Pediatric Hyperglycemic Hyperosmolar Syndrome: A Comprehensive Approach to Diagnosis, Management, and Complications Utilizing Novel Summarizing Acronyms
Abstract
:1. Introduction
2. Review of HHS and DKA
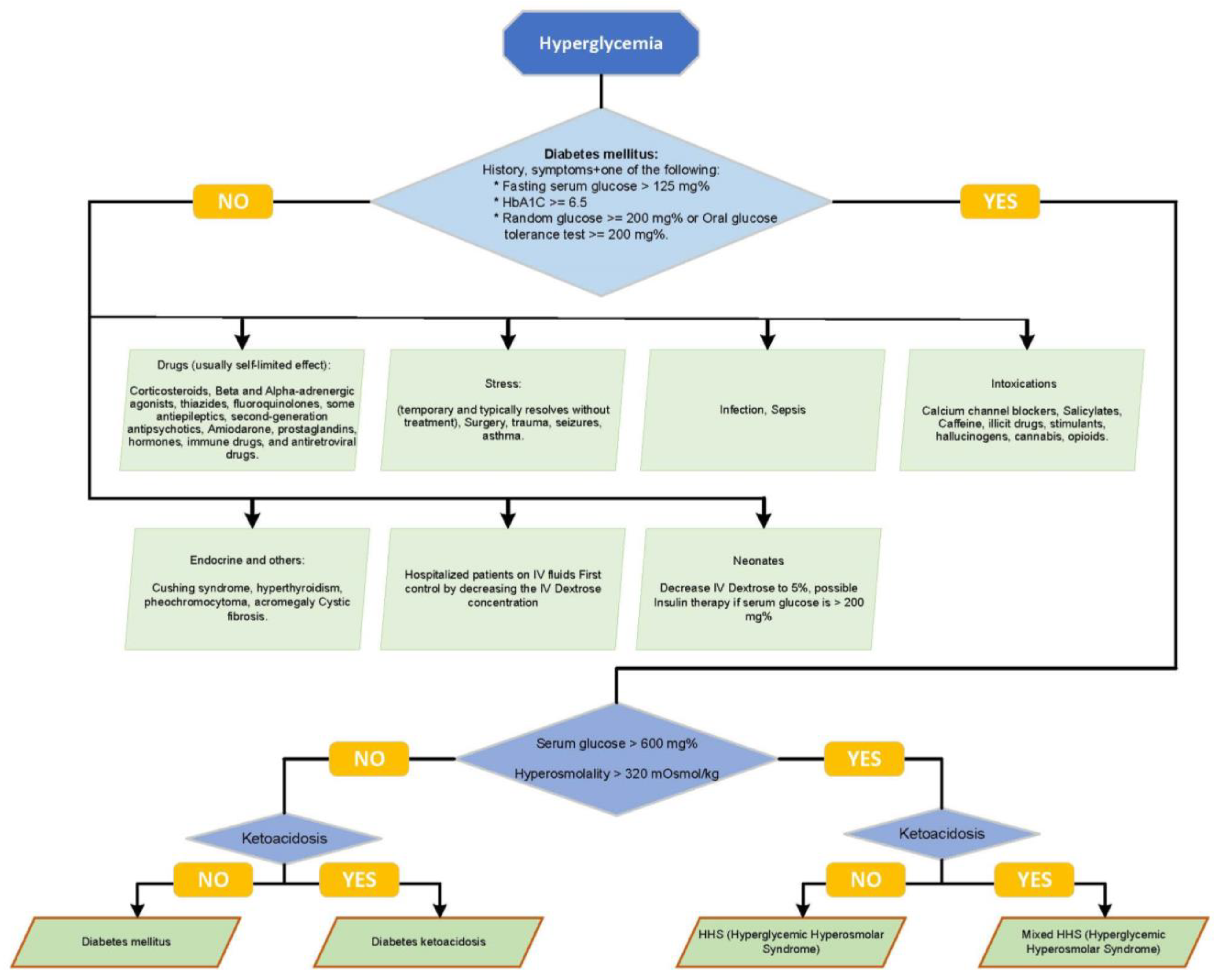
3. The Challenge of Differentiating HHS and DKA at Clinical Presentation
4. Complications in HHS and DKA
5. Treatment of HHS
- -
- ‘DI’—Delay and Decrease Insulin Administration: HHS patients require a later initiation and a lower dose of insulin compared to those with DKA. This stage emphasizes the need to manage the decline in serum glucose, primarily through fluid resuscitation, before insulin therapy is initiated.
- -
- ‘FF’— Fluid replacement emphasis: Fluid management is pivotal in HHS due to severe dehydration, which is often double that observed in DKA.
- -
- ‘ER’—Electrolyte replacement, especially potassium, phosphate, and magnesium, are more pronounced in HHS and necessitate frequent monitoring and replacement, initially as often as every 2 h.
- -
- ‘EN’—Encephalopathy: HHS can potentially lead to encephalopathy, underlining the need for controlled serum osmolality reduction. The regular monitoring of clinical and biochemical parameters, including close hourly glucose monitoring, is essential during treatment.
- -
- ‘CE’—Cerebral Edema: While rare in HHS, cerebral edema requires prompt and aggressive therapy when it occurs.
- -
- ‘S’—Systemic Multiorgan Failure: HHS can potentially lead to systemic multiorgan failure. For ease of awareness and screening, we categorize the associated risks into three mnemonic groups: the 3Rs (renal failure, respiratory distress, rhabdomyolysis), the 3Hs (heart failure, hypercoagulation, hyperthermia), and AP (arrhythmias, pancreatitis). Early recognition and management of those complications are crucial for improving patient outcomes.
- -
- Premature insulin use in under-resuscitated HHS patients with vasospasm may cause hypotension due to a prominent extravascular fluid shift. A rapid decline in the serum glucose of >100 mg/dL may occur in the first hours of fluid expansion alone [17]. Therefore, insulin infusion should be held temporarily if it was already initiated. Some authors advise adding low glucose (2.5–5%) if a significant decrease continues [47]. Once the serum glucose begins to fall at a rate below 50 mg/dL/h (2.7 mmol/L/h), an insulin drip is added to fluid therapy at a lower dose of 0.05–0.025 unit/kg/h. This approach contrasts with that used for patients with severe ketoacidosis typical of DKA, where insulin infusion is initiated earlier and at a higher dose (around 0.1 unit/kg/h) [48]. The initial goal of hyperglycemia level control for HHS is between 200 and 300 mg/dL, with an IV insulin drip titrated at a lower rate than in DKA. In comparison, the blood glucose goal for DKA on IV Insulin is 150–200 mg/dL. A hypernatremic sodium decrease of 0.5 meq/L/h is advocated in some studies [49]. Generally, electrolyte losses in HHS, including losses in potassium, phosphate, and magnesium, are higher than in DKA. Potassium losses are extreme despite the possible initial hyperkalemia secondary to acidosis ion shift [50]. If the serum potassium is <5 mEq/L with adequate renal function, it is recommended that patients are provided with 40 mEq/L of potassium phosphate/KCl. Magnesium may be needed for hypomagnesemia or hypocalcemia [40,43,45,47,48].
6. Prognosis
7. Mixed HHS and DKA
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013, 36 (Suppl. S1), S11–S66. [Google Scholar] [CrossRef] [PubMed]
- Gosmanov, A.R.; Gosmanova, E.O.; Kitabchi, A.E. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Chiang, J.L.; Maahs, D.M.; Garvey, K.C.; Hood, K.K.; Laffel, L.M.; Weinzimer, S.A.; Wolfsdorf, J.I.; Schatz, D. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, 2026–2044. [Google Scholar] [CrossRef] [PubMed]
- Pasquel, F.J.; Umpierrez, G.E. Hyperosmolar hyperglycemic state: A historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014, 37, 3124–3131. [Google Scholar] [CrossRef]
- Hassan, E.M.; Mushtaq, H.; Mahmoud, E.E.; Chhibber, S.; Saleem, S.; Issa, A.; Nitesh, J.; Jama, A.B.; Khedr, A.; Boike, S.; et al. Overlap of diabetic ketoacidosis and hyperosmolar hyperglycemic state. World J. Clin. Cases 2022, 10, 11702–11711. [Google Scholar] [CrossRef] [PubMed]
- Broadley, L.; Clark, K.; Ritchie, G. Prevention and management of hyperglycaemic crisis. Nurs. Stand. 2019, 34, 75–82. [Google Scholar] [CrossRef]
- Agrawal, S.; Baird, G.L.; Quintos, J.B.; Reinert, S.E.; Gopalakrishnan, G.; Boney, C.M.; Topor, L.S. Pediatric Diabetic Ketoacidosis With Hyperosmolarity: Clinical Characteristics and Outcomes. Endocr. Pract. 2018, 24, 726–732. [Google Scholar] [CrossRef]
- Kharode, I.; Coppedge, E.; Antal, Z. Care of Children and Adolescents with Diabetes Mellitus and Hyperglycemia in the Inpatient Setting. Curr. Diab. Rep. 2019, 19, 85. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Fisher, J.N.; Murphy, M.B.; Stentz, F.B. Thirty years of personal experience in hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J. Clin. Endocrinol. Metab. 2008, 93, 1541–1552. [Google Scholar] [CrossRef]
- Shahramian, I.; Rahimi, P.O.; Radvar, S. Hyperosmolar hyperglycemic state in children: Case report and review of the literature. J. Klin. Endokrinol. Stoffwechs. 2022, 15, 60–62. [Google Scholar] [CrossRef]
- Sperling, M.A. 21-Diabetes Mellitus. In Sperling Pediatric Endocrinology, 5th ed.; Sperling, M.A., Ed.; Elsevier: Philadelphia, PA, USA, 2021; pp. 814–883. [Google Scholar]
- Maruyama, K.; Chujo, D. Tacrolimus-induced diabetic ketoacidosis with subsequent rapid recovery of endogenous insulin secretion after cessation of tacrolimus: A case report with review of literature. Medicine 2019, 98, e16992. [Google Scholar] [CrossRef]
- Yu, M.; Yu, L.; Wang, Z.X. Diagnosis and Management of Kearns-Sayre Syndrome Rely on Comprehensive Clinical Evaluation. Chin. Med. J. 2016, 129, 2519–2520. [Google Scholar] [CrossRef] [PubMed]
- Bassham, B.; Estrada, C.; Abramo, T. Hyperglycemic hyperosmolar syndrome in the pediatric patient: A case report and review of the literature. Pediatr. Emerg. Care 2012, 28, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Fayfman, M.; Pasquel, F.J.; Umpierrez, G.E. Management of Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. Med. Clin. N. Am. 2017, 101, 587–606. [Google Scholar] [CrossRef]
- Wolf, R.A.; Haw, J.S.; Paul, S.; Faulkner, M.S.; Cha, E.; Findley, M.; Khan, F.; Webster, S.M.; Alexopoulos, A.-S.; Mehta, K.; et al. Hospital admissions for hyperglycemic emergencies in young adults at an inner-city hospital. Diabetes Res. Clin. Pract. 2019, 157, 107869. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, A.; Kondamudi, N.P. Hyperosmolar Hyperglycemic Syndrome. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mercer, S.; Hanks, L.; Ashraf, A. Rhabdomyolysis in Pediatric Patients with Diabetic Ketoacidosis or Hyperglycemic Hyperosmolar State: A Case Series. Glob. Pediatr. Health 2016, 3, 2333794x16671391. [Google Scholar] [CrossRef]
- Gosmanov, A.R.; Kitabchi, A.E. Diabetic Ketoacidosis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Goguen, J.; Gilbert, J. Hyperglycemic Emergencies in Adults. Can. J. Diabetes 2018, 42, S109–S114. [Google Scholar] [CrossRef]
- Nugent, B.W. Hyperosmolar hyperglycemic state. Emerg. Med. Clin. 2005, 23, 629–648. [Google Scholar] [CrossRef]
- Liamis, G.; Liberopoulos, E.; Barkas, F.; Elisaf, M. Diabetes mellitus and electrolyte disorders. World J. Clin. Cases WJCC 2014, 2, 488. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Murphy, M.B.; Kitabchi, A.E. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectr. 2002, 15, 28–36. [Google Scholar] [CrossRef]
- Muir, A.B.; Quisling, R.G.; Yang, M.C.K.; Rosenbloom, A.L. Cerebral edema in childhood diabetic ketoacidosis: Natural history, radiographic findings, and early identification. Diabetes Care 2004, 27, 1541–1546. [Google Scholar] [CrossRef]
- English, P.; Williams, G. Hyperglycaemic crises and lactic acidosis in diabetes mellitus. Postgrad. Med. J. 2004, 80, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Vavilala, M.S.; Richards, T.L.; Roberts, J.S.; Chiu, H.; Pihoker, C.; Bradford, H.; Deeter, K.; Marro, K.I.; Shaw, D. Change in blood–brain barrier permeability during pediatric diabetic ketoacidosis treatment. Pediatr. Crit. Care Med. 2010, 11, 332. [Google Scholar] [CrossRef]
- Rose, K.L. Cerebral Edema in Diabetic Ketoacidosis. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2009. [Google Scholar]
- Blank, S.P.; Blank, R.M.; Campbell, L. What Is the Optimal Speed of correction of the Hyperosmolar Hyperglycemic State in Diabetic Ketoacidosis? An Observational Cohort Study of US Intensive Care Patients. Endocr. Pract. 2022, 28, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Sasbón, J.S.; Arroyo, H. Toxic Metabolic Encephalopathy. Pediatric Critical Care Medicine: Volume 2: Respiratory, Cardiovascular and Central Nervous Systems; Northwestern University: Evanston, IL, USA, 2014; pp. 627–642. [Google Scholar]
- Pituitary, P. CHAPTER 10. Williams Textbook of Endocrinology E-Book; Elsevier: Amsterdam, The Netherlands, 2011; p. 291. [Google Scholar]
- Adrogué, H.J.; Madias, N.E. Fluid-electrolyte and acid-base disorders complicating diabetes mellitus. In Diseases of the Kidney and Urinary Tract, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 2353–2378. [Google Scholar]
- Glaser, N.S.; Wootton-Gorges, S.L.; Marcin, J.P.; Buonocore, M.H.; DiCarlo, J.; Neely, E.; Barnes, P.; Bottomly, J.; Kuppermann, N. Mechanism of cerebral edema in children with diabetic ketoacidosis. J. Pediatr. 2004, 145, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Roldán, T.; Salamanca, O. Hyperglycemic Crises: A General Review. Open Access J. Biomed. Sci. 2022, 4, 2280–2287. [Google Scholar]
- Woodrow, G.; Brownjohn, A.; Turney, J. The clinical and biochemical features of acute renal failure due to rhabdomyolysis. Ren. Fail. 1995, 17, 467–474. [Google Scholar] [CrossRef]
- Diercks, D.B.; Shumaik, G.M.; A Harrigan, R.; Brady, W.J.; Chan, T.C. Electrocardiographic manifestations: Electrolyte abnormalities. J. Emerg. Med. 2004, 27, 153–160. [Google Scholar] [CrossRef]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef]
- Tegazzin, V.; Scutari, E.; Treves, S.; Zorzato, F. Chlorocresol, an additive to commercial succinylcholine, induces contracture of human malignant hyperthermia-susceptible muscles via activation of the ryanodine receptor calcium sup 2+ channel. J. Am. Soc. Anesthesiol. 1996, 84, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Umpierrez, G.E.; Murphy, M.B.; Barrett, E.J.; Kreisberg, R.A.; Malone, J.I.; Wall, B.M. Hyperglycemic crises in patients with diabetes mellitus. Diabetes Care 2003, 26, S109. [Google Scholar]
- Chiasson, J.-L.; Aris-Jilwan, N.; Bélanger, R.; Bertrand, S.; Beauregard, H.; Ekoé, J.-M.; Fournier, H.; Havrankova, J. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Can. Med. Assoc. J. 2003, 168, 859–866. [Google Scholar]
- Magee, M.F.; Bhatt, B.A. Management of decompensated diabetes: Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Crit. Care Clin. 2001, 17, 75–106. [Google Scholar] [CrossRef]
- Rewers, A. Acute Metabolic Complications in Diabetes; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2021.
- Glaser, N.; Fritsch, M.; Priyambada, L.; Rewers, A.; Cherubini, V.; Estrada, S.; Wolfsdorf, J.I.; Codner, E. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr. Diabetes 2022, 23, 835–856. [Google Scholar] [CrossRef]
- Assadi, F. Pediatric Fluid, Electrolyte, and Acid-Base Disorders: A Case-Based Approach; Elsevier Health Sciences: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Fülöp, T.; Zsom, L.; Rodríguez, R.D.; Chabrier-Rosello, J.O.; Hamrahian, M.; Koch, C.A. Therapeutic hypernatremia management during continuous renal replacement therapy with elevated intracranial pressures and respiratory failure. Rev. Endocr. Metab. Disord. 2019, 20, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Van Ness-Otunnu, R.; Hack, J.B. Hyperglycemic crisis. J. Emerg. Med. 2013, 45, 797–805. [Google Scholar] [CrossRef]
- Pope, A.; French, G.; Longnecker, D.E. Experience with and Complications of Fluid Resuscitation. In Fluid Resuscitation: State of the Science for Treating Combat Casualties and Civilian Injuries; National Academies Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Fleisher, G.R.; Ludwig, S. Textbook of Pediatric Emergency Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Calimag, A.P.P.; Chlebek, S.; Lerma, E.V.; Chaiban, J.T. Diabetic ketoacidosis. Dis. Mon. 2023, 69, 101418. [Google Scholar] [CrossRef] [PubMed]
- Human, T. Current therapeutic options for hyponatremia: Indications, limitations, and confounding variables. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2011, 31, 18S–24S. [Google Scholar] [CrossRef]
- Zeitler, P.; Haqq, A.; Rosenbloom, A.; Glaser, N. Hyperglycemic hyperosmolar syndrome in children: Pathophysiological considerations and suggested guidelines for treatment. J. Pediatr. 2011, 158, 9–14.e2. [Google Scholar] [CrossRef]
- Dirksen, S.J.H.; Larach, M.G.; Rosenberg, H.; Brandom, B.W.; Parness, J.; Lang, R.S.; Gangadharan, M.; Pezalski, T. Future directions in malignant hyperthermia research and patient care. Anesth. Analg. 2011, 113, 1108. [Google Scholar] [CrossRef]
- Wang, K.-L.; Chu, P.-H.; Lee, C.-H.; Pai, P.-Y.; Lin, P.-Y.; Shyu, K.-G.; Chang, W.-T.; Chiu, K.-M.; Huang, C.-L.; Lee, C.-Y.; et al. Management of venous thromboembolisms: Part I. The consensus for deep vein thrombosis. Acta Cardiol. Sin. 2016, 32, 1. [Google Scholar]
- Beltran, G. Diabetic emergencies: New strategies for an old disease. Emerg. Med. Pract. 2014, 16, 1–19. [Google Scholar] [PubMed]
- Nambam, B.; Menefee, E.; Gungor, N.; Mcvie, R. Severe complications after initial management of hyperglycemic hyperosmolar syndrome and diabetic ketoacidosis with a standard diabetic ketoacidosis protocol. J. Pediatr. Endocrinol. Metab. 2017, 30, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.; Bennett, K.; Silke, B. Serum osmolarity as an outcome predictor in hospital emergency medical admissions. Eur. J. Intern. Med. 2012, 23, e39–e43. [Google Scholar] [CrossRef]
- Yosypiv, I.V. Diabetes in Children and Adolescents. Diabetes Kidney Dis. 2014, 63–75. [Google Scholar]
- Rosenbloom, A.L. Hyperglycemic hyperosmolar state: An emerging pediatric problem. J. Pediatr. 2010, 156, 180–184. [Google Scholar] [CrossRef]
- Price, A.; Losek, J.; Jackson, B. Hyperglycaemic hyperosmolar syndrome in children: Patient characteristics, diagnostic delays and associated complications. J. Paediatr. Child Health 2016, 52, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Zahran, N.A.; Zeller, W.P.; Charnogursky, G.A. Hyperglycemia and multiple organ failure in a male adolescent. J. Clin. Transl. Endocrinol. Case Rep. 2023, 28, 100140. [Google Scholar] [CrossRef]
- Wolfsdorf, J.; Craig, M.E.; Daneman, D.; Dunger, D.; Edge, J.; Lee, W.; Rosenbloom, A.; Sperling, M.; Hanas, R. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr. Diabetes 2009, 10 (Suppl. S12), 118–133. [Google Scholar] [CrossRef]
- Akinlade, A.T.; Ogbera, A.O.; Fasanmade, O.A.; Olamoyegun, M.A. Serum C-peptide assay of patients with hyperglycemic emergencies at the Lagos State University Teaching Hospital (LASUTH), Ikeja. Int. Arch. Med. 2014, 7, 50. [Google Scholar] [CrossRef]
- Philips, B. Diabetic hyperglycaemic crises. In Core Topics in Endocrinology in Anaesthesia and Critical Care; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]


|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahran, N.A.; Jadidi, S. Pediatric Hyperglycemic Hyperosmolar Syndrome: A Comprehensive Approach to Diagnosis, Management, and Complications Utilizing Novel Summarizing Acronyms. Children 2023, 10, 1773. https://doi.org/10.3390/children10111773
Zahran NA, Jadidi S. Pediatric Hyperglycemic Hyperosmolar Syndrome: A Comprehensive Approach to Diagnosis, Management, and Complications Utilizing Novel Summarizing Acronyms. Children. 2023; 10(11):1773. https://doi.org/10.3390/children10111773
Chicago/Turabian StyleZahran, Naser Amin, and Shaheen Jadidi. 2023. "Pediatric Hyperglycemic Hyperosmolar Syndrome: A Comprehensive Approach to Diagnosis, Management, and Complications Utilizing Novel Summarizing Acronyms" Children 10, no. 11: 1773. https://doi.org/10.3390/children10111773
APA StyleZahran, N. A., & Jadidi, S. (2023). Pediatric Hyperglycemic Hyperosmolar Syndrome: A Comprehensive Approach to Diagnosis, Management, and Complications Utilizing Novel Summarizing Acronyms. Children, 10(11), 1773. https://doi.org/10.3390/children10111773






