Acute Pupillary Disorders in Children: A 10-Year Retrospective Study of 101 Patients
Abstract
:1. Introduction
2. Materials and Methods
- 1)
- High/intermediate priority: includes patients classified as “code red” (critical medical status) and “code yellow” (severe status, risk of evolution to critical condition);
- 2)
- Low/nonurgent priority: includes patients classified as “code green” (fair status, stable vital signs) or “code white” (good status, nonurgent consultation).
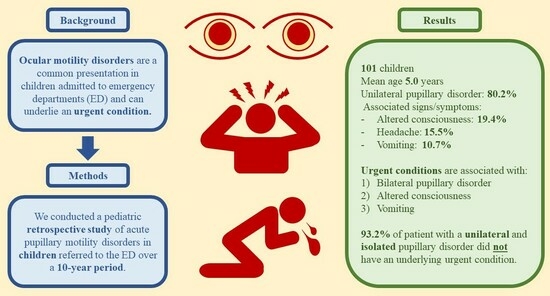
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Raucci, U.; Parisi, P.; Vanacore, N.; Ferro, V.; Garone, G.; Sancetta, F.; Petroni, S.; Pro, S.; Rossi, R.; Reale, A.; et al. A cohort study on acute ocular motility disorders in pediatric emergency department. Ital. J. Pediatr. 2018, 44, 62. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.R.; McClelland, C.M.; Lee, M.S. An approach to anisocoria. Curr. Opin. Ophthalmol. 2016, 27, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.V. Eye strabismus. In The Textbook of Pediatric Emergency Medicine, 6th ed.; Fleisher, G.R., Ludwig, S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 281–286. [Google Scholar]
- Nye, C. A child’s vision. Pediatr. Clin. N. Am. 2014, 61, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, H.Z.; Colpak, I.A.; Kansu, T. A diagnostic challenge: Dilated pupil. Curr. Opin. Ophthalmol. 2013, 24, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Conferenza Permanente Per i Rapporti tra lo Stato le Regioni e le Province Autonome di Trento e Bolzano. Accordo Tra il Ministro della Salute, le Regioni e le Province Autonome sul Documento di Linee-Guida sul Sistema di Emergenza Sanitaria Concernente: “Triage Intraospedaliero (Valutazione Gravità All’ingresso) e Chirurgia della Mano e Microchirurgia nel Sistema Dell’emergenza–Urgenza Sanitaria.” Gazzetta Ufficiale Serie Generale n.285. Available online: https://www.gazzettaufficiale.it/eli/id/2001/12/07/01A12203/sg (accessed on 20 March 2020).
- Falardeauj, J. Anisocoria. Int. Ophthalmol. Clin. 2019, 59, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Payne, W.N.; Blair, K.; Barrett, M.J. Anisocoria. In StatPearls; StatPearls Publishing: Treasure Island FL, USA, 2022. [Google Scholar]
- Rasmidatta, S.; Fukushima, A.; Bando, M. Ipratropium bromide-effects on the eye. J. Jpn. Ophthalmol. Soc. (Nippon. Ganka. Gakkai. Zasshi) 1980, 84, 2053–2060. [Google Scholar]
- Samaniego, F.; Newman, L.S. Migratory anisocoria--a novel clinical entity. Am. Rev. Respir. Dis. 1986, 134, 844. [Google Scholar] [PubMed]
- Sharma, N.S.; Ooi, J.L.; Papalkar, D.; Sharma, S.; Francis, I.C.; Fung, V.S. Pharmacological mydriasis secondary to ipratropium bromide: A cause of unilateral dilated pupil. J. Clin. Neurosci. 2008, 15, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Parisi, P.; Francia, A. A female with central anticholinergic syndrome responsive to neostigmine. Pediatr. Neurol. 2000, 23, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Havelius, U.; Asman, P. Accidental mydriasis from exposure to Angel’s trumpet (Datura suaveolens). Acta. Ophthalmol. Scand. 2002, 80, 33233–33235. [Google Scholar] [CrossRef]
- Firestone, D.; Sloane, C. Not your everyday anisocoria: Angel’s trumpet ocular toxicity. J. Emerg. Med. 2007, 33, 21–24. [Google Scholar] [CrossRef]
- Andreola, B.; Piovan, A.; Da Dalt, L.; Filippini, R.; Cappelletti, E. Unilateral mydriasis due to Angel’s trumpet. Clin. Toxicol. 2008, 46, 329–331. [Google Scholar] [CrossRef]
- Macchiaiolo, M.; Vignati, E.; Gonfiantini, M.V.; Grandin, A.; Romano, M.T.; Salata, M.; Valentini, D.; Villani, A. An unusual case of anisocoria by vegetal intoxication: A case report. Ital. J. Pediatr. 2010, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, E. Pupillary Disturbances Due to Poisonings. In The Pupil; Springer: New York, NY, USA, 1985; pp. 77–87. [Google Scholar]
- Iyer, S.; Tay, E.T.; Maslyanskaya, S. Anisocoria from Cocaine Exposure: A Case Report. J. Emerg. Med. 2019, 56, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Servidio, A.G.; Peri, F.; Tenore, A.; Cesca, L.; Diplotti, L.; Dall’Amico, R.; Barbi, B. A well-appearing infant with a sudden anisocoria. Arch. Dis. Child. Educ. Pract. Ed. 2022, 107, 116–117. [Google Scholar] [CrossRef] [PubMed]

| Total Number—No. | 103 a,b |
|---|---|
| Age—mean ± SD (range) | 5.0 ± 4.5 (0.07–15.96) |
| Males—no. (%) | 62 (60.2) |
| Female-to-male ratio | 1:1.5 |
| Triage | |
| High/intermediate priority | 64 (62.1) |
| Low priority | 39 (37.9) |
| Reason for entering the ED—no. (%) | |
| Pupillary disorder | 63 (61.2) |
| Other | 38 (37.6) |
| Time from onset (days)—median ± IQR (range) | 1 ± 2 (0.13–90) |
| History of similar episode—no. (%) | 7 (6.8) |
| Intoxication—no. (%) | 50 (48.5) |
| Accidental | 42 (84.0) |
| Voluntary | 8 (16.0) |
| Exposure to toxic substance—no. (%) | |
| Contact | 38 (76.0) |
| Ingestion | 12 (24.0) |
| Type of toxic substance | |
| Poisonous plants | 9 (18.0) |
| Chemical agents | 5 (10.0) |
| Drugs | 37 (72.0) |
| Pupillary disorder—no. (%) | |
| Mydriasis | 94 (93.1) |
| Myosis | 5 (5.0) |
| Unspecified anisocoria | 2 (1.9) |
| Unilateral | 81 (80.2) |
| Bilateral | 20 (19.8) |
| Isolated pupillary disorder—no. (%) | 60 (59.4) |
| Other ocular signs/symptoms—no. (%) | 16 (15.5) |
| Loss of vision | 8 (7.8) |
| Strabismus | 5 (4.9) |
| Ptosis | 3 (2.9) |
| Papillary edema | 3 (2.9) |
| Nonocular signs/symptoms—no. (%) | 35 (34.0) |
| Impaired consciousness | 20 (19.4) |
| Headache | 16 (15.5) |
| Vomiting | 11 (10.7) |
| Fever | 5 (4.9) |
| Focal deficits | 3 (2.9) |
| Ataxia | 3 (2.9) |
| Seizure | 2 (1.9) |
| Neck stiffness | 2 (1.9) |
| Hypotonia | 2 (1.9) |
| Vertigo | 1 (0.9) |
| Comorbidities—no. (%) | 36 (35.0) |
| Hospitalization—no. (%) | 52 (50.5) |
| Length of stay (days)—median ± IQR (range) | 4 ± 13 (0–72) |
| Discharge—no. (%) | 51 (49.5) |
| Medical imaging—no. (%) | 52 (50.5) |
| Head CT scan only | 31 (30.1) |
| Head MRI only | 4 (3.9) |
| Both head CT and MRI | 13 (10.0) |
| VEP/ERG | 9 (8.7) |
| Specialist advice—no. (%) | 85 (82.5) |
| Ophthalmologist | 58 (56.3) |
| Neurologist | 51 (49.5) |
| Intensive care physician | 19 (18.4) |
| Neurosurgeon | 10 (9.7) |
| Neuropsychiatrist | 4 (3.9) |
| Diagnosis | |
| Urgent condition—no. (%) | 39 (38.6) |
| Systemic intoxication | 10 (9.9) |
| CNS tumor | 7 (6.9) |
| CNS infection | 5 (4.9) |
| Ocular disease | 5 (4.9) |
| Cerebrovascular disease | 3 (2.9) |
| Other encephalopathy * | 3 (2.9) |
| Malfunction of VPS | 2 (1.9) |
| Cranial neuropathy | 2 (1.9) |
| Optical neuritis | 2 (1.9) |
| Nonurgent condition—no. (%) | 62 (61.4) |
| Local intoxication | 38 (37.6) |
| No diagnosis | 24 (23.8) |
| UC | Non UC | p-Value | |
|---|---|---|---|
| Total number—no. | 39 | 62 | |
| Age—mean ± SD (range) | 6.6 ± 5.3 (0.1–16.0) | 4.0 ± 3.7 (0.1–14.3) | 0.011 |
| Males—no. (%) | 20 (51.3) | 40 (64.5) | 0.216 |
| Triage | |||
| High/intermediate priority | 30 (76.9) | 34 (54.8) | 0.025 |
| Low priority | 9 (23.1) | 28 (45.2) | 0.025 |
| Reason for entering the ED—no. (%) | |||
| Pupillary disorder | 10 (25.6) | 53 (85.5) | <0.001 |
| Other | 29 (74.4) | 9 (14.5) | <0.001 |
| Time from onset (days)—median ± IQR (range) | 1 ± 2 (1–30) | 1 ± 0 (0.13–90) | 0.013 |
| History of similar episode—no. (%) | 4 (57.1) | 3 (42.9) | 0.257 |
| Intoxication—no. (%) | 11 (28.2) | 39 (62.9) | 0.001 |
| Accidental | 5 (45.5) | 37 (94.9) | 0.001 |
| Voluntary | 6 (94.9) | 2 (5.1) | 0.001 |
| Exposure to toxic substance—no. (%) | |||
| Contact | 0 (0) | 38 (97.4) | <0.001 |
| Ingestion | 11 (100) | 1 (2.6) | <0.001 |
| Type of toxic substance—no. (%) | |||
| Poisonous plants | 0 (0) | 9 (23.1) | 0.177 |
| Chemical agents | 5 (45.5) | 0 (0) | <0.001 |
| Drugs | 7 (63.6) | 30 (76.9) | 0.445 |
| Pupillary disorder—no. (%) | |||
| Mydriasis | 36 (92.3) | 58 (93.5) | 1.000 |
| Myosis | 3 (7.7) | 2 (3.2) | 0.372 |
| Unilateral | 21 (53.8) | 60 (96.8) | <0.001 |
| Bilateral | 18 (46.2) | 2 (3.2) | <0.001 |
| Unspecified | 0 (0) | 2 (3.2) | 0.521 |
| Isolated pupillary disorder—no. (%) | 6 (15.4) | 54 (87.1) | <0.001 |
| Other ocular signs/symptoms—no. (%) | 14 (35.9) | 2 (3.2) | <0.001 |
| Loss of vision | 6 (15.4) | 2 (3.2) | 0.052 |
| Strabismus | 5 (12.8) | 0 (0) | 0.007 |
| Ptosis | 3 (7.7) | 0 (0) | 0.055 |
| Papillary edema | 3 (7.7) | 0 (0) | 0.055 |
| Nonocular signs/symptoms—no. (%) | 28 (71.8) | 7 (11.3) | <0.001 |
| Impaired consciousness | 19 (48.7) | 1 (1.6) | <0.001 |
| Headache | 10 (25.6) | 6 (9.7) | 0.032 |
| Vomiting | 10 (25.6) | 1 (1.6) | <0.001 |
| Fever | 4 (10.3) | 1 (1.6) | 0.072 |
| Focal deficits | 3 (7.7) | 0 (0) | 0.055 |
| Ataxia | 3 (7.7) | 0 (0) | 0.055 |
| Seizure | 2 (5.1) | 0 (0) | 0.147 |
| Neck stiffness | 2 (5.1) | 0 (0) | 0.147 |
| Hypotonia | 2 (5.1) | 0 (0) | 0.147 |
| Dizziness | 0 (0) | 1 (1.6) | 1.000 |
| Comorbidities—no. (%) | 12 (30.8) | 24 (38.7) | 0.497 |
| Hospitalization—no. (%) | 36 (92.3) | 16 (25.8) | <0.001 |
| Length of stay (days)—median ± IQR (range) | 9 ± 17 (0–72) | 1 ± 1 (0–11) | |
| Discharged (no hospitalization)—no. (%) | 3 (7.7) | 46 (74.2) | <0.001 |
| Medical imaging—no. (%) | 28 (71.8) | 24 (38.7) | 0.001 |
| Head CT scan only | 13 (33.3) | 18 (29.0) | 0.648 |
| Head MRI only | 1 (2.6) | 3 (4.8) | 1.000 |
| Both head CT and MRI | 12 (30.8) | 1 (1.6) | <0.001 |
| VEP/ERG | 7 (17.9) | 2 (3.2) | 0.026 |
| Variables | aOR | C.I. 95% | p-Value |
|---|---|---|---|
| Age (years) | 1.014 | 0.842–1.221 | 0.883 |
| Time from onset (days) | 1.000 | 0.939–1.065 | 0.997 |
| Reason for entering the ED (other) | 1.946 | 0.311–12.164 | 0.477 |
| Intoxication (yes) | 0.321 | 0.057–1.814 | 0.199 |
| Pupillary disorder (bilateral) | 31.227 | 3.327–293.056 | 0.003 |
| Other ocular signs/symptoms (yes) | 26.603 | 3.206–220.741 | 0.002 |
| Impaired consciousness (yes) | 11.602 | 0.807–166.693 | 0.071 |
| Headache (yes) | 0.131 | 0.011–1.620 | 0.113 |
| Vomiting (yes) | 28.064 | 1.277–616.633 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garone, G.; Roversi, M.; Pisani, M.; La Penna, F.; Musolino, A.; Cristaldi, S.; Musolino, A.M.; Roberto, A.; Petrocelli, G.; Reale, A.; et al. Acute Pupillary Disorders in Children: A 10-Year Retrospective Study of 101 Patients. Children 2023, 10, 1739. https://doi.org/10.3390/children10111739
Garone G, Roversi M, Pisani M, La Penna F, Musolino A, Cristaldi S, Musolino AM, Roberto A, Petrocelli G, Reale A, et al. Acute Pupillary Disorders in Children: A 10-Year Retrospective Study of 101 Patients. Children. 2023; 10(11):1739. https://doi.org/10.3390/children10111739
Chicago/Turabian StyleGarone, Giacomo, Marco Roversi, Mara Pisani, Francesco La Penna, Antonio Musolino, Sebastian Cristaldi, Anna Maria Musolino, Amanda Roberto, Gianni Petrocelli, Antonino Reale, and et al. 2023. "Acute Pupillary Disorders in Children: A 10-Year Retrospective Study of 101 Patients" Children 10, no. 11: 1739. https://doi.org/10.3390/children10111739
APA StyleGarone, G., Roversi, M., Pisani, M., La Penna, F., Musolino, A., Cristaldi, S., Musolino, A. M., Roberto, A., Petrocelli, G., Reale, A., Midulla, F., Villani, A., & Raucci, U. (2023). Acute Pupillary Disorders in Children: A 10-Year Retrospective Study of 101 Patients. Children, 10(11), 1739. https://doi.org/10.3390/children10111739