors, such as the skin intervention, heredity for asthma or infant sex, had influence on our findings.
4.5. Clinical Implications for Future Research
With some exceptions, developmental factors for impaired lung function and asthma from early infancy, childhood and later adulthood are not well understood [
61]. Based on the significant inverse associations between high TEWL and
tPTEF, our results suggest a possible link between the skin barrier- and infant lung function. One might thereby speculate that reduced skin barrier may influence lung function in early infancy, contributing as one of the missing pieces of the puzzle of understanding lung function development. As children and adults with obstructive airway diseases generally reach
tPTEF earlier [
39,
40,
41], and in this study infants with a lower
tPTEF also had a lower
tPTEF/
tE, it is possible that a lower
tPTEF might better capture infants with an obstructive breathing pattern. However, as
tPTEF seldomly have been described in the literature it is debatable whether the parameters are sensitive markers for lung function and asthma. Further studies are necessary to establish if our findings could be of relevance for the identification and prediction of infants and older children at risk of developing asthma.
5. Conclusions
While no associations between high TEWL, eczema or FLG mutations and lower tPTEF/tE were found in healthy infants at three months of age, we are first to report that a high TEWL inversely was associated with tPTEF, indicating a possible link between the skin barrier- and lung function in early infancy. Future studies are necessary to determine the relationship between the skin barrier function and lung function development in general, as well as in children at risk of developing asthma.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/children10010088/s1, Table S1: Summary statistics of TFV loop parameters in the 899 included three-month-old infants, as well as infants with lower
tPTEF/
tE and
tPTEF. Table S2. 1,2: Linear regression models of high TEWL, eczema,
FLG mutations on the continuous
tPTEF/
tE (2.1) and
tPTEF (2.2), in the 899 three-month-old infants; Table S3. 1,2: Interaction effects in logistic regression models of high TEWL, eczema,
FLG mutations and the skin intervention on lower
tPTEF/
tE (3.1) and
tPTEF (3.2), in the 899 three-month-old infants; Figure S1. (a,b): The distribution of the mean TEWL and the continuous
tPTEF/
tE (a) as well as
tPTEF (b) at three months of age. Black dots represent infants with lower
tPTEF/
tE and
tPTEF, respectively.
Author Contributions
Conceptualization, M.F., H.K.G., K.E.S.B., E.M.R., H.O.S., C.S., K.C.L.C. and B.N.; methodology, M.F., H.K.G., K.E.S.B., E.M.R., C.S., K.C.L.C. and B.N.; software, R.V.; formal analysis, M.F.; investigation, H.K.G., K.E.S.B. and E.M.R.; data curation, M.F., H.K.G., A.H., K.E.S.B. and R.V.; writing—original draft preparation, M.F.; writing—review and editing, M.F., H.K.G., A.H., K.E.S.B., C.A., C.M.J., E.M.R., H.O.S., A.C.S., R.V., C.S., K.C.L.C. and B.N.; visualization, M.F., H.K.G., K.E.S.B., E.M.R., C.S., K.C.L.C. and B.N.; supervision, C.A., C.S., K.C.L.C. and B.N.; project administration, E.M.R., H.O.S., C.S., K.C.L.C. and B.N.; funding acquisition, E.M.R., H.O.S., C.S., K.C.L.C. and B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study is a part of a PhD project and M.F. has received funding as a PhD student from Karolinska Institutet, Sweden, and The Swedish Heart-Lung Foundation, Sweden. The PreventADALL study was supported by a number of public and private funding bodies with no influence on design, conduct or analyses: The Regional Health Board South East, The Norwegian Research Council, Oslo University Hospital, The University of Oslo, Health and Rehabilitation Norway, The Foundation for Healthcare and Allergy Research in Sweden–Vårdalstiftelsen, The Swedish Asthma- and Allergy Association’s Research Foundation, The Swedish Research Council–the Initiative for Clinical Therapy Research, The Swedish Heart-Lung Foundation, SFO-V Karolinska Institutet, Østfold Hospital Trust, The European Union (MeDALL project), by unrestricted grants from the Norwegian Association of Asthma and Allergy, The Kloster foundation, Thermo-Fisher, Uppsala, Sweden (through supplying allergen reagents) and Fürst Medical Laboratory, Oslo, Norway (through performing IgE analyses), Norwegian Society of Dermatology and Venerology, Arne Ingel’s legat, Region Stockholm (ALF-project and individual grants), Forte, Swedish Order of Freemasons Foundation Barnhuset, The Sven Jerring Foundation, The Hesselman foundation, The Magnus Bergvall foundation, The Konsul Th C Bergh’s Foundation, The Swedish Society of Medicine, The King Gustaf V 80th Birthday Foundation, KI grants, The Cancer and Allergy Foundation, The Pediatric Research Foundation at Astrid Lindgren Children’s Hospital, The Samaritan Foundation for Pediatric research, Barnestiftelsen at Oslo University Hospital, Roche, The Frithjof Nansen Institute.
Institutional Review Board Statement
Informed Consent Statement
Informed written consent was collected from all mothers at enrolment, and from parent(s) at infant inclusion.
Data Availability Statement
Participants of this study were not asked to consent for open access data from third parties.
Acknowledgments
We sincerely thank all families participating in the PreventADALL study and the study personnel contributing in enrolling, performing lung function measurements and managing the study: Hilde Aaneland, Samina Asad, Anna Asarnoj, Ann Berglind, Jessica Björk, Maria Bradley, Oda C. Lødrup Carlsen, Åshild Wik Despriée, Kim M. A. Endre, Thea Aspelund Fatnes, Peder A. Granlund, Malén Gudbrandsgard, Sandra Götberg, Gunilla Hedlin, Mari Rønning Kjendsli, Ina Kreyberg, Linn Landro, Caroline-Aleksi Olsson Mägi, Nora Nilsson, Monika Nordenbrand, Live S. Nordhagen, Carina M. Saunders, Kajsa Sedergren, Natasha Sedergren, Päivi Söderman, Liv Julie Sørdal, Sandra Ganrud Tedner, Ellen Tegnerud, Magdalena R. Værnesbranden, Johanna Wiik and in memoriam Kai-Håkon Carlsen.
Conflicts of Interest
The authors have no financial relationships relevant to this article to disclose. Eva Maria Rehbinder has received honoraria for lectures from Sanofi Genzyme, Leo Pharma, Novartis, Norwegian Psoriasis and Eczema Association, Norwegian Asthma and Allergy Association and Karin C. Lødrup Carlsen reports that her institution has received honorarium and travel costs from Thermo Fisher Scientific for international symposium participation.
References
- Fuchs, O.; Bahmer, T.; Rabe, K.F.; von Mutius, E. Asthma transition from childhood into adulthood. Lancet Respir. Med. 2017, 5, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Scherzer, R.; Grayson, M.H. Heterogeneity and the origins of asthma. Ann. Allergy Asthma Immunol. 2018, 121, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Teach, S.J. Asthma. Pediatr. Rev. 2019, 40, 549–567. [Google Scholar] [CrossRef]
- Lau, S.; Matricardi, P.M.; Wahn, U.; Lee, Y.A.; Keil, T. Allergy and atopy from infancy to adulthood: Messages from the German birth cohort MAS. Ann. Allergy Asthma Immunol. 2019, 122, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in asthma: New understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441. [Google Scholar] [CrossRef]
- Szefler, S.J.; Chipps, B. Challenges in the treatment of asthma in children and adolescents. Ann. Allergy Asthma Immunol. 2018, 120, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Poddighe, D.; Brambilla, I.; Licari, A.; Marseglia, G.L. Omalizumab in the Therapy of Pediatric Asthma. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Koppelman, G.H. Biologic Therapies for Severe Asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Subbarao, P.; Mandhane, P.J.; Sears, M.R. Asthma: Epidemiology, etiology and risk factors. Cmaj 2009, 181, E181–E190. [Google Scholar] [CrossRef]
- Duijts, L. Fetal and infant origins of asthma. Eur. J. Epidemiol. 2012, 27, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Bobolea, I.; Arismendi, E.; Valero, A.; Agustí, A. Early Life Origins of Asthma: A Review of Potential Effectors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Halken, S. Prevention of allergic disease in childhood: Clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2004, 15 (Suppl. S16), 4–5, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Hsu, C.K.; Akiyama, M.; Shimizu, H. Filaggrin: An emerging star in atopic march. J. Formos. Med. Assoc. Taiwan Yi Zhi 2008, 107, 429–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Van den Wijngaart, L.S.; Roukema, J.; Merkus, P.J. Respiratory disease and respiratory physiology: Putting lung function into perspective: Paediatric asthma. Respirology 2015, 20, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, A.; Sampath, V.; Nadeau, K.C. Early intervention of atopic dermatitis as a preventive strategy for progression of food allergy. Allergy Asthma Clin. Immunol. 2021, 17, 30. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Kusari, A.; Han, A.M.; Schairer, D.; Eichenfield, L.F. Atopic Dermatitis: New Developments. Dermatol. Clin. 2019, 37, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [Green Version]
- Wärnberg Gerdin, S.; Lie, A.; Asarnoj, A.; Borres, M.P.; Lødrup Carlsen, K.C.; Färdig, M.; Konradsen, J.R.; Monceyron Jonassen, C.; Olsson Mägi, C.A.; Rehbinder, E.M.; et al. Impaired skin barrier and allergic sensitization in early infancy. Allergy 2022, 77, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Boralevi, F.; Hubiche, T.; Léauté-Labrèze, C.; Saubusse, E.; Fayon, M.; Roul, S.; Maurice-Tison, S.; Taïeb, A. Epicutaneous aeroallergen sensitization in atopic dermatitis infants–determining the role of epidermal barrier impairment. Allergy 2008, 63, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kabashima, S.; Inoue, E.; Sasaki, T.; Niizeki, H.; Saito, H.; Matsumoto, K.; et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2016, 65, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Beck, L.A.; de Benedetto, A. Skin barrier defects in atopic dermatitis: From old idea to new opportunity. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2022, 71, 3–13. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Nagao, K.; Amagai, M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J. Clin. Investig. 2012, 122, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Marenholz, I.; Esparza-Gordillo, J.; Lee, Y.A. The genetics of the skin barrier in eczema and other allergic disorders. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 426–434. [Google Scholar] [CrossRef]
- Flohr, C.; England, K.; Radulovic, S.; McLean, W.H.; Campbel, L.E.; Barker, J.; Perkin, M.; Lack, G. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br. J. Dermatol. 2010, 163, 1333–1336. [Google Scholar] [CrossRef]
- Hoyer, A.; Rehbinder, E.M.; Färdig, M.; Asad, S.; Lødrup Carlsen, K.C.; Endre, K.M.A.; Granum, B.; Haugen, G.; Hedlin, G.; Monceyron Jonassen, C.; et al. Filaggrin mutations in relation to skin barrier and atopic dermatitis in early infancy. Br. J. Dermatol. 2022, 186, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 2008, 121, 1203–1209. [Google Scholar] [CrossRef]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenn, M.W.; Ellis, A.K. The clinical relevance of filaggrin mutations: Effect on allergic disease. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2016, 117, 483–489. [Google Scholar] [CrossRef]
- Palmer, C.N.; Ismail, T.; Lee, S.P.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Smith, F.J.; McLean, W.H.; Mukhopadhyay, S. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J. Allergy Clin. Immunol. 2007, 120, 64–68. [Google Scholar] [CrossRef]
- Haland, G.; Carlsen, K.C.; Sandvik, L.; Devulapalli, C.S.; Munthe-Kaas, M.C.; Pettersen, M.; Carlsen, K.H. Reduced lung function at birth and the risk of asthma at 10 years of age. N. Engl. J. Med. 2006, 355, 1682–1689. [Google Scholar] [CrossRef] [Green Version]
- Lodrup Carlsen, K.C.; Mowinckel, P.; Hovland, V.; Haland, G.; Riiser, A.; Carlsen, K.H. Lung function trajectories from birth through puberty reflect asthma phenotypes with allergic comorbidity. J. Allergy Clin. Immunol. 2014, 134, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Stocks, J.; Dezateux, C.A.; Jackson, E.A.; Hoo, A.F.; Costeloe, K.L.; Wade, A.M. Analysis of tidal breathing parameters in infancy: How variable is TPTEF:TE? Am. J. Respir. Crit. Care Med. 1994, 150, 1347–1354. [Google Scholar] [CrossRef]
- Anık, A.; Uysal, P. Impaired Lung Functions Using Tidal Breath Analysis in High-risk Infants with Recurrent Wheezing. TIP 2020, 1, 49–54. [Google Scholar] [CrossRef]
- Beydon, N.; Davis, S.D.; Lombardi, E.; Allen, J.L.; Arets, H.G.; Aurora, P.; Bisgaard, H.; Davis, G.M.; Ducharme, F.M.; Eigen, H.; et al. An official American Thoracic Society/European Respiratory Society statement: Pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 2007, 175, 1304–1345. [Google Scholar] [CrossRef] [Green Version]
- Lødrup Carlsen, K.C.; Rehbinder, E.M.; Skjerven, H.O.; Carlsen, M.H.; Fatnes, T.A.; Fugelli, P.; Granum, B.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; et al. Preventing Atopic Dermatitis and ALLergies in Children-the PreventADALL study. Allergy 2018, 73, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Bains, K.E.S.; Gudmundsdóttir, H.K.; Färdig, M.; Amnö, E.; Jonassen, C.M.; Nordlund, B.; Rehbinder, E.M.; Skjerven, H.O.; Rueegg, C.S.; Vettukattil, R.; et al. Infant lung function: Criteria for selecting tidal flow-volume loops. ERJ Open Res. 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Berents, T.L.; Carlsen, K.C.; Mowinckel, P.; Skjerven, H.O.; Kvenshagen, B.; Rolfsjord, L.B.; Bradley, M.; Lieden, A.; Carlsen, K.H.; Gaustad, P.; et al. Skin Barrier Function and Staphylococcus aureus Colonization in Vestibulum Nasi and Fauces in Healthy Infants and Infants with Eczema: A Population-Based Cohort Study. PloS ONE 2015, 10, e0130145. [Google Scholar] [CrossRef] [Green Version]
- Rehbinder, E.M.; Advocaat Endre, K.M.; Lødrup Carlsen, K.C.; Asarnoj, A.; Stensby Bains, K.E.; Berents, T.L.; Carlsen, K.H.; Gudmundsdóttir, H.K.; Haugen, G.; Hedlin, G.; et al. Predicting Skin Barrier Dysfunction and Atopic Dermatitis in Early Infancy. J. Allergy Clin. Immunol. Pract. 2020, 8, 664–673. [Google Scholar] [CrossRef]
- Lodrup Carlsen, K.C.; Carlsen, K.H.; Nafstad, P.; Bakketeig, L. Perinatal risk factors for recurrent wheeze in early life. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 1999, 10, 89–95. [Google Scholar] [CrossRef]
- Hevroni, A.; Goldman, A.; Blank-Brachfeld, M.; Abu Ahmad, W.; Ben-Dov, L.; Springer, C. Use of tidal breathing curves for evaluating expiratory airway obstruction in infants. J. Asthma 2018, 55, 1331–1337. [Google Scholar] [CrossRef]
- Morris, M.J.; Lane, D.J. Tidal expiratory flow patterns in airflow obstruction. Thorax 1981, 36, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endre, K.M.A.; Landrø, L.; LeBlanc, M.; Gjersvik, P.; Lødrup Carlsen, K.C.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; Nordlund, B.; Rudi, K.; et al. Diagnosing atopic dermatitis in infancy using established diagnostic criteria: A cohort study. Br. J. Dermatol. 2022, 186, 50–58. [Google Scholar] [CrossRef]
- Löffler, H.; Effendy, I. Skin susceptibility of atopic individuals. Contact Dermat. 1999, 40, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Nijsten, T.; van Meel, E.R.; Erler, N.S.; Piketty, C.; de Jong, N.W.; Pasmans, S.; de Jongste, J.C.; Duijts, L. Eczema phenotypes and risk of allergic and respiratory conditions in school age children. Clin. Transl. Allergy 2020, 10, 7. [Google Scholar] [CrossRef]
- Håland, G.; Carlsen, K.H.; Devulapalli, C.S.; Pettersen, M.; Mowinckel, P.; Lødrup Carlsen, K.C. Lung function development in the first 2 yr of life is independent of allergic diseases by 2 yr. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2007, 18, 528–534. [Google Scholar] [CrossRef]
- Brenninkmeijer, E.E.; Schram, M.E.; Leeflang, M.M.; Bos, J.D.; Spuls, P.I. Diagnostic criteria for atopic dermatitis: A systematic review. Br. J. Dermatol. 2008, 158, 754–765. [Google Scholar] [CrossRef]
- Amat, F.; Soria, A.; Tallon, P.; Bourgoin-Heck, M.; Lambert, N.; Deschildre, A.; Just, J. New insights into the phenotypes of atopic dermatitis linked with allergies and asthma in children: An overview. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Basu, K.; Inglis, S.K.; Bremner, S.A.; Ramsay, R.; Abd, A.; Rabe, H.; Strange, E.; Phillips, V.; Seddon, P.; Tavendale, R.; et al. Filaggrin gene defects are associated with eczema, wheeze, and nasal disease during infancy: Prospective study. J. Allergy Clin. Immunol. 2020, 146, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Albang, R.; Maier, D.; Duran-Tauleria, E.; Mena, G.; Gimeno-Santos, E.; Sola, I.; Garcia-Aymerich, J.; Guerra, S.; Stein, R.T.; et al. Systematic Review on the Definition of Allergic Diseases in Children: The MeDALL Study. Int. Arch. Allergy Immunol. 2015, 168, 110–121. [Google Scholar] [CrossRef]
- Magnusson, L.L.; Olesen, A.B.; Wennborg, H.; Olsen, J. Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin. Exp. Allergy: J. Br. Soc. Allergy Clin. Immunol. 2005, 35, 1550–1556. [Google Scholar] [CrossRef] [Green Version]
- Just, J.; Bourgoin-Heck, M.; Amat, F. Clinical phenotypes in asthma during childhood. Clin. Exp. Allergy: J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 848–855. [Google Scholar] [CrossRef]
- Den Dekker, H.T.; Sonnenschein-van der Voort, A.M.M.; de Jongste, J.C.; Anessi-Maesano, I.; Arshad, S.H.; Barros, H.; Beardsmore, C.S.; Bisgaard, H.; Phar, S.C.; Craig, L.; et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J. Allergy Clin. Immunol. 2016, 137, 1026–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byberg, K.K.; Eide, G.E.; Forman, M.R.; Júlíusson, P.B.; Øymar, K. Body mass index and physical activity in early childhood are associated with atopic sensitization, atopic dermatitis and asthma in later childhood. Clin. Transl. Allergy 2016, 6, 33. [Google Scholar] [CrossRef]
- Melen, E.; Guerra, S. Recent advances in understanding lung function development. F1000Research 2017, 6, 726. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).