Effect of Clemastine on Neurophysiological Outcomes in an Ovine Model of Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Umbilical Cord Occlusion Model
2.3. Drug Treatment and Pharmacokinetic Analysis
2.4. Neurobehavioral Outcomes
2.5. Biochemical Markers of Inflammation
2.6. Power Analysis, Sample Size Calculation, Attrition Rate, Blinding, and Randomization
2.7. Statistical Analysis
3. Results
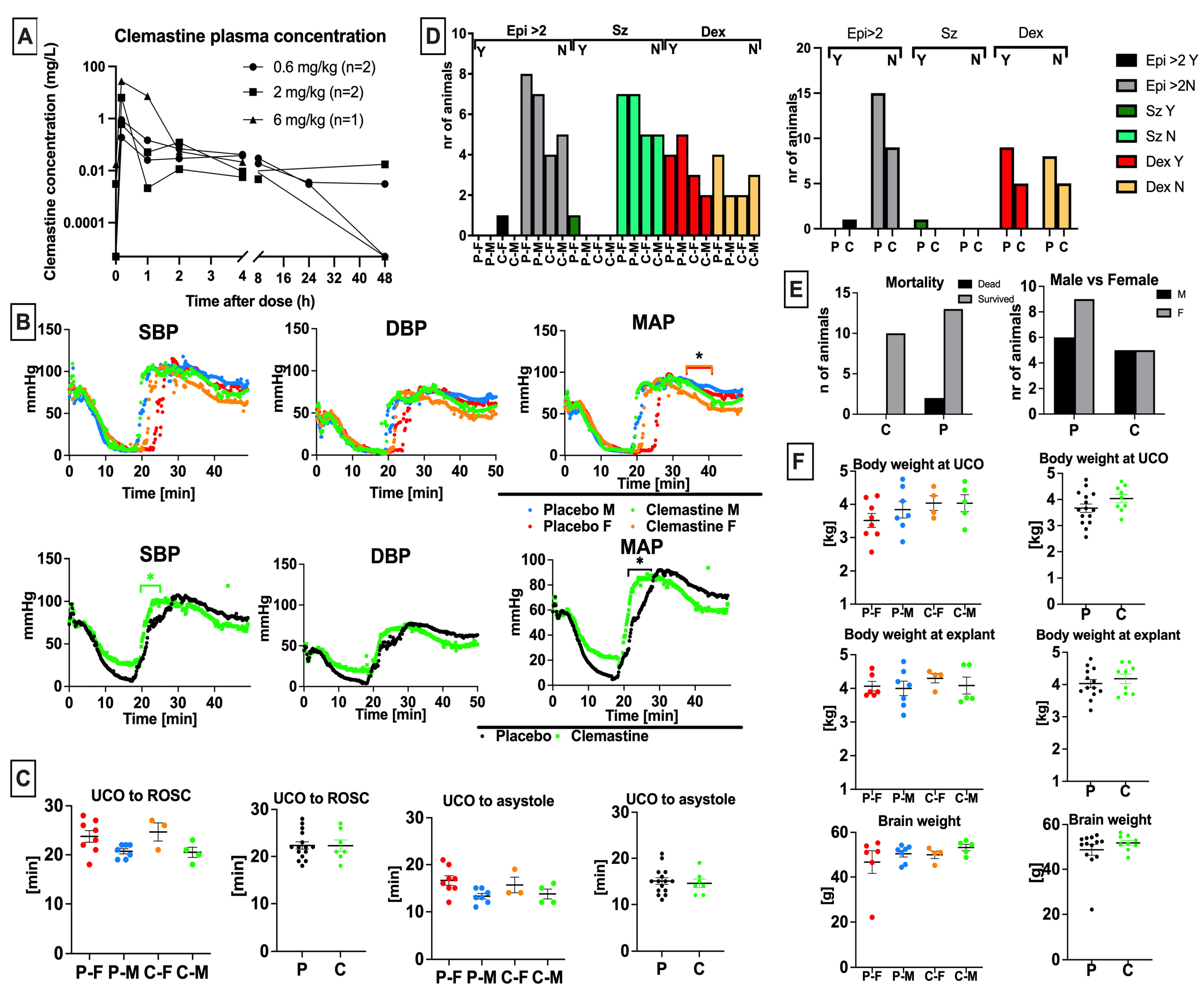
3.1. Clemastine Pharmacokinetics and Dose Selection
3.2. Clemastine Safety and Toxicity
3.3. Clemastine Effect on Biochemical Markers
3.4. Clemastine Effect on Peripheral Blood Cells and Inflammatory Indices
3.5. Clemastine Effect on Neurological Outcomes
3.6. Markers Associated with Total Neurological Outcomes Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Silverman, A.J.; Vannucci, S.J. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 2009, 40, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Ziemka-Nalecz, M.; Jaworska, J.; Zalewska, T. Insights Into the Neuroinflammatory Responses after Neonatal Hypoxia-Ischemia. J. Neuropathol. Exp. Neurol. 2017, 76, 644–654. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, R.; Li, G. Drug repurposing: Clemastine fumarate and neurodegeneration. Biomed. Pharmacother. 2023, 157, 113904. [Google Scholar] [CrossRef]
- Qian, H.; Shu, C.; Xiao, L.; Wang, G. Histamine and histamine receptors: Roles in major depressive disorder. Front. Psychiatry 2022, 13, 825591. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Schrage, R.; Mohr, K. Allosteric modulators targeting CNS muscarinic receptors. Neuropharmacology 2018, 136 Pt C, 427–437. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Bernardo, A.; Magnaghi, V.; Minghetti, L.; Tata, A.M. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev. Neurobiol. 2012, 72, 713–728. [Google Scholar] [CrossRef]
- Scarr, E. Muscarinic receptors: Their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci. Ther. 2012, 18, 369–379. [Google Scholar] [CrossRef]
- Radu, B.M.; Osculati AM, M.; Suku, E.; Banciu, A.; Tsenov, G.; Merigo, F.; Di Chio, M.; Banciu, D.D.; Tognoli, C.; Kacer, P.; et al. All muscarinic acetylcholine receptors (M1-M5) are expressed in murine brain microvascular endothelium. Sci. Rep. 2017, 7, 5083. [Google Scholar] [CrossRef]
- Palma, A.; Chara, J.C.; Montilla, A.; Otxoa-de-Amezaga, A.; Ruíz-Jaén, F.; Planas, A.M.; Matute, C.; Pérez-Samartín, A.; Domercq, M. Clemastine Induces an Impairment in Developmental Myelination. Front. Cell Dev. Biol. 2022, 10, 841548. [Google Scholar] [CrossRef]
- Yadav, S.K.; Soin, D.; Ito, K.; Dhib-Jalbut, S. Insight into the mechanism of action of dimethyl fumarate in multiple sclerosis. J. Mol. Med. 2019, 97, 463–472. [Google Scholar] [CrossRef]
- Blair, H.A. Dimethyl Fumarate: A Review in Relapsing-Remitting MS. Drugs 2019, 79, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Mitchell, S.; Ciechanowicz, S.; Savage, S.; Wang, T.; Ji, X.; Ma, D. Argon protects against hypoxic-ischemic brain injury in neonatal rats through activation of nuclear factor (erythroid-derived 2)-like. Oncotarget 2016, 7, 25640–25651. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y.; Wei, X.; Fan, T. Neuroprotective effect of licochalcone A against oxygen-glucose deprivation/reperfusion in rat primary cortical neurons by attenuating oxidative stress injury and inflammatory response via the SIRT1/Nrf2 pathway. J. Cell. Biochem. 2018, 119, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, B.; Rampakakis, E.; Gilbert, G.; Fezoua, A.; Wintermark, P. Myelination may be impaired in neonates following birth asphyxia. Neuroimage Clin. 2021, 31, 102678. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Niu, J.; Hoi, K.K.; Zhao, C.; Caganap, S.D.; Henry, R.G.; Dao, D.Q.; Zollinger, D.R.; Mei, F.; Shen, Y.A.; et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain 2018, 141, 85–98. [Google Scholar] [CrossRef]
- Wittstatt, J.; Reiprich, S.; Küspert, M. Crazy Little Thing Called Sox-New Insights in Oligodendroglial Sox Protein Function. Int. J. Mol. Sci. 2019, 20, 2713. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.J.; Yang, N.; Chen, X.J.; Huang, N.X.; Zhang, J.; Wu, Y.; Liu, Z.; Gao, X.; Li, T.; et al. Enhancing Oligodendrocyte Myelination Rescues Synaptic Loss and Improves Functional Recovery after Chronic Hypoxia. Neuron 2018, 99, 689–701.e5. [Google Scholar] [CrossRef]
- Tong, L.Y.; Deng, Y.B.; Du, W.H.; Zhou, W.Z.; Liao, X.Y.; Jiang, X. Clemastine Promotes Differentiation of Oligodendrocyte Progenitor Cells through the Activation of ERK1/2 via Muscarinic Receptors after Spinal Cord Injury. Front. Pharmacol. 2022, 13, 914153. [Google Scholar] [CrossRef]
- Sabir, H.; Maes, E.; Zweyer, M.; Schleehuber, Y.; Imam, F.B.; Silverman, J.; White, Y.; Pang, R.; Pasca, A.M.; Robertson, N.J.; et al. Comparing the efficacy in reducing brain injury of different neuroprotective agents following neonatal hypoxia-ischemia in newborn rats: A multi-drug randomized controlled screening trial. Sci. Rep. 2023, 13, 9467. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Pan, T.T.; Xu, D.E.; Huang, X.; Tang, Y.; Huang, W.; Chen, R.; Lu, L.; Chi, H.; Ma, Q.H. Clemastine Ameliorates Myelin Deficits via Preventing Senescence of Oligodendrocytes Precursor Cells in Alzheimer’s Disease Model Mouse. Front. Cell Dev. Biol. 2021, 9, 733945. [Google Scholar] [CrossRef]
- Du, W.; Deng, Y.; Jiang, R.; Tong, L.; Li, R.; Jiang, X. Clemastine Enhances Myelination, Delays Axonal Loss and Promotes Functional Recovery in Spinal Cord Injury. Neurochem. Res. 2022, 47, 503–515. [Google Scholar] [CrossRef]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017, 390, 2481–2489. [Google Scholar] [CrossRef]
- Schran, H.F.; Petryk, L.; Chang, C.T.; O’Connor, R.; Gelbert, M.B. The pharmacokinetics and bioavailability of clemastine and phenylpropanolamine in single-component and combination formulations. J. Clin. Pharmacol. 1996, 36, 911–922. [Google Scholar] [CrossRef]
- Xie, D.; Ge, X.; Ma, Y.; Tang, J.; Wang, Y.; Zhu, Y.; Gao, C.; Pan, S. Clemastine improves hypomyelination in rats with hypoxic–ischemic brain injury by reducing microglia-derived IL-1β via P38 signaling pathway. J. Neuroinflamm. 2020, 17, 57. [Google Scholar] [CrossRef]
- Mike, J.K.; Wu, K.Y.; White, Y.; Pathipati, P.; Ndjamen, B.; Hutchings, R.S.; Losser, C.; Vento, C.; Arellano, K.; Vanhatalo, O.; et al. Defining Longer-Term Outcomes in an Ovine Model of Moderate Perinatal Hypoxia-Ischemia. Dev. Neurosci. 2022, 44, 277–294. [Google Scholar] [CrossRef]
- Parisi, G.F.; Leonardi, S.; Ciprandi, G.; Corsico, A.; Licari, A.; Miraglia del Giudice, M.; Peroni, D.; Salpietro, C.; Marseglia, G.L. Antihistamines in children and adolescents: A practical update. Allergol. Immunopathol. 2020, 48, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Fein, M.N.; Fischer, D.A.; O’Keefe, A.W.; Sussman, G.L. CSACI position statement: Newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin. Immunol. 2019, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Ngo, E.; Spigset, O.; Lupattelli, A.; Panchaud, A.; Annaert, P.; Allegaert, K.; Nordeng, H. Antihistamine use during breastfeeding with focus on breast milk transfer and safety in humans: A systematic literature review. Basic Clin. Pharmacol. Toxicol. 2022, 130, 171–181. [Google Scholar] [CrossRef]
- Arora, I.; Bhandekar, H.; Lakra, A.; Lakra, M.S.; Khadse, S.S. Filling the Gaps for Feeding Difficulties in Neonates with Hypoxic-Ischemic Encephalopathy. Cureus 2022, 14, e28564. [Google Scholar] [CrossRef]
- Ridley, J.M.; Milnes, J.T.; Hancox, J.C.; Witchel, H.J. Clemastine, a conventional antihistamine, is a high potency inhibitor of the HERG K+ channel. J. Mol. Cell Cardiol. 2006, 40, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Giesinger, R.E.; Levy, P.T.; Ruoss, J.L.; El Dib, M.; Mohammad, K.; Wintermark, P.; McNamara, P.J. Cardiovascular management following hypoxic–ischemic encephalopathy in North America: Need for physiologic consideration. Pediatr. Res. 2021, 90, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Nagasawa, Y.; Hagiwara-Nagasawa, M.; Omura, K.; Aimoto, M.; Takahara, A. Torsadogenic potential of a novel remyelinating drug clemastine for multiple sclerosis assessed in the rabbit proarrhythmia model. J. Pharmacol. Sci. 2020, 144, 123–128. [Google Scholar] [CrossRef]
- Lal, A. Effect of a few histamine(1)-antagonists on blood glucose in patients of allergic rhinitis. Indian J. Otolaryngol. Head Neck Surg. 2000, 52, 193–195. [Google Scholar] [CrossRef]
- Fowden, A.L.; Mundy, L.; Silver, M. Developmental regulation of glucogenesis in the sheep fetus during late gestation. J. Physiol. 1998, 508 Pt 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, X.; Hou, L.; Wang, X.; Zhao, C.; Du, Y. Study on the relationship between nephrotic syndrome and atopic diseases in childhood. Front. Pediatr. 2022, 10, 992862. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, J.; Lee, H.; Wu, C.; Dong, K.; Liu, Z.; Cui, L.; Song, H.; Ding, Y.; Meng, R. Immunocytes Rapid Responses Post-ischemic Stroke in Peripheral Blood in Patients with Different Ages. Front. Neurol. 2022, 13, 887526. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, F.M.; Watson, R.W.; O’Neill, A.; Blanco, A.; Donoghue, V.; Molloy, E.J. Persistent systemic monocyte and neutrophil activation in neonatal encephalopathy. J. Matern. Neonatal Med. 2016, 29, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, Y.; Feng, J.; Guo, Y. Identifying the pattern of immune related cells and genes in the peripheral blood of ischemic stroke. J. Transl. Med. 2020, 18, 296. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, Q.; Xia, X.; Yao, B.; Liang, S.; Chen, Z.; Wu, M. The correlation between novel peripheral blood cell ratios and 90-day mortality in patients with acute ischemic stroke. PLoS ONE 2020, 15, e0238312. [Google Scholar] [CrossRef]
- Smida, T.; Koller, A.C.; Menegazzi, J.J.; Salcido, D.D. Early cytotoxic lymphocyte localization to the brain following resuscitation in a porcine model of asphyxial cardiac arrest: A pilot study. Resusc. Plus 2021, 6, 100125. [Google Scholar] [CrossRef]
- Povroznik, J.M.; Engler-Chiurazzi, E.B.; Nanavati, T.; Pergami, P. Absolute lymphocyte and neutrophil counts in neonatal ischemic brain injury. SAGE Open Med. 2018, 6, 2050312117752613. [Google Scholar] [CrossRef] [PubMed]
- Mülling, K.; Fischer, A.J.; Siakaeva, E.; Richter, M.; Bordbari, S.; Spyra, I.; Köster, C.; Hermann, D.M.; Gunzer, M.; Felderhoff-Müser, U.; et al. Neutrophil dynamics, plasticity and function in acute neurodegeneration following neonatal hypoxia–ischemia. Brain Behav. Immun. 2021, 92, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Köster, C.; Crasmöller, M.; Abberger, H.; Hansen, W.; Felderhoff-Müser, U.; Bendix, I. Peripheral T Cell Depletion by FTY720 Exacerbates Hypoxic-Ischemic Brain Injury in Neonatal Mice. Front. Immunol. 2018, 9, 1696. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Increased Systemic Immune-Inflammation Index Predicts Disease Severity and Functional Outcome in Acute Ischemic Stroke Patients. Neurologist 2023, 28, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pandey, S.; Shen, R.; Xu, Y.; Zhang, Q. Increased Systemic Immune-Inflammation Index Is Associated with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage Patients. Front. Neurol. 2021, 12, 745175. [Google Scholar] [CrossRef]
- Su, W.J.; Zhang, T.; Jiang, C.L.; Wang, W. Clemastine Alleviates Depressive-Like Behavior Through Reversing the Imbalance of Microglia-Related Pro-inflammatory State in Mouse Hippocampus. Front. Cell. Neurosci. 2018, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Zeng, S.; Chen, Y.; Liao, D.; Lai, M.; Wang, Z.; Wang, Y.; Xiao, S. Clemastine promotes recovery of neural function and suppresses neuronal apoptosis by restoring balance of pro-inflammatory mediators in an experimental model of intracerebral hemorrhage. Int. J. Med. Sci. 2021, 18, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-κB Signaling and Inflammation—Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar]
- Manukjan, N.; Majcher, D.; Leenders, P.; Caiment, F.; van Herwijnen, M.; Smeets, H.J.; Suidgeest, E.; van der Weerd, L.; Vanmierlo, T.; Jansen, J.F.A.; et al. Hypoxic oligodendrocyte precursor cell-derived VEGFA is associated with blood–brain barrier impairment. Acta Neuropathol. Commun. 2023, 11, 128. [Google Scholar] [CrossRef]
- Bano, S.; Chaudhary, V.; Garga, U.C. Neonatal Hypoxic-ischemic Encephalopathy: A Radiological Review. J. Pediatr. Neurosci. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Schrock, J.W.; Glasenapp, M.; Drogell, K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin. Neurol. Neurosurg. 2012, 114, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-F.; Deng, M.-L. Prognostic impact of blood urea nitrogen/creatinine ratio changes in patients with acute ischemic stroke. Clin. Neurol. Neurosurg. 2022, 215, 107204. [Google Scholar] [CrossRef] [PubMed]
- Murden, S.; Borbélyová, V.; Laštůvka, Z.; Mysliveček, J.; Otáhal, J.; Riljak, V. Gender differences involved in the pathophysiology of the perinatal hypoxic-ischemic damage. Physiol. Res. 2019, 68 (Suppl. S3), S207–S217. [Google Scholar] [CrossRef]
- Mirza, M.A.; Ritzel, R.; Xu, Y.; McCullough, L.D.; Liu, F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J. Neuroinflamm. 2015, 12, 32. [Google Scholar] [CrossRef] [PubMed]



| Function | Neurological Milestone | Score |
|---|---|---|
| Motor function | Walking | 4 |
| Standing | 3 | |
| Four limbs | 2 | |
| Front/hind limbs | 1 | |
| No movement/Spastic | 0 | |
| Feeding | Nurses normally | 1 |
| Suckling well once finds the bottle | 0.5 | |
| Requires assistance to find bottle; a few good suckles | 0.25 | |
| Minimal suckle, tube fed | 0 | |
| Activity at rest | Lifts the head up, alert active | 1 |
| Wakes up with stimulation, attempts to lift the head | 0.5 | |
| Sleepy; no head lift with stimulation | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mike, J.K.; White, Y.; Hutchings, R.S.; Vento, C.; Ha, J.; Iranmahboub, A.; Manzoor, H.; Gunewardena, A.; Cheah, C.; Wang, A.; et al. Effect of Clemastine on Neurophysiological Outcomes in an Ovine Model of Neonatal Hypoxic-Ischemic Encephalopathy. Children 2023, 10, 1728. https://doi.org/10.3390/children10111728
Mike JK, White Y, Hutchings RS, Vento C, Ha J, Iranmahboub A, Manzoor H, Gunewardena A, Cheah C, Wang A, et al. Effect of Clemastine on Neurophysiological Outcomes in an Ovine Model of Neonatal Hypoxic-Ischemic Encephalopathy. Children. 2023; 10(11):1728. https://doi.org/10.3390/children10111728
Chicago/Turabian StyleMike, Jana Krystofova, Yasmine White, Rachel S. Hutchings, Christian Vento, Janica Ha, Ariana Iranmahboub, Hadiya Manzoor, Anya Gunewardena, Cheryl Cheah, Aijun Wang, and et al. 2023. "Effect of Clemastine on Neurophysiological Outcomes in an Ovine Model of Neonatal Hypoxic-Ischemic Encephalopathy" Children 10, no. 11: 1728. https://doi.org/10.3390/children10111728








