Effect of Obesity on the Respiratory Parameters in Children with Obstructive Sleep Apnea Syndrome
Abstract
:1. Introduction
1.1. Obstructive Sleep Apnea Syndrome (OSAS)
1.2. Obesity and OSAS
1.3. Investigation of OSAS in Children
1.4. Literary Review
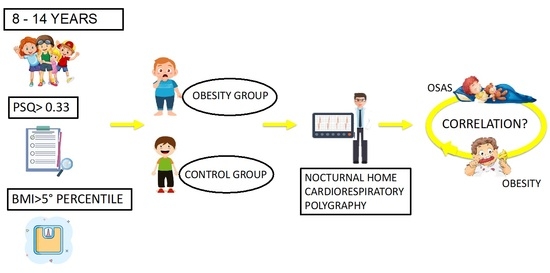
1.5. Aim
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
2.2. Sample Recruitment
2.3. Clinical Examination
2.4. Pediatric Sleep Questionnaire
2.5. Cardiorespiratory Polygraphy
2.6. Statistical Analysis
3. Results
3.1. Comparison Analysis of PSQ between the Obesity Group and the Control Group
3.2. Correlation Analysis of PSQ with Z-Score BMI and Respiratory Parameters
3.3. Correlation Analysis between BMI and Respiratory Parameters
3.4. Distribution of the Entity of OSAS and SaO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nespoli, L.; Caprioglio, A.; Brunetti, L.; Nosetti, L. Obstructive sleep apnea syndrome in childhood. Early Hum. Dev. 2013, 89 (Suppl. S3), s33–s37. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Magliulo, G. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, S.L.; Franckx, H.; Van Gaal, L.; De Backer, W.; Desager, K. The effect of weight loss on sleep-disordered breathing in obese teenagers. Obesity 2009, 17, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Andersen, I.G.; Holm, J.C.; Homøe, P. Obstructive sleep apnea in children and adolescents with and without obesity. Eur. Arch. Otorhinolaryngol. 2019, 276, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Lushington, K.; Couper, R.; Martin, J.; van den Heuvel, C.; Pamula, Y.; Kennedy, D. Obesity and Risk of Sleep Related Upper Airway Obstruction in Caucasian Children. J. Clin. Sleep Med. 2008, 4, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.J.; Thormaehlen, S.; Kennedy, J.D.; Pamula, Y.; van den Heuvel, C.J.; Lushington, K.; Martin, A.J. Differences in the Association Between Obesity and Obstructive Sleep Apnea Among Children and Adolescents. J. Clin. Sleep Med. 2009, 5, 506–511. [Google Scholar] [CrossRef]
- Su, M.S.; Zhang, H.L.; Cai, X.H.; Lin, Y.; Liu, P.N.; Zhang, Y.B.; Xiao, Y.F. Obesity in children with different risk factors for obstructive sleep apnea: A community-based study. Eur. J. Pediatr. 2016, 175, 211–220. [Google Scholar] [CrossRef]
- Beebe, D.W. Neurobehavioral morbidity associated with disordered breathing during sleep in children: A comprehensive review. Sleep 2006, 29, 1115–1134. [Google Scholar] [CrossRef]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric Sleep Questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Chervin, R.D.; Weatherly, R.A.; Garetz, S.L.; Ruzicka, D.L.; Giordani, B.J.; Hodges, E.K.; Guire, K.E. Pediatric Sleep Questionnaire: Prediction of Sleep Apnea and Outcomes. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 216–222. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Clinical Practice Guideline: Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, 576. [Google Scholar] [CrossRef] [PubMed]
- Umano, G.R.; Rondinelli, G.; Luciano, M.; Pennarella, A.; Aiello, F.; Mangoni di Santo Stefano, G.S.R.; Miraglia del Giudice, E. Pediatric Sleep Questionnaire Predicts Moderate-to-Severe Obstructive Sleep Apnea in Children and Adolescents with Obesity. Children 2022, 9, 1303. [Google Scholar] [CrossRef]
- Micheal, J.; Sateia, M.D. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar]
- Kaditis, A.G.; Alvarez, M.L.A.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Verhulst, S. Obstructive sleep disordered breathing in 2- to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2015, 47, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Pena-Zarza, J.A.; Osona-Rodriguez de Torres, B.; Gil-Sanchez, J.A.; Figuerola-Mulet, J. Utility of the Pediatric Sleep Questionnaire and Pulse Oximetry as Screening Tools in Pediatric Patients with Suspected Obstructive Sleep Apnea Syndrome. Sleep Discord 2012, 2012, 819035. [Google Scholar] [CrossRef] [PubMed]
- Wing, Y.K.; Hui, S.H.; Pak, W.M.; Ho, C.K.; Cheung, A.; Li, A.M.; Fok, T.F. A controlled study of sleep related disordered breathing in obese children. Arch. Dis. Child. 2003, 88, 1043–1047. [Google Scholar] [CrossRef]
- Arens, R.; Sin, S.; Nandalike, K.; Rieder, J.; Khan, U.I.; Freeman, K.; Shifteh, K. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2011, 183, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cicognani, A. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. (Version 4.1) [Computer Software]. 2021. Available online: https://cran.r-project.org (accessed on 1 January 2022).
- Andersen, I.G.; Holm, J.C.; Homøe, P. Impact of weight-loss management on children and adolescents with obesity and obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 57–62. [Google Scholar] [CrossRef]
- Xu, Z.; An, J.; Li, Y.; Shen, K. A case-control study of obstructive sleep apnea-hypopnea syndrome in obese and nonobese Chinese children. Chest 2008, 133, 684–689. [Google Scholar] [CrossRef]
- Brietzke, S.E.; Katz, E.S.; Roberson, D.W. Cam history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypoapnea syndrome? A systematic review of the literarure. Otolaringol. Head Neck Surg. 2004, 131, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, Y.; Tai, J.; Feng, G.; Ge, W.; Zheng, L.; Ni, X. Risk factors of obstructive sleep apnea syndrome in children. J. Otolaryngol. Head Neck Surg. 2020, 49, 11. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Su, Y.X.; Wu, Y.C.; Chang, J.Z.C.; Tu, Y.K. Management of paediatric obstructive sleep apnoea: A systematic review and network meta-analysis. Int. J. Paediatr. Dent. 2019, 30, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Korobkin, R.; Winkle, R. A review of 50 children with obstructive sleep apnea syndrome. Lung 1981, 159, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Álvarez, M.L.; Cordero-Guevara, J.A.; Terán-Santos, J.; Gonzalez-Martinez, M.; José Jurado-Luque, M.; Corral-Peñafiel, J.; Duran-Cantolla, J.; Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea in Obese Community-Dwelling Children: The NANOS Study. Sleep 2014, 37, 943–949. [Google Scholar]
- Khosla, T.; Lowe, C.R. Indices of obesity derived from body weight and heigh. Nihon Rinsho 1995, 53, 147–153. [Google Scholar]




| Obesity Group | Control Group | p | |
|---|---|---|---|
| Subjects | 33 | 23 | 0.88 |
| Male | 19 | 11 | 0.47 |
| Female | 14 | 12 | 0.47 |
| Mean Age ± Sd (years) | 10.47 ± 2.93 | 8.72 ± 2.25 | 0.02 |
| BMI ± Sd (kg/m2) | 34.95 ± 7.27 | 17.69 ± 2.31 | <0.00 |
| Z-score BMI | 3.77 ± 0.87 | 0.63 ± 0.95 | <0.00 |
| Obesity Group | Control Group | p | |
|---|---|---|---|
| Osas Severity | 0.97 | ||
| Snoring (14.3%) | 5 (15.2%) | 3 (13%) | |
| Mild (33.9%) | 11 (33.3%) | 8 (34.7%) | |
| Moderate (32.2%) | 10 (30.3%) | 8 (34.7%) | |
| Severe (19.6%) | 7 (21.2%) | 4 (17.6%) | |
| AHI | 7.04 ± 5.80 | 6.23 ± 4.31 | 0.46 |
| ODI | 9.67 ± 10.30 | 3.72 ± 6.29 | 0.02 |
| Nadir O2 | 86.45 ± 6.76 | 88.94 ± 9.40 | 0.36 |
| SaO2 | 92.67 ± 1.27 | 96.2 ± 1.57 | 0.38 |
| PSQ | 0.53 ± 0.13 | 0.47 ± 0.15 | 0.15 |
| r2 | p | |
|---|---|---|
| Z-score BMI | 0.02 | 0.87 |
| AHI | 0.02 | 0.76 |
| SaO2 | 0.01 | 0.61 |
| ODI | 0.03 | 0.81 |
| Nadir O2 | 0.02 | 0.66 |
| r2 | p | |
|---|---|---|
| AHI | 0.01 | <0.00 |
| SaO2 | 0.09 | <0.00 |
| ODI | 0.04 | 0.56 |
| NADIR O2 | 0.04 | <0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caliendo, C.; Femiano, R.; Umano, G.R.; Martina, S.; Nucci, L.; Perillo, L.; Grassia, V. Effect of Obesity on the Respiratory Parameters in Children with Obstructive Sleep Apnea Syndrome. Children 2023, 10, 1874. https://doi.org/10.3390/children10121874
Caliendo C, Femiano R, Umano GR, Martina S, Nucci L, Perillo L, Grassia V. Effect of Obesity on the Respiratory Parameters in Children with Obstructive Sleep Apnea Syndrome. Children. 2023; 10(12):1874. https://doi.org/10.3390/children10121874
Chicago/Turabian StyleCaliendo, Carolina, Rossella Femiano, Giuseppina Rosaria Umano, Stefano Martina, Ludovica Nucci, Letizia Perillo, and Vincenzo Grassia. 2023. "Effect of Obesity on the Respiratory Parameters in Children with Obstructive Sleep Apnea Syndrome" Children 10, no. 12: 1874. https://doi.org/10.3390/children10121874
APA StyleCaliendo, C., Femiano, R., Umano, G. R., Martina, S., Nucci, L., Perillo, L., & Grassia, V. (2023). Effect of Obesity on the Respiratory Parameters in Children with Obstructive Sleep Apnea Syndrome. Children, 10(12), 1874. https://doi.org/10.3390/children10121874