Management of Choanal Atresia: National Recommendations with a Comprehensive Literature Review
Abstract
:1. Causes of Nasal Obstruction in an Infant
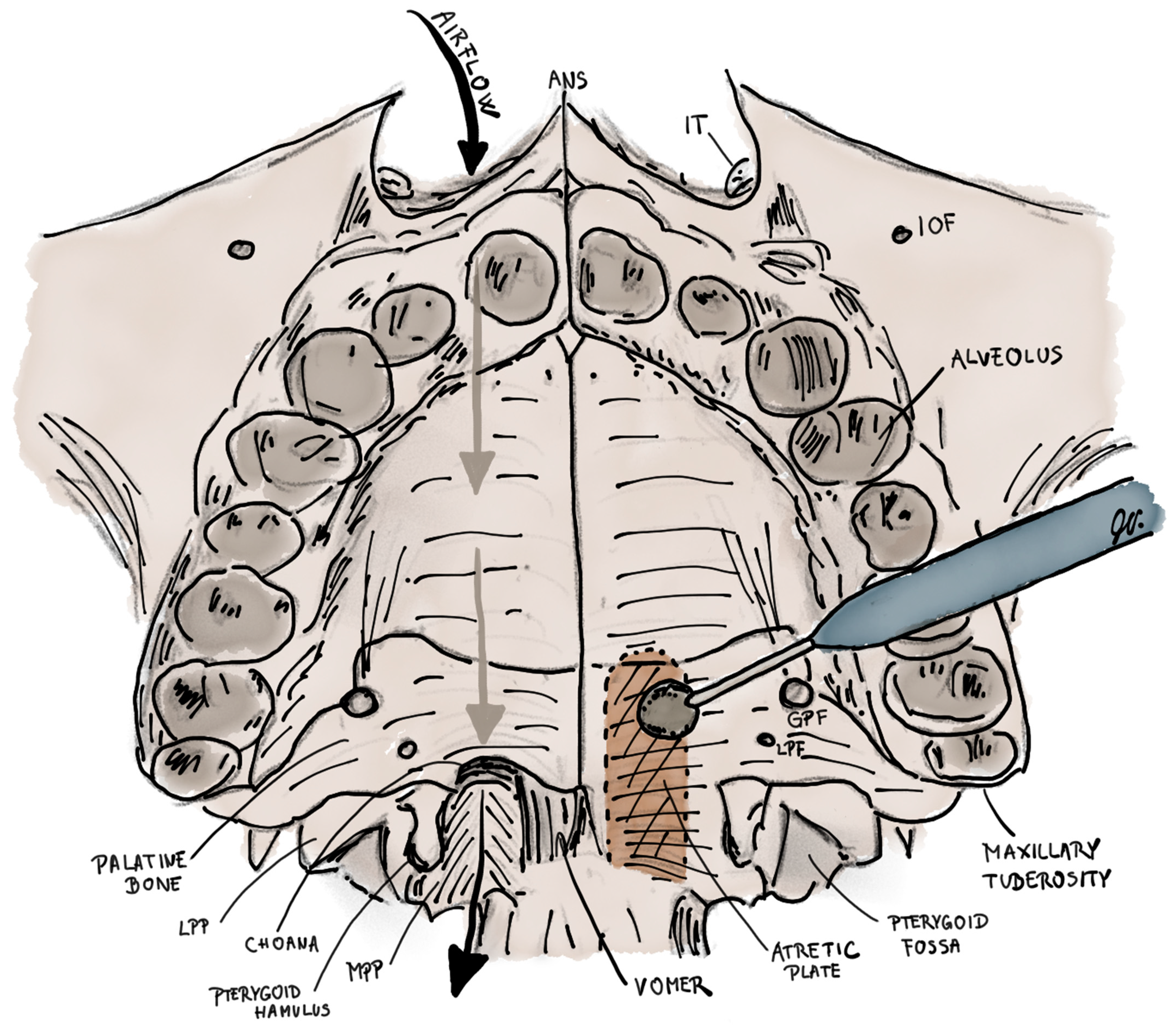
2. Epidemiology, Pathogenesis and Anatomical Characteristics of Choanal Atresia
3. The Clinical Picture of Choanal Atresia
3.1. Bilateral Choanal Atresia
3.2. Unilateral Choanal Atresia
4. Materials and Methods
5. Results
5.1. Diagnosis of Choanal Atresia
5.1.1. Bilateral Choanal Atresia
5.1.2. Unilateral Choanal Atresia
5.2. Treatment
5.2.1. Bilateral Choanal Atresia
Airway Management
Surgical Treatment
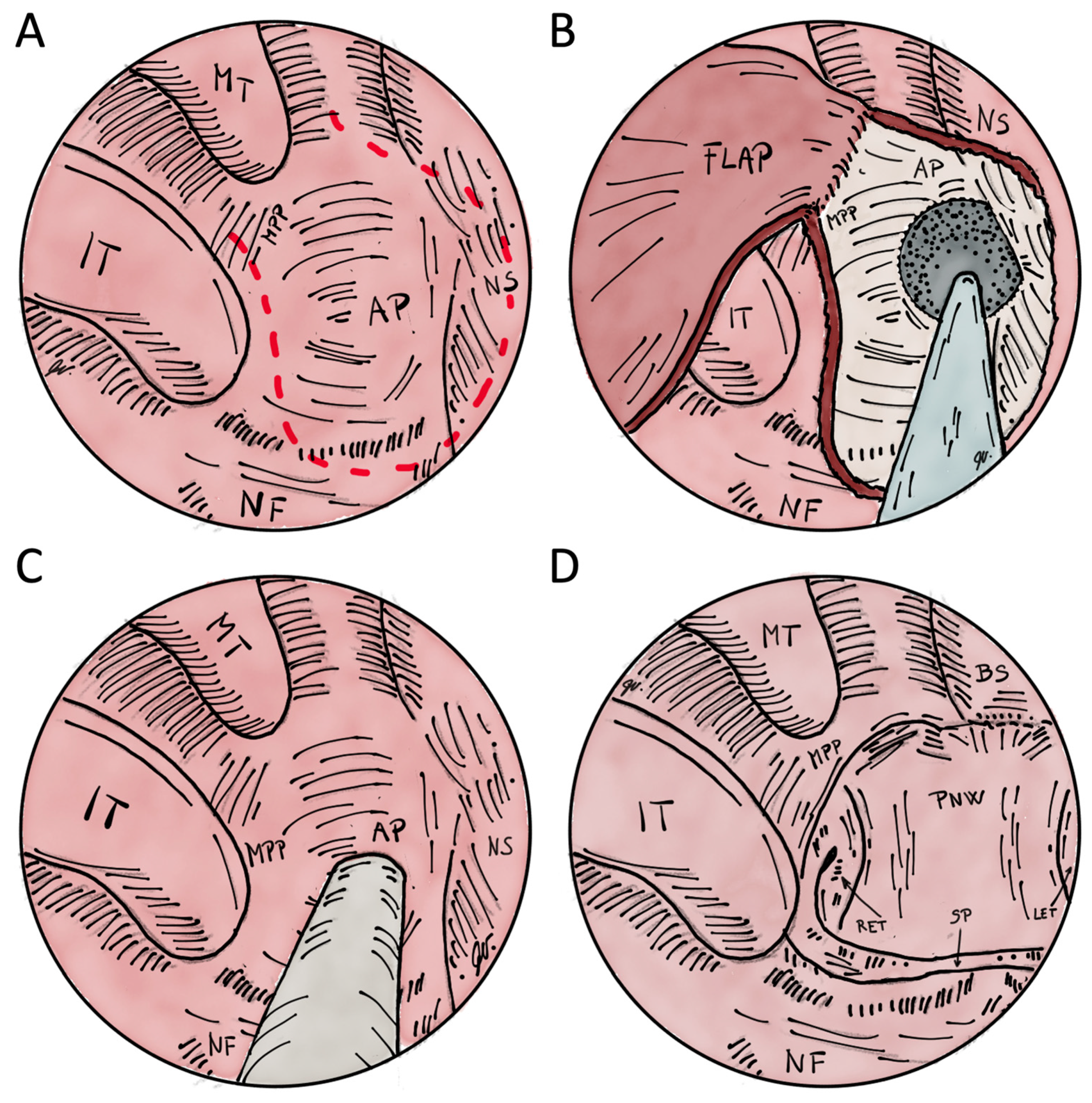
Transnasal Endoscopic Choanoplasty
Transnasal Perforation
Transnasal Endoscopically Assisted Perforation
Transpalatal Resection
5.2.2. Unilateral Choanal Atresia
5.3. Postoperative Management
5.3.1. Bilateral Choanal Atresia
Stented
Non-Stented
5.3.2. Unilateral Choanal Atresia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajan, R.; Tunkel, D.E. Choanal Atresia and Other Neonatal Nasal Anomalies. Clin. Perinatol. 2018, 45, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Roehm, C.E.; Lawrason, A.; Valdez, T.A. Nasal Obstruction in Newborn. In Encyclopedia of Otolaryngology, Head and Neck Surgery; Kountakis, S.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1746–1757. [Google Scholar]
- Gnagi, S.H.; Schraff, S.A. Nasal Obstruction in Newborns. Pediatr. Clin. 2013, 60, 903–922. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.M. Current Updates on Choanal Atresia. Front. Pediatr. 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, J.R.; Harmon, P.; Shirley, W.P.; Hill, J.S.; Woolley, A.L.; Wiatrak, B.J. Operative Management of Choanal Atresia: A 15-Year Experience. JAMA Otolaryngol. –Head Neck Surg. 2013, 139, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiji, I.; Kalezi, Z.E.; Abdulshakoor, A.; Tarimo, J.F.; Leiya, R.; Zuechner, A.; Naburi, H.E.; Massawe, A.; Manji, K.P. Pfeiffer Syndrome Type 2; A Case Report of Cranio-Orbitofaciostenosis with Bilateral Choanal Atresia at Muhimbili National Hospital, Tanzania. Clin. Case Rep. 2020, 8, 1613–1617. [Google Scholar] [CrossRef]
- Childs, A.J.; Mabin, D.C.; Turnpenny, P.D. Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome Presenting with Bilateral Choanal Atresia and Rectal Stenosis. Am. J. Med. Genet. Part A 2020, 182, 1939–1943. [Google Scholar] [CrossRef]
- Kita, M.; Kuwata, Y.; Usui, T. Familial Congenital Choanal Atresia with GATA3 Associated Hypoparathyroidism-Deafness-Renal Dysplasia Syndrome Unidentified on Auditory Brainstem Response. Auris Nasus Larynx 2019, 46, 808–812. [Google Scholar] [CrossRef]
- Sung, J.Y.; Cho, K.-S.; Bae, Y.C.; Bae, S.H. Image-Guided Navigation Surgery for Bilateral Choanal Atresia with a Tessier Number 3 Facial Cleft in an Adult. Arch. Craniofacial Surg. 2020, 21, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Hengerer, A.S.; Brickman, T.M.; Jeyakumar, A. Choanal Atresia: Embryologic Analysis and Evolution of Treatment, a 30-Year Experience. Laryngoscope 2008, 118, 862–866. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Shiffman, R.N.; Robertson, P. Clinical Practice Guideline Development Manual, Third Edition: A Quality-Driven Approach for Translating Evidence into Action. Otolaryngol. –Head Neck Surg. 2013, 148, S1–S55. [Google Scholar] [CrossRef]
- Gersak, K.; Fras, Z.; Rems, M. Do We Know What Makes a Good Clinical Guideline? Zdr. Vestn. 2016, 85, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Moreddu, E.; Rizzi, M.; Adil, E.; Balakrishnan, K.; Chan, K.; Cheng, A.; Daniel, S.J.; de Alarcon, A.; Hart, C.; Hartnick, C.; et al. International Pediatric Otolaryngology Group (IPOG) Consensus Recommendations: Diagnosis, Pre-Operative, Operative and Post-Operative Pediatric Choanal Atresia Care. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Cedin, A.C.; Atallah, Á.N.; Andriolo, R.B.; Cruz, O.L.; Pignatari, S. Surgery for Congenital Choanal Atresia. Cochrane Database Syst. Rev. 2012, CD008993. [Google Scholar] [CrossRef] [PubMed]
- El-Begermy, M.M.; Samir, M.M.; Fawaz, S.A.; Elkelany, W.G. Effect of the Type of Surgery, Use of Intraoperative Topical Mitomycin C or Stenting on the Outcome of Choanal Atresia Repair: A Systematic Review and Meta-Analysis. Egypt. J. Otolaryngol. 2016, 32, 255–263. [Google Scholar] [CrossRef]
- Murray, S.; Luo, L.; Quimby, A.; Barrowman, N.; Vaccani, J.-P.; Caulley, L. Immediate versus Delayed Surgery in Congenital Choanal Atresia: A Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2019, 119, 47–53. [Google Scholar] [CrossRef]
- Bartel, R.; Levorato, M.; Adroher, M.; Cardelus, S.; Diaz, A.; Lacima, J.; Vazquez, C.; Veneri, A.; Wienberg, P.; Claveria, M.A.; et al. Performance of Endoscopic Repair with Endonasal Flaps for Congenital Choanal Atresia. A Systematic Review. Acta Otorrinolaringol. 2021, 72, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Strychowsky, J.E.; Kawai, K.; Moritz, E.; Rahbar, R.; Adil, E.A. To Stent or Not to Stent? A Meta-Analysis of Endonasal Congenital Bilateral Choanal Atresia Repair. Laryngoscope 2016, 126, 218–227. [Google Scholar] [CrossRef]
- Durmaz, A.; Tosun, F.; Yldrm, N.; Sahan, M.; Kvrakdal, C.; Gerek, M. Transnasal Endoscopic Repair of Choanal Atresia: Results of 13 Cases and Meta-Analysis. J. Craniofacial Surg. 2008, 19, 1270–1274. [Google Scholar] [CrossRef]
- Alsubaie, H.M.; Almosa, W.H.; Al-Qahtani, A.S.; Margalani, O. Choanal Atresia Repair with Stents and Flaps: A Systematic Review Article. Allergy Rhinol. 2021, 12, 21526567211058052. [Google Scholar] [CrossRef]
- Gundle, L.; Ojha, S.; Hendry, J.; Rosen, H. Stenting versus Stentless Repair for Bilateral Choanal Atresia: A Systematic Review of the Literature. Int. J. Pediatr. Otorhinolaryngol. 2021, 151, 110926. [Google Scholar] [CrossRef]
- Tomoum, M.O.; Askar, M.H.; Mandour, M.F.; Amer, M.A.; Saafan, M.E. Stentless Mirrored L-Shaped Septonasal Flap versus Stented Flapless Technique for Endoscopic Endonasal Repair of Bilateral Congenital Choanal Atresia: A Prospective Randomised Controlled Study. J. Laryngol. Otol. 2018, 132, 329–335. [Google Scholar] [CrossRef]
- Saafan, M.E. Endoscopic Management of Congenital Bilateral Posterior Choanal Atresia: Value of Using Stents. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Al-Ammar, A. Effect of Use of Mitomycin C on the Outcome of Choanal Atresia Repair. Saudi Med. J. 2007, 28, 1537–1540. [Google Scholar] [PubMed]
- ElSalmawy, A.; Abdelfattah, G.; Farag, T.; Farahat, A. Role of Proton Pump Inhibitor in Healing after Choanal Atresia Repair: A Randomized Control Trial. Egypt. J. Otolaryngol. 2022, 38, 44. [Google Scholar] [CrossRef]
- Hand, I.L.; Shellhaas, R.A.; Milla, S.S. Routine Neuroimaging of the Preterm Brain. Pediatrics 2020, 146, e2020029082. [Google Scholar] [CrossRef]
- Marston, A.P.; Patel, T.; Nguyen, S.A.; White, D.R. Short-Term Risk Factor Profile of Pediatric Choanal Atresia Repair Using ACS-NSQIP National Database. Ann. Otol. Rhinol. Laryngol. 2019, 128, 855–861. [Google Scholar] [CrossRef]
- Zoizner-Agar, G.; Rotsides, J.M.; Shao, Q.; Rickert, S.; Ward, R.; Greifer, M.; April, M. Proton Pump Inhibitor Administration in Neonates and Infants. Lack of Consensus—An ASPO Survey. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110200. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence Working Group* Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 18 March 2021).
- WHO Reproductive Health Library. WHO Recommendations on Newborn Health: Guidelines Approved by the WHO Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mason, K.; Royan, D.; Daya, H.; Pepper, C.; Tweedie, D. Use of a Modified Stethoscope to Assess Paediatric Nasal Airflow in Suspected Choanal Atresia, Nasal Stents or Nasopharyngeal Airways. Clin. Otolaryngol. 2020, 45, 654–655. [Google Scholar] [CrossRef] [Green Version]
- Elsheikh, E.; El-Anwar, M.W. False Computed Tomography Findings in Bilateral Choanal Atresia. Int. Arch. Otorhinolaryngol. 2016, 20, 163–165. [Google Scholar] [CrossRef] [Green Version]
- Wineland, A.; Menezes, M.D.; Shimony, J.S.; Shinawi, M.S.; Hullar, T.E.; Hirose, K. Prevalence of Semicircular Canal Hypoplasia in Patients with CHARGE Syndrome: 3C Syndrome. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 168–177. [Google Scholar] [CrossRef]
- Walsh, J.; Rastatter, J. Neonatal Tracheostomy. Clin. Perinatol. 2018, 45, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Stavrakas, M.; Ray, J. Lasers in Rhinology—An Update. Ear Nose Throat J. 2020, 100, 77S–82S. [Google Scholar] [CrossRef] [PubMed]
- De Vincentiis, G.C.; Panatta, M.L.; De Corso, E.; Marini, G.; Bianchi, A.; Giuliani, M.; Sitzia, E.; Tucci, F.M. Endoscopic Treatment of Choanal Atresia and Use of Balloon Dilation: Our Experience. Acta Otorhinolaryngol. Ital. 2020, 40, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L.J.; Smith, M.M.; de Alarcon, A.; Epperson, M.; Born, H.; Hart, C.K. Use of Steroid-Eluting Stents after Endoscopic Repair of Choanal Atresia: A Case Series with Review. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1003–1010. [Google Scholar] [CrossRef]
- Patel, V.A.; Ramadan, J.; Carr, M.M. Congenital Choanal Atresia Repair: An Analysis of Adverse Perioperative Events. Otolaryngol. Head Neck Surg. 2018, 159, 920–926. [Google Scholar] [CrossRef] [PubMed]






| Congenital tumours | ● Neoplasms: chordoma, teratoma, haemangioma, rhabdomyosarcoma, craniopharyngioma, hamartoma, histiocytoma, hemangiopericytoma, lipoma, vascular malformation, congenital squamous cell carcinoma, teratoid, lymphoma ● Cysts: dacryocystocele, incisive canal cyst, dentigerous cyst, mucocele, Thornwaldt’s cyst ● Midline masses: nasal dermoid, glioma, meningoencephalocele, encephalocele |
| Congenital anatomical abnormalities | ● Nasal agenesis or partial dysgenesis ● Anterior nasal stenosis ● Midline nasal stenosis ● Pyriform aperture stenosis ● Choanal atresia/stenosis ● Craniofacial syndromes: CHARGE, Binder, Crouzon, Apert, Treacher-Collins, Pfeiffer, Kabuki |
| Traumatic | ● Nasal tip depression ● Iatrogenic stenosis ● Nasal septum fracture/dislocation ● Nasal septum haematoma/abscess ● Foreign body |
| Inflammatory | ● Rhinitis medicamentosa: mother’s or infant’s medications ● Neonatal rhinitis: meconium, allergy, GER, nasal milk regurgitation, infections (Staphylococcus aureus, Chlamydia trachomatis, Treponema pallidum, RSV etc.) |
| Metabolic diseases | ● Hypothyroidism |
| Nr | Recommendation | L | Gr | Strength |
|---|---|---|---|---|
| 1,2 | In the case of respiratory distress in a neonate, a saline test should be performed to confirm nasal obstruction, followed by a test with an aspiration catheter of size Fr 6–8 [13] | IIa | C | Recommended |
| 3 | The otorhinolaryngological examination should include examination of the nose and face, anterior rhinoscopy, toilet, anemization, epimucosal anaesthesia of the nose and examination with a flexible or rigid endoscope [13]. | I | A | Strongly recommended |
| 4 | In the case of bilateral CA, CT of the facial structures, nose, paranasal cavities and skull base should be performed with a slice thickness of 2–5 mm [4,13] | I | A | Strongly recommended |
| 5 | Head ultrasound should be performed for bilateral CA [26] | I | C | Strongly recommended |
| 9 | In the case of unilateral CA, a CT of the facial structures, nose, paranasal cavities, and skull base should be performed with a slice thickness of 2–5 mm [13] | IIa | D | Recommended |
| 6, 7 | In the case of bilateral CA, congenital abnormalities should be ruled out, so a clinical geneticist and a cardiologist should be involved because of heart diseases that would put the child at risk during the procedure [13] | I | B | Strongly recommended |
| 8, 10 | In the case of unilateral or bilateral CA, an audiovestibulological evaluation should be performed to assess hearing and treatment by a phoniatrician in case of additional swallowing problems [13] | I | D | Strongly recommended |
| 11 | Bilateral CA should be surgically treated between the 10th and 13th day of the neonate’s age, even in the case of prematurity (Murray et al. 2019; Marston et al. 2019) [16,27] | I | A | Strongly recommended |
| 12 | Transnasal endoscopic choanoplasty should be performed in all patients with bilateral CA in whom an endoscopic approach is possible [13] | I | A | Strongly recommended |
| 13 | If a stent is used, it should be removed within seven days [18] | IIa | A | Recommended |
| 14 | In case of associated craniofacial abnormalities, neuronavigation should be used [4,9] | IIa | C | Recommended |
| 15 | Transnasal perforation with stent insertion can be performed in patients with thin bony-membranous bilateral CA or stenosis and in patients in whom transnasal endoscopically assisted perforation is not possible due to insufficient nasal lumen [4] | IIb | D | Optional |
| 16 | Transnasal endoscopically assisted perforation with stent insertion can be performed when transnasal endoscopic choanoplasty of bilateral CA is not possible due to anatomical conditions [4,13] | IIb | C | Optional |
| 17 | Transpalatal resection should not be the primary method of treatment for unilateral or bilateral CA [4,13] | III | A | Recommended against |
| 18 | Surgical treatment of unilateral CA should be performed after the baby is six months old [13] | IIa | A | Recommended |
| 19 | Transnasal endoscopic choanoplasty should be performed in unilateral CA. The extent of the resection should be large enough so that it is not necessary to insert a stent [13] | I | A | Strongly recommended |
| 20 | In the case of bilateral CA, where stents were used for a nasal passage, they should be changed endoscopically several times a week in the first and second postoperative weeks * | IIa | D | Recommended |
| 21 | Discharge from the hospital should be planned for the second postoperative week or as soon as possible after surgical treatment of bilateral CA * | IIa | D | Recommended |
| 22, 23 | In the case of bilateral CA, stents can be changed on an outpatient basis in the second postoperative week. They can be changed twice in the third week and once from the fourth week onwards * | IIb | D | Optional |
| 24 | In the case of bilateral CA, the stent should be removed in the fourth postoperative week, and nasal patency should be reevaluated a week later * | I | D | Strongly recommended |
| 25 | Photo documentation of the lumen of the choana should be done for unilateral or bilateral CA * | IIa | D | Recommended |
| 26 | A child with bilateral CA should be monitored for at least two years after the procedure or until the end of growth * | I | C | Strongly recommended |
| 27 | In case of problems with nasal patency and more than 50% reduction of the choanal lumen, a revision transnasal endoscopic choanoplasty should be performed [13] | IIa | A | Recommended |
| 28 | A proton pump inhibitor should be used daily for the first two months after unilateral or bilateral CA surgery [25] | IIa | C | Recommended |
| 29 | During the postoperative treatment of the infant, drops of saline solution and nasal glucocorticoid with low systemic absorption should be used daily intranasally for the first two months [13,28] | I | C | Strongly recommended |
| 30 | Antibiotic intranasal drops should be used exceptionally in the first postoperative week or later when noticeable purulent discharge appears * | IIa | D | Recommended |
| 31 | The first postoperative examination with assessment of nasal breathing with the mouth closed and feeding by mouth without pauses for breathing should be performed on the first postoperative day of bilateral CA treatment * | IIa | D | Recommended |
| 32 | On the second postoperative day of treatment for bilateral CA, discharge from the hospital can be planned * | IIb | D | Optional |
| 33 | Endoscopy should be performed one week after surgery, two weeks after surgery, and then four weeks after surgery for bilateral CA * | IIa | D | Recommended |
| 34 | Discharge from the hospital can be planned on the first postoperative day of treatment for unilateral CA * | IIa | D | Recommended |
| Step of Literature Review: Article Types | Database, a Search Query |
|---|---|
| 1. step: systematic reviews, meta-analyses, clinical guidelines, recommendations | Cochrane Library: choan* AND atresia in Title Abstract Keyword–(Word variations have been searched) |
| Web of Science: TI = (choan* AND atresia) AND (TI = ((systematic review) OR (meta*) ) OR TS = ((systematic review) OR meta* OR recommend* OR guideline*)) | |
| Scopus: (TITLE(choan* and atresia) AND TITLE-ABS-KEY((systematic AND review) OR (meta*) OR recommend* OR guideline*)) | |
| Pubmed: (choan*[Title] AND atresia[Title]) AND ((systematic review)[Title/Abstract] OR meta*[Title/Abstract] OR recommend *[Title/Abstract] OR guideline*[Title/Abstract]) | |
| 2. step: randomized controlled clinical studies | Web of Science: TI = (choan* AND atresia) AND TI = (randomi*) |
| Pubmed: (choan*[Title] AND atresia[Title]) AND (randomi*[Title/Abstract]) | |
| Scopus: (TITLE(choan* AND atresia) AND TITLE-ABS-KEY(randomi*)) | |
| Literature review from systematic reviews, meta-analyses, clinical guidelines and recommendations included after 1st step of the literature review | |
| 3. step: other types of clinical articles | Literature review from studies included after the 1st and 2nd steps of the literature review. |
| Evidence Grade | Type of Studies | The Preponderance of Benefit or Harm | Balance of Benefit and Harm |
|---|---|---|---|
| A (high-quality) | the data are derived from consistent level 1 studies | Strongly recommended | Optional |
| B (moderate quality) | the data are derived from consistent level 2 or 3 studies or extrapolations from level 1 studies | Recommended or strongly recommended | Optional |
| C (low quality) | the data are derived from level 4 studies or extrapolations from level 2 or 3 studies | Recommended | Optional |
| D (very low quality) | the data are derived from level 5 evidence or troublingly inconsistent or inconclusive studies of any level | Optional | No recommendation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbančič, J.; Vozel, D.; Battelino, S.; Boršoš, I.; Bregant, L.; Glavan, M.; Iglič, Č.; Jenko, K.; Lanišnik, B.; Soklič Košak, T. Management of Choanal Atresia: National Recommendations with a Comprehensive Literature Review. Children 2023, 10, 91. https://doi.org/10.3390/children10010091
Urbančič J, Vozel D, Battelino S, Boršoš I, Bregant L, Glavan M, Iglič Č, Jenko K, Lanišnik B, Soklič Košak T. Management of Choanal Atresia: National Recommendations with a Comprehensive Literature Review. Children. 2023; 10(1):91. https://doi.org/10.3390/children10010091
Chicago/Turabian StyleUrbančič, Jure, Domen Vozel, Saba Battelino, Imre Boršoš, Lev Bregant, Matic Glavan, Črtomir Iglič, Klemen Jenko, Boštjan Lanišnik, and Tanja Soklič Košak. 2023. "Management of Choanal Atresia: National Recommendations with a Comprehensive Literature Review" Children 10, no. 1: 91. https://doi.org/10.3390/children10010091
APA StyleUrbančič, J., Vozel, D., Battelino, S., Boršoš, I., Bregant, L., Glavan, M., Iglič, Č., Jenko, K., Lanišnik, B., & Soklič Košak, T. (2023). Management of Choanal Atresia: National Recommendations with a Comprehensive Literature Review. Children, 10(1), 91. https://doi.org/10.3390/children10010091







