An Update in Cystic Fibrosis-Related Diabetes in Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
3. Definition
4. Epidemiology
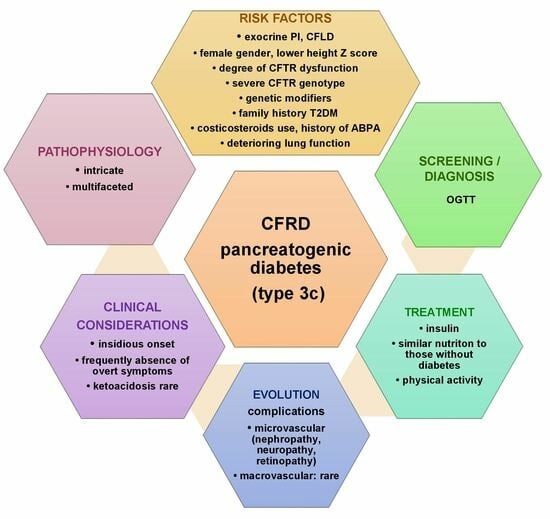
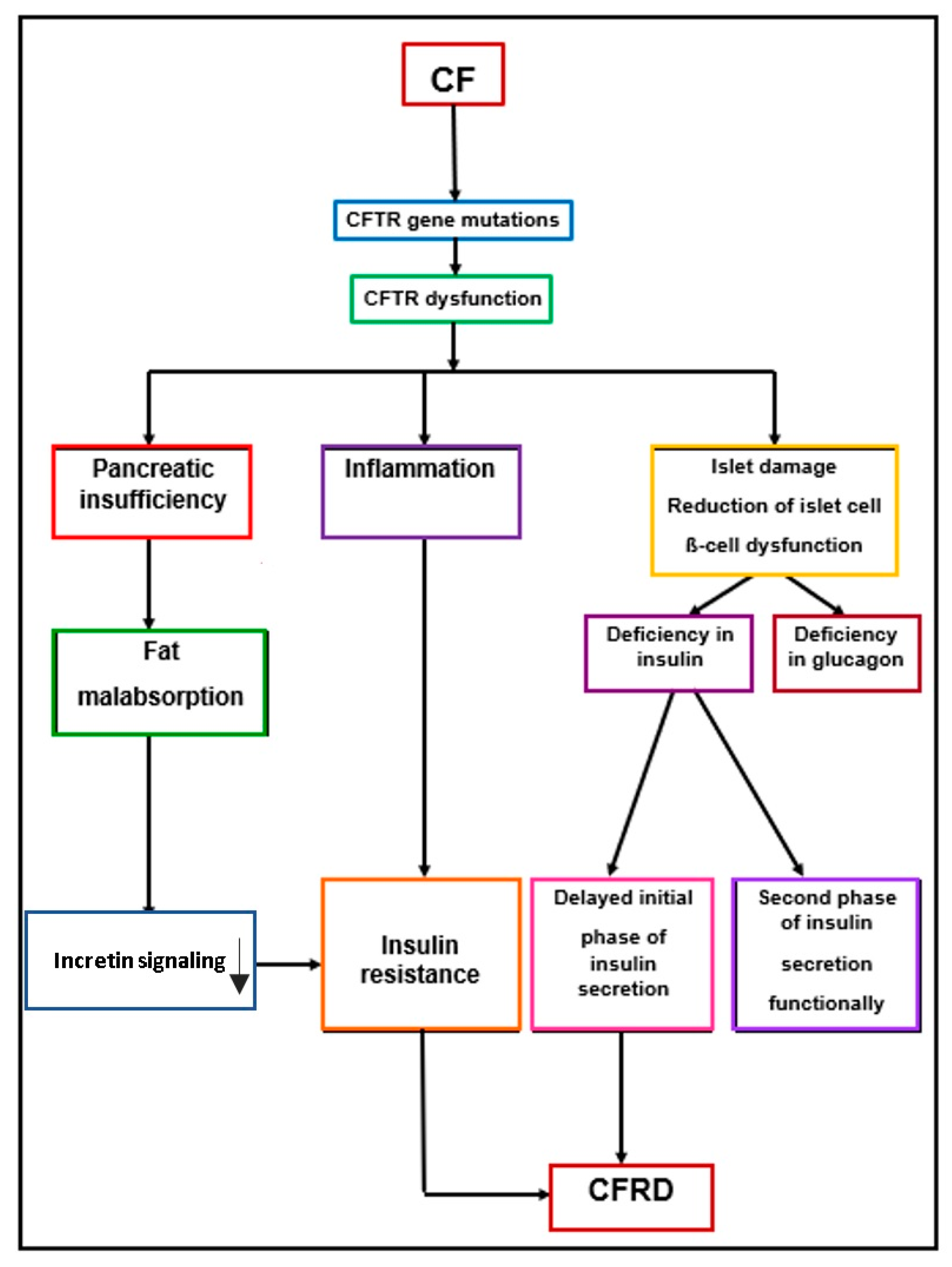
5. Pathophysiology of CFRD
6. Clinical Considerations
7. Screening and Diagnosis
7.1. Oral Glucose Tolerance Test (OGTT)
- Normal glucose tolerance (NGT): Characterized by fasting plasma glucose levels (FPG) of <5.6 mmol/L (<100 mg/dL) and plasma glucose at 2 h of <7.8 mmol/L (<140 mg/dL).
- Indeterminate glucose tolerance (INDET): Manifests when both the fasting and 2-h plasma glucose levels are normal, but the 1-h plasma glucose value is ≥11.1 mmol/L (≥200 mg/dL).
- Impaired fasting glucose (IFG): Indicates fasting glucose levels between 5.6–7 mmol/L (100–126 mg/dL) with 2-h plasma glucose remaining normal.
- Impaired glucose tolerance (IGT): Characterized by fasting glucose levels between 5.6–7 mmol/L (100–126 mg/dL) and 2-h plasma glucose levels between 7.8–11.0 mmol/L (140–199 mg/dL).
- CFRD without fasting hyperglycemia: Features fasting glucose levels of <7 mmol/L (<126 mg/dL) and 2-h plasma glucose levels ≥ 11.1 mmol/L (≥200 mg/dL).
- Time-consuming process: The OGTT demands a significant amount of time for both patients and medical staff.
- Fasting requirement: The patients must fast before undergoing the test, which can be burdensome.
- Morning testing: It is typically conducted in the morning, restricting the flexibility of timing.
- Taste and tolerance issues: Some patients may find the taste of the glucose solution unpalatable or may have difficulties tolerating it.
- Multiple blood draws: The procedure involves a substantial number of punctures or blood sample collections.
7.2. Fasting Plasma Glucose (FPG)
7.3. Glycated Hemoglobin (HbA1c)
7.4. Other Screening Methods
7.5. Continuous Glucose Monitoring (CGM)
8. Complications Development
9. Treatment of CFRD
9.1. Insulin Therapy
9.2. Nutritional Treatment
9.3. Oral Hypoglycemic Agents
9.3.1. Repaglinide
9.3.2. Incretins
9.3.3. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
9.3.4. Metformin
9.4. Physical Activity
10. The Role of CFTR Modulators in Patients with CFRD
10.1. The Role of Ivacaftor in CFRD
10.2. The Role of Lumacaftor/Ivacaftor in CFRD
10.3. The Role of Elexacaftor/Tezacaftor/Ivacaftor (ELX/TEZ/IVA) on CFRD
| Study | Year | Authors | Study Type | Study Group Size (Patients) | Age (Years) | Modulator | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Effect of IVA on insulin secretion | 2013 | Bellin et al. | Pilot study | 5 | 6–52 | Ivacaftor | Improvement of insulin secretion | [83] |
| The effects of Ivacaftor on growth and pancreatic function | 2019 | Emery et al. | Retrospective | 28 | 2–17 | Ivacaftor | Mild decrease seen in random blood glucose, but without significance | [84] |
| Disease progression in patients treated with IVA | 2020 | Volkova et al. | Observational, prospective, multicenter | 635 | Ivacaftor | Lung function better preserves lower frequencies of exacerbations and hospitalizations; improved nutritional status | [16] | |
| Effect of LUM/ IVA on glucose in CF | 2019 | Li et al. | Prospective | 9 | 11–15.6 | Lumacaftor/ Ivacaftor | Worsening in HbA1c and fasting plasma glucose; no significant improvements in the HbA1c levels, OGTT results, or CGM data after 29 weeks of treatment | [86] |
| Effect of LUM/IVA on glucose abnormalities in CF | 2020 | Misgault et al. | Observational, prospective, multicenter | 40 | 24 ± 10 | Lumacaftor/ Ivacaftor | Improvement of abnormalities in glucose tolerance after 1 year of LUM/IVA treatment | [80] |
| Effect of LUM/IVA on the insulin secretion in CF | 2021 | Moheet et al. | Prospective | 39 | 22 ± 10 | Lumacaftor/ Ivacaftor | No changes in fasting glucose, 2-h glucose, insulin, or time to peak insulin after 3, 6, and 12 months of treatment. Did not lead to improvements in insulin secretion or glucose tolerance | [87] |
| Effect of modulators on glucose in CF | 2022 | Korten et al. | Observational | 16 | ≥12 | Elexacaftor, Tezacaftor, Ivacaftor | Improvement of glucose tolerance measured by the OGTT | [81] |
| Impact of CFTR modulators on glucose metabolism. | 2022 | Piona et al. | Prospective, observational | 21 | ≥6 | Elexacaftor, Tezacaftor, Ivacaftor or Lumacaftor/ Ivacaftor | CFTR modulators do not significantly ameliorate glucose homeostasis and/or any of its direct determinants; insulin sensitivity worsened in the group treated with LUM/IVA | [88] |
| The effect of elexacaftor-tezacaftor-ivacaftor (ETI) on glucose tolerance | 2022 | Choudhari et al. | Retrospective | 12 | ≥12 | Elexacaftor, Tezacaftor, Ivacaftor | HbA1c values suffered minimally changes without significant differences comparative with the control group formed by children with CF who do not receive CFTR modulators | [89] |
| Effects of CFTR modulators on the FEV1, weight, BMI, HbA1c, and daily insulin dose | 2023 | Lurquin et al. | Retrospective | 17 | 37 ± 12 | Elexacaftor, Tezacaftor, Ivacaftor | Decrease in insulin doses; positive effects on BMI and FEV1 | [23] |
| Glucose regulation after the initiation of CFTR modulator treatment | 2023 | Park et al. | Case series/ presentation | 7 | >12 | Elexacaftor, Tezacaftor, Ivacaftor | Positive impact on glycemic control and insulin requirements | [90] |
11. Research Gaps and Future Directions
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coderre, L.; Debieche, L.; Plourde, J.; Rabasa-Lhoret, R.; Lesage, S. The Potential Causes of Cystic Fibrosis-Related Diabetes. Front. Endocrinol. 2021, 12, 702823. [Google Scholar] [CrossRef] [PubMed]
- Granados, A.; Chan, C.L.; Ode, K.L.; Moheet, A.; Moran, A.; Holl, R. Cystic fibrosis-related diabetes: Pathophysiology, screening and diagnosis. J. Cyst. Fibros. 2019, 18, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Pozo, L.; Bello, F.; Mendez, Y.; Surani, S. Cystic fibrosis-related diabetes: The unmet need. World J. Diabetes 2020, 11, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Iafusco, F.; Maione, G.; Rosanio, F.M.; Mozzillo, E.; Franzese, A.; Tinto, N. Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors. Diagnostics 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Olesen, H.V.; Drevinek, P.; Gulmans, V.A.; Hatziagorou, E.; Jung, A.; Mei-Zahav, M.; Stojnic, N.; Thomas, M.; Zoli, A. Cystic fibrosis-related diabetes in Europe: Prevalence, risk factors and outcome. J. Cyst. Fibros. 2020, 19, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ballmann, M. Cystic Fibrosis Related Diabetes. In Cystic Fibrosis. Facts, Management and Advances; Mohite, P., Reed, A., Simon, A., Eds.; IntechOpen: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Brodsky, J.; Dougherty, S.; Makani, R.; Rubenstein, R.C.; Kelly, A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care 2011, 34, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Hope, E.; Thurston, J.; Vigers, T.; Pyle, L.; Zeitler, P.S.; Nadeau, K.J. Hemoglobin A1c Accurately Predicts Continuous Glucose Monitoring–Derived Average Glucose in Youth and Young Adults With Cystic Fibrosis. Diabetes Care 2018, 41, 1406–1413. [Google Scholar] [CrossRef]
- Elidottir, H.; Diemer, S.; Eklund, E.; Hansen, C. Abnormal glucose tolerance and lung function in children with cystic fibrosis. Comparing oral glucose tolerance test and continuous glucose monitoring. J. Cyst. Fibros. 2021, 20, 779–784. [Google Scholar] [CrossRef]
- Kayani, K.; Mohammed, R.; Mohiaddin, H. Cystic Fibrosis-Related Diabetes. Front. Endocrinol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Chan, C.L. Continuous glucose monitoring in cystic fibrosis–Benefits, limitations, and opportunities. J. Cyst. Fibros. 2021, 20, 725–726. [Google Scholar] [CrossRef]
- Moheet, A.; Moran, A. New Concepts in the Pathogenesis of Cystic Fibrosis-Related Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Mogoi, M.; Pop, L.L.; Dediu, M.; Ciuca, I.M. Oral Glucose Tolerance Test in Patients with Cystic Fibrosis Compared to the Overweight and Obese: A Different Approach in Understanding the Results. Children 2022, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Sovtic, A. Diagnosis of cystic fibrosis-related diabetes: Too early or too late? J. Bras. Pneumol. 2022, 48, e20220069. [Google Scholar] [CrossRef] [PubMed]
- Hadjiliadis, D.; Madill, J.; Chaparro, C.; Tsang, A.; Waddell, T.K.; Singer, L.G.; Hutcheaon, M.A.; Keshavjee, S.; Tullis, D.E. Incidence and prevalence of diabetes mellitus in patients with cystic fibrosis undergoing lung transplantation before and after lung transplantation. Clin. Transplant 2005, 19, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.; Moy, K.; Evans, J.; Campbell, D.; Tian, S.; Simard, C.; Higgins, M.; Konstan, W.M.; Sawicki, S.G.; Elbert, A.; et al. Disease progression in patients with cystic fibrosis treated with ivacaftor. J. Cyst. Fibros. 2020, 19, 68–79. [Google Scholar] [CrossRef]
- Adler, I.A.; Shine, S.F.B.; Chamnan, P.; Haworth, S.C.; Bilton, D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: Results from a British cohort of children and adults. Diabetes Care 2008, 31, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Blackman, S.M.; Hsu, S.; Ritter, S.E.; Naughton, K.M.; Wright, F.A.; Drumm, M.L.; Knowles, M.R.; Cutting, G.R. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009, 52, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the CysticFibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef]
- Sidhaye, A.; Goldswieg, B.; Kaminski, B.; Blackman, S.M.; Kelly, A. Endocrine complications after solid-organ transplant in cystic fibrosis. J. Cyst. Fibros. 2019, 18, S111–S119. [Google Scholar] [CrossRef]
- Khare, S.; Desimone, M.; Kasim, N.; Chan, L.C. Cystic fibrosis-related diabetes: Prevalence, screening, and diagnosis. J. Clin. Transl. Endocrinol. 2022, 27, 100290. [Google Scholar] [CrossRef]
- Blackman, M.S.; Commander, W.C.; Watson, C.; Arcarea, M.K.; Strug, J.L.; Stonebraker, R.J.; Wright, A.F.; Rommens, M.J.; Sun, L.; Pace, G.R.; et al. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes 2013, 62, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Lurquin, F.; Gohy, S.; Hermans, P.M.; Preumont, V. Combined CFTR modulator therapies are linked with anabolic benefits and insulin-sparing in cystic fibrosis-related diabetes. J. Clin. Transl. Endocrinol. 2023, 33, 100320. [Google Scholar] [CrossRef] [PubMed]
- Ticona, J.H.; Lapinel, N.; Wang, J. Future Comorbidities in an Aging Cystic Fibrosis Population. Life 2023, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Montemari, A.L.; Manco, M.; Fiocchi, A.G.; Bartoli, M.; Facchiano, F.; Tabolacci, C.; Scatigna, M.; Ciciriello, F.; Alghisi, F.; Montemitro, E.; et al. An inflammatory Signature of Glucose Impairment in Cystic Fibrosis. J. Inflamm. Res. 2022, 15, 5677–5685. [Google Scholar] [CrossRef]
- Davern, R.; Balan, G.; Kilcoyne, C.; Coveney, C.; Devine, H.; Walsh, J.; Higgins, M.; Hatunic, M. Cystic Fibrosis-Related Diabetes Mellitus and Pregnancy: A Retrospective Study. Diabetes Ther. 2022, 13, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Jones, G.H.; Dyce, P.; Jackson, V.; Nazareth, D.; Walshaw, M.J. Loss of incretin effect contributes to postprandial hyperglycaemia in cystic fibrosis-related diabetes. Diabet Med. 2019, 36, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.J.; Aramandla, R.; Poffenberger, G.; Fayolle, C.; Thames, A.R.; Bautista, A.; Spigelman, A.; Babon, J.A.; DeNicola, M.; Dadi, P.; et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight. 2018, 3, e98240. [Google Scholar] [CrossRef]
- Gojsina, B.; Minic, P.; Todorovic, S.; Soldatovic, I.; Sovtic, A. Continuous Glucose Monitoring as a Valuable Tool in the Early Detection of Diabetes Related to Cystic Fibrosis. Front. Pediatr. 2021, 9, 659728. [Google Scholar] [CrossRef]
- Putman, S.M.; Norris, W.A.; Hull, L.R.; Rickels, R.M.; Sussel, L.; Blackman, M.S.; Chan, L.C.; Ode, K.L.; Daley, T.; Stecenko, A.A.; et al. Cystic Fibrosis–Related Diabetes Workshop: Research Priorities Spanning Disease Pathophysiology, Diagnosis, and Outcomes. Diabetes Care 2023, 46, 1112–1123. [Google Scholar] [CrossRef]
- Schiaffini, R.; Pampanini, V. Diabetes and prediabetes in children with cystic fibrosis. Curr. Opin. Pediatr. 2023, 35, 481–485. [Google Scholar] [CrossRef]
- Perrem, L.; Stanojevic, S.; Solomon, M.; Carpenter, S.; Ratjen, F. Incidence and risk factors of paediatric cystic fibrosis-related diabetes. J. Cyst. Fibros. 2019, 18, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Foppiani, A.; Bisogno, A.; Gambazza, S.; Daccò, V.; Nazzari, E.; Leone, A.; Giana, A.; Mari, A.; Battezzati, A. Lumacaftor/ivacaftor in cystic fibrosis: Effects on glucose metabolism and insulin secretion. J. Endocrinol. Investig. 2021, 44, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.A.; Pyle, L.; Ha, J.; Sherman, A.; Cree-Green, M.; Sagel, D.S.; Nadeau, J.K.; Chan, L.C. Predictive Value of 1-Hour Glucose Elevations during Oral Glucose Tolerance Testing for Cystic Fibrosis-Related Diabetes. Hindawi Pediatr. Diabetes 2023, 2023, 4395556. [Google Scholar] [CrossRef]
- Hicks, R.; Ode, K.L.; Vigers, T.; Chan, C.L. A provider survey of cystic fibrosis related diabetes screening and management practices at North American CF centers. Front. Endocrinol. 2023, 14, 1183288. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Pillay, K.; Becker, D.; Granados, A.; Hameed, S.; Acerini, C.L. ISPAD clinical practice consensus guidelines 2018: Management of cystic fibrosisrelated diabetes in children and adolescents. Pediatr. Diabetes. 2018, 19 (Suppl. S27), 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ode, K.L.; Ballman, M.; Battezzati, A.; Brennan, A.; Chan, L.C.; Hameed, S.; Heba, M.I.; Kelly, A.; Moran, M.A.; Rabasa-Lhoret, R.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1212–1228. [Google Scholar] [CrossRef]
- Battezzati, A.; Battezzati, P.M.; Costantini, D.; Seia, M.; Zazzeron, L.; Russo, M.C.; Daccò, V.; Bertoli, S.; Crosignani, A.; Colombo, C. Spontaneous hypoglycemia in patients with cystic fibrosis. Eur. J. Endocrinol. 2007, 156, 369–376. [Google Scholar] [CrossRef]
- Tommerdahl, K.L.; Brinton, J.T.; Vigers, T.; Nadeau, K.J.; Zeitler, P.S.; Chan, C.L. Screening for cystic fibrosis-related diabetes and prediabetes: Evaluating 1,5-anhydroglucitol, fructosamine, glycated albumin, and hemoglobin A1c. Pediatr. Diabetes 2019, 20, 1080–1086. [Google Scholar] [CrossRef]
- Boudreau, V.; Coriati, A.; Desjardins, K.; Rabasa-Lhoret, R. Glycated hemoglobin cannot yet be proposed as a screening tool for cystic fibrosis related diabetes. J. Cyst. Fibros. 2016, 15, 258–260. [Google Scholar] [CrossRef]
- Racine, F.; Shohoudi, A.; Boudreau, V.; Nguyen, C.Q.T.; Denis, M.H.; Desjardins, K.; Reynaud, Q.; Rabasa-Lhoret, R.; Mailhot, G. Glycated Hemoglobin as a First-line Screening Test for Cystic Fibrosis—Related Diabetes and Impaired Glucose Tolerance in Children With Cystic Fibrosis: A Validation Study. Can. J. Diabetes 2021, 45, 768–774. [Google Scholar] [CrossRef]
- Lam, Y.G.; Doll-Shankaruk, M.; Dayton, J.; Rodriguez-Capote, K.; Higgins, N.T.; Thomas, D.; Mulchey, K.; Smith, P.M.; Brown, E.N.; Leung, M.W.; et al. The use of fructosamine in cystic fibrosis-related diabetes (CFRD) screening. J. Cyst. Fibros. 2018, 17, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Y.; Wang, J.M.; Zhao, X.L.; Yang, C.; Jiang, X.S.; Chen, Y.M.; Chen, C.Q.; Li, Z.Y. Glycated albumin as a biomarker for diagnosis of diabetes mellitus: A systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 9520–9534. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Ode, K.L.; Granados, A.; Moheet, A.; Moran, A.; Hameed, S. Continuous glucose monitoring in cystic fibrosis—A practical guide. J. Cyst. Fibros. 2019, 18, S25–S31. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, S.M.P.; Hindmarsh, P.; Hill, N.R.; Matthews, D.R.; George, S.; Greally, P.; Canny, G.; Slattery, D.; Murphy, N.; Roche, E.; et al. Validation of Continuous Glucose Monitoring in Children and Adolescents with Cystic Fibrosis: A prospective cohort study. Diabetes Care 2009, 32, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Scully, J.K.; Marchetti, P.; Sawicki, S.G.; Uluer, A.; Cernadas, M.; Cagnina, E.R.; Kennedy, C.J.; Putman, M.S. The effect of elexacaftor/tezacaftor/ivacaftor (ETI) on glycemia in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, A.; Gauthier, B.; Rosner, V.; Weiss, L.; Moreau, F.; Constantinescu, A.A.; Kessler, R. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J. Cyst. Fibros. 2014, 13, 478–484. [Google Scholar] [CrossRef]
- Banavath, L.N.; Kumar, R.; Dayal, D.; Yadav, J.; Sachdeva, N.; Mathew, J.L.; Vaidya, P.C.; Singh, M. Glucose intolerance in children with cystic fibrosis: A developing country’s perspective. J. Pediatr. Endocrinol. Metab. 2018, 31, 1139–1146. [Google Scholar] [CrossRef]
- Tofé, S.; Moreno, J.C.; Máiz, L.; Alonso, M.; Escobar, H.; Barrio, R. Insulin-secretion abnormalities and clinical deterioration related to impaired glucose tolerance in cystic fibrosis. Eur. J. Endocrinol. 2005, 152, 241–247. [Google Scholar] [CrossRef]
- Hameed, S.; Morton, J.R.; Jaffé, A.; Field, P.I.; Belessis, Y.; Yoong, T.; Katz, T.; Verge, C.F. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care 2010, 33, 221–226. [Google Scholar] [CrossRef]
- Burgess, J.C.; Bridges, N.; Banya, W.; Gyi, K.; Hodson, M.; Bilton, D.; Simmonds, N. HbA1c as a screening tool for cystic fibrosis related diabetes. J. Cyst. Fibros. 2016, 15, 251–257. [Google Scholar] [CrossRef]
- Gilmour, J.; Sykes, J.; Etchells, E.; Tullis, E. Cystic Fibrosis-Related Diabetes Screening in Adults: A Gap Analysis and Evaluation of Accuracy of Glycated Hemoglobin Levels. Can. J. Diabetes 2019, 43, 13–18. [Google Scholar] [CrossRef] [PubMed]
- León, M.C.; Bilbao Gassó, L.; Moreno-Galdó, A.; Campos Martorell, A.; Gartner Tizzano, S.; Fernández, D.Y.; Carrascosa Lezcano, A. Oral glucose tolerance test and continuous glucose monitoring to assess diabetes development in cystic fibrosis patients. Endocrinol. Diabetes Nutr. 2018, 65, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zorron, M.; Marson, F.A.L.; Morcillo, A.M.; Gonçalves, A.C.; El Beck, M.S.; Ribeiro, J.D.; Ribeiro, A.F. Can continuous glucose monitoring predict cystic fibrosis-related diabetes and worse clinical outcome? J. Bras. Pneumol. 2022, 48, e20210307. [Google Scholar] [CrossRef] [PubMed]
- Prentice, B.J.; Ooi, C.Y.; Verge, C.F.; Hameed, S.; Widger, J. Glucose abnormalities detected by continuous glucose monitoring are common in young children with Cystic Fibrosis. J. Cyst. Fibros. 2020, 19, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Valles, F.; Arranz Martín, J.A.; Girón, R.M.; Knott-Torcal, C.; Sampedro-Nuñez, M.A.; Martin-Adan, J.C.; Jiménez-Díaz, J.; Marazuela, M. Continuous Glucose Monitoring as an Additional Tool in Early Cystic Fibrosis-Related Diabetes Monitoring and in Evaluation of Short-Term Sitagliptin Response. Biomedicines 2023, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Vagg, T.; Kumar, S.; Soldatos, G.; Ranasinha, S.; Teede, H.; Pallin, M. Continuous glucose monitoring versus self-monitoring of blood glucose in the management of cystic fibrosis related diabetes: A systematic review and meta-analysis. J. Cyst. Fibros. 2022, 22, 39–49. [Google Scholar] [CrossRef]
- Patel, M.; McCracken, C.; Daley, T.; Stecenko, A.; Linnemann, R. Trajectories of oral glucose tolerance testing in cystic fibrosis. Pediatr. Pulmonol. 2021, 56, 901–909. [Google Scholar] [CrossRef]
- Suppakitjanusant, P.; Kasemkosin, N.; Sivapiromrat, K.A.; Weinstein, S.; Ongphiphadhanakul, B.; Hunt, R.W.; Sueblinvong, V.; Tangpricha, V. Predicting glycemic control status and high blood glucose levels through voice characteristic analysis in patients with cystic fibrosis-related diabetes (CFRD). Sci. Rep. 2023, 13, 8617. [Google Scholar] [CrossRef]
- Schwarzenberg, S.J.; Thomas, W.; Olsen, W.T.; Grover, T.; Walk, D.; Milla, C.; Moran, A. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007, 30, 1056–1061. [Google Scholar] [CrossRef]
- Sandouk, Z.; Khan, F.; Khare, S.; Moran, A. Cystic fibrosis related diabetes (CFRD) prognosis. J. Clin. Transl. Endocrinol. 2021, 26, 100278. [Google Scholar] [CrossRef]
- Totani, L.; Plebani, R.; Piccoli, A.; Di Silvestre, S.; Lanuti, P.; Recchiuti, A.; Cianci, E.; Dell’Elba, G.; Sacchetti, S.; Patruno, S.; et al. Mechanisms of endothelial cell dysfunction in cystic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3243–3253. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, O.W.; Stoate, A.L.E.; Dobson, L.; Williams, C.A. The Effect of Dysglycaemia on Changes in Pulmonary and Aerobic Function in Cystic Fibrosis. Front. Physiol. 2022, 13, 834664. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.W.J.; Alison, M.; Morton, M.A.; Kok, W.S.; Pijl, H.; Conway, P.S.; Heijerman, G.M.H. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J. Cyst. Fibros. 2008, 7, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Kempegowda, P.; Sunsoa, H.; Chandan, S.J.; Quinn, M.L.; Amrelia, M.P.; Atta, S.N.; Amir, S.; The, Y.S.; Chaudhry, S.; de Bray, A.; et al. Retinopathy and microalbuminuria are common microvascular complications in cystic fibrosis-related diabetes. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820966428. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Della-Manna, T.; Albuquerque, C.T.M. Cystic fibrosis-related diabetes: An update on pathophysiology, diagnosis, and treatment. J. Pediatr. Endocrinol. Metab. 2020, 33, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Ode, K.L.; Chan, C.L.; Granados, A.; Moheet, A.; Moran, A.; Brennan, A.L. Cystic fibrosis related diabetes: Medical management. J. Cyst. Fibros. 2019, 18, S10–S18. [Google Scholar] [CrossRef]
- Mozzillo, E.; Franceschi, R.; Piona, C.; Passanisi, S.; Casertano, A.; Pjetraj, D.; Maltoni, G.; Calcaterra, V.; Cauvin, V.; Cherubini, V.; et al. Diabetes and prediabetes in children with cystic fibrosis: A systematic review of the literature and recommendations of the Italian Society for Pediatric Endocrinology and Diabetes (ISPED). Front. Endocrinol. 2021, 29, 673539. [Google Scholar] [CrossRef]
- Scheuing, N.; Badenhoop, M.; Konrad, K.; Lilienthal, E.; Laubner, K.; Naeke, A.; Rami-Merhar, B.; Thon, A.; Wiemann, D.; Holl, R.W. Why is insulin pump treatment rarely used in adolescents and young adults with cystic fibrosis-related diabetes? Pediatr. Diabetes 2015, 16, 10–15. [Google Scholar] [CrossRef]
- Kaminski, B.A.; Goldsweig, B.K.; Sidhaye, A.; Blackman, S.M.; Schindler, T.; Moran, A. Cystic fibrosis related diabetes: Nutrition and growth considerations. J. Cyst. Fibros. 2019, 18, S32–S37. [Google Scholar] [CrossRef]
- Ballmann, M.; Hubert, D.; Assael, B.M.; Staab, D.; Hebestreit, A.; Naelich, L.; Nickolay, T.; Prinz, N.; Holl, R. Repaglinide versus insulin for newly diagnosed diabetes in patients with cystic fibrosis: A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 114–121. [Google Scholar] [CrossRef]
- Geyer, M.C.; Sullivan, T.; Tai, A.; Morton, J.M.; Edwards, S.; Martin, A.J.; Perano, S.J.; Gagliardi, L.; Rayner, C.K.; Horowitz, M.; et al. Exenatide corrects postprandial hyperglycaemia in young people with cystic fibrosis and impaired glucose tolerance: A randomized crossover trial. Diabetes Obes. Metab. 2019, 21, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Gnanapragasam, H.; Mustafa, N.; Bierbrauer, M.; Providence, T.A.; Dandona, P. Semaglutide in Cystic Fibrosis-Related Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2341–2344. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Khan, M.S.; Lansang, C. The effect of cystic fibrosis transmembrane conductance regulator modulators on impaired glucose tolerance and cystic fibrosis related diabetes. J. Clin. Transl. Endocrinol. 2022, 29, 100301. [Google Scholar] [CrossRef] [PubMed]
- Kapouni, N.; Moustaki, M.; Douros, K.; Loukou, I. Efficacy and Safety of Elexacaftor-Tezacaftor-Ivacaftor in the Treatment of Cystic Fibrosis: A Systematic Review. Children 2023, 10, 554. [Google Scholar] [CrossRef]
- Schmid, K.; Fink, K.; Holl, R.W.; Hebestreit, H.; Ballmann, M. Predictors for future cystic fibrosis-related diabetes by oral glucose tolerance test. J. Cyst. Fibros. 2014, 13, 80–85. [Google Scholar] [CrossRef]
- Regard, L.; Martin, C.; Da Silva, J.; Burgel, P.R. CFTR Modulators: Current Status and Evolving Knowledge. Semin. Respir. Crit. Care Med. 2023, 44, 186–195. [Google Scholar] [CrossRef]
- Salazar-Barragan, M.; Taub, D.R. The Effects of Elexacaftor, Tezacaftor, and Ivacaftor (ETI) on Blood Glucose in Patients With Cystic Fibrosis: A Systematic Review. Cureus 2023, 15, e41697. [Google Scholar] [CrossRef]
- Merjaneh, L.; Hasan, S.; Kasim, N.; Ode, K.L. The role of modulators in cystic fibrosis related diabetes. J. Clin. Transl. Endocrinol. 2022, 27, 100286. [Google Scholar] [CrossRef]
- Misgault, B.; Chatron, E.; Quitterie, R.; Touzet, S.; Abely, S.; Melly, L.; Dominique, S.; Troussier, F.; Ronsin-Pradel, O.; Gerardin, M.; et al. Effect of one-year lumacaftor-ivacaftor treatment on glucose tolerance abnormalities in cystic fibrosis patients. J. Cyst. Fibros. 2020, 19, 712–716. [Google Scholar] [CrossRef]
- Korten, I.; Kieninger, E.; Krueger, L.; Bullo, M.; Flück, C.E.; Latzin, P.; Casaulta, C.; Boettcher, C. Short-Term Effects of Elexacaftor/Tezacaftor/Ivacaftor Combination on Glucose Tolerance in Young People With Cystic Fibrosis—An Observational Pilot Study. Front. Pediatr. 2022, 10, 852551. [Google Scholar] [CrossRef]
- Connett, G.J. Lumacaftor-ivacaftor in the treatment of cystic fibrosis: Design, development and place in therapy. Drug Des. Dev. Ther. 2019, 13, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Laguna, T.; Leschyshyn, J.; Regelmann, W.; Dunitz, J.; Billings, J.; Moran, A. Insulin secretion improves in cystic fibrosis following Ivacaftor correction of CFTR: A small pilot study. Pediatr. Diabetes 2013, 14, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.; Mullane, D.; Chroinin, M.N. The effects of Ivacaftor on pancreatic function in pediatric patients with cystic fibrosis gating mutations. Arch. Dis. Child. 2019, 104 (Suppl. S3), A1–A428. [Google Scholar] [CrossRef]
- Jordan, K.D.; Zemanick, E.T.; Taylor-Cousar, J.L.; Hoppe, J.E. Managing cystic fibrosis in children aged 6-11yrs: A critical review of elexacaftor/tezacaftor/ivacaftor combination therapy. Expert Rev. Respir. Med. 2023, 17, 97–108. [Google Scholar] [CrossRef]
- Li, A.; Vigers, T.; Pyle, L.; Zemanick, E.; Nadeau, K.; Sagel, S.D.; Chan, C.L. Continuous glucose monitoring in youth with cystic fibrosis treated with lumacaftor-ivacaftor. J. Cyst. Fibros. 2019, 18, 144–149. [Google Scholar] [CrossRef]
- Moheet, A.; Beisang, D.; Zhang, L.; Sagel, D.S.; Van Dalfsen, M.J.; Heltshee Sonya, L.; Frederick, C.; Mann, M.; Antos, N.; Billings, J.; et al. Lumacaftor/Ivacaftor Therapy Fails to Increase Insulin Secretion in F508del/F508del CF Patients. J. Cyst. Fibros. 2021, 20, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Piona, C.; Mozzillo, E.; Tosco, A.; Volpi, S.; Rosanio, F.M.; Cimbalo, C.; Franzese, A.; Raia, V.; Zusi, C.; Emiliani, F.; et al. Impact of CFTR Modulators on Beta-Cell Function in Children and Young Adults with Cystic Fibrosis. J. Clin. Med. 2022, 11, 4149. [Google Scholar] [CrossRef]
- Choudhari, P.; Ham, M.R. Effects Of Elexacaftor-tezacaftor-ivacaftor On Glucose Tolerance And Other Metabolic Parameters In Children With Cystic Fibrosis. J. Endocr. Soc. 2023, 6 (Suppl. S1), A595–A596. [Google Scholar] [CrossRef]
- Park, J.; Walsh, A.; Kerr, S.; Woodland, C.; Southward, S.; Deakin, M.; Senniappan, S.; Thursfield, R. Improvements in glucose regulation in children and young people with cystic fibrosis-related diabetes following initiation of elexacaftor/tezacaftor/ivacaftor. Horm. Res. Paediatr. 2023. [Google Scholar] [CrossRef]
| CFRD | T1DM | T2DM | |
|---|---|---|---|
| Prevalence in CF patients | 22.2% (ECFS registries) | 0.2% | 11% |
| Maximum age of onset | Young adults | Children and adolescents | Adults |
| Onset | - Subtle and gradual - Frequent absence of overt symptoms | Acute | Insidious |
| Risk factors | - Exocrine pancreatic insufficiency (PI) - Female gender - Degree of residual CFTR function - Severe CFTR genotype - Genetic modifiers - Family history of T2DM - CF-related liver disease (CFLD) - Solid organ transplantation - Systemic corticosteroids use - Calcineurin inhibitors - Deteriorating lung function - History of allergic bronchopulmonary aspergillosis (ABPA) - Gastrostomy tube feedings - Lower childhood height z- score | - Family history of T1DM - Infectious agents - Intestinal microbiota - Dietary factors - Genetics - Age - Geography | - Family history of T2DM - Overweight - Prediabetes - Age > 45 years - Gestational diabetes - African American, Hispanic or Latino, or American Indian |
| Clinical symptoms | - Polyuria - Polydipsia - Sensations of fatigue - Delayed onset of puberty | - Polyuria - Polydipsia - Polyphagia - Loss of weight | - Increased thirst and hunger - Frequent urination - Unintended weight loss - Fatigue - Frequent infections - Acanthosis nigricans |
| Weight | Normal/Underweight | Normal | Overweight/Obese |
| Antibodies | Rare | Yes | No |
| Pathogenesis | - Inflammation, insulin resistance - Islet damage - Reduction in islet cells - β-cells dysfunction | - Autoimmune destruction of the β-cells of the endocrine pancreas | - Insulin resistance and reduced secretion of insulin by the β-cells |
| Insulin secretion | Reduced | Reduced to absent | Severely reduced |
| Sensitivity to insulin | Somewhat reduced | Somewhat reduced | Severely reduced |
| Screening/diagnosis | Oral glucose tolerance test (OGTT) | FPG ≥ 126 mg/dL (7.0 mmol/L), or a 2-h plasma glucose level ≥ 200 mg/dL (11.1 mmol/L) during a 1.75 g/kg oral glucose tolerance test (OGTT), or a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis | HbA1c |
| Risk of ketoacidosis | Rare | Yes | Rare |
| Insulin | Yes | Yes | Yes, if diet, oral antihyperglycemic agents, and insulin secretagogues are without results |
| Dietary recommendation | - Hypercaloric - Hyperproteic - Normoglucidic - Liposoluble vitamins | - Carbohydrate monitoring. - Normal balanced diet to allow the harmonious development of the child. | - Monitoring carbohydrates and calories. - Promotion of decreased weight |
| Physical activity | Yes | Yes | |
| Macrovascular complications | No/Rare | Yes | Yes |
| Microvascular complications (retinopathy, nephropathy, and neuropathy) | Yes (less frequent and severe) | Yes | Yes |
| Causes of death | Pulmonary involvement | Cardiovascular disease; nephropathy | Cardiovascular disease |
| Method of Screening | Study | Year | Authors | Results | Ref. |
|---|---|---|---|---|---|
| OGTT | Insulin secretion abnormalities | 2005 | Tofé et al. | Hypoglycemia signifies a disruption in insulin secretion regulation. | [49] |
| OGTT | Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain | 2010 | Hameed et al. | A peak blood glucose level < 8.2 mmol/L (147 mg/dL) is associated with a decline in the weight z-score and lung function over the preceding 12 months. | [50] |
| OGTT | Relation between 1-h plasma glucose during the OGTT and pulmonary function | 2011 | Brodsky et al. | Elevated blood glucose levels 1-h after the OGTT could serve as an early marker for the decline in respiratory function. | [7] |
| OGTT | AGT in children with CF and its relationship with the duration and severity of CF | 2018 | Banavath et al. | The majority of children with CF for >3 years and/or age >6 years developed AGT. | [48] |
| AGT in children aged 6 to 9 years can identify those at a higher risk of developing CFRD. | |||||
| OGTT; FPG | Screening methods for the diagnosis of CFRD | 2019 | Granados et al. | The OGTT is primarily designed for detecting T2DM. | [2] |
| The 2-h blood glucose level and fasting glucose level in the OGTT may not be as relevant in the context of CF as it is in T2DM. | |||||
| Under these circumstances, an FPG of 7.0 mmol/L (126 mg/dL) or a postprandial blood glucose level of 11.1 mmol/L (200 mg/dL) may raise suspicion of CFRD. | |||||
| OGTT | Screening rate | 2021 | Racine et al. | A screening rate of 53%, with variations from 29.5% among children born before 1993 to 76.7% for those born after. | [41] |
| OGTT | Predictive value of 1-hour glucose elevations during the OGTT | 2023 | Lorenz et al. | The elevations in the 1-h glucose levels >140 mg/dL were linked to a progression toward CFRD in the subsequent 5 years. | [34] |
| The 1-h glucose measurement distinguishes individuals at high risk from those at low risk of CFRD development. | |||||
| OGTT; HbA1c; CGM | Comparison between the OGTT and HbA1c | 2018 | Chan et al. | The limited agreement between the OGTT and HbA1c should not diminish the utility of HbA1c. | [8] |
| HbA1c exhibited a notable correlation with multiple glucose measurements during CGM. | |||||
| FPG; HbA1c | Utility of different methods of diagnosis for CFR | 2022 | Sovtic | FPG concentrations can persist within the normal range for an extended duration in approximately half of CFRD patients. | [14] |
| HbA1c exhibits a sensitivity of merely 50% and does not correlate adequately with the mean plasma glucose levels. | |||||
| HbA1c—limited predictive value | |||||
| HbA1c | Sensitivity and specificity of HbA1c in CFRD | 2016 | Burgess et al. | Lower sensitivity (68.2%) and specificity (60.5%) | [51] |
| HbA1c; GA; OGTT | Investigation of alternate glycemic markers as screening tests for (CFRD) | 2019 | Tommerdahl et al. | The value of HbA1c 5.5% was found to differentiate patients with more stable lung functions from those with more impaired lung functions. | [39] |
| GA exhibited a significant correlation with the 2-h OGTT values. | |||||
| HbA1c | The applicability of HbA1c as a method of diagnosis in children with CFRD | 2019 | Gilmour et al. | Limited data was available regarding the applicability of HbA1c between 5.5–6.4% for diagnosing CFRD in children. | [52] |
| FSF | Utility of FSF in screening for CFRD | 2018 | Lam et al. | In situations where the HbA1C measurements may be unreliable due to red blood cell turnover, FSF emerges as a reliable alternative for tracking the clinical outcomes. | [42] |
| GA | Utility of GA in the screening for CFRD | 2021 | Xiong et al. | GA should be considered an additional test rather than a substitute for HbA1c or OGTT. | [43] |
| HbA1c; OGTT; CGM | Correlations between HbA1c, OGTT, and CGM | 2021 | Gojsina et al. | The HbA1c levels were lower in the CGM–CFRD subgroup compared to the OGTT–CFRD subgroup. | [29] |
| CGM; OGTT | Comparison between the results of the OGTT and those obtained through CGM | 2018 | Leon et al. | CGM was effective in identifying glucose fluctuations that had gone unnoticed in the OGTT. | [53] |
| CGM; OGTT | The effectiveness of CGM in predicting the onset of CFRD | 2022 | Zorron et al. | The CGM is useful for identifying abnormalities in glucose metabolism that the OGTT might not detect. | [54] |
| CGM offers the advantage of providing a more detailed characterization of glucose patterns. | |||||
| CGM | Utility of CGM for identifying carbohydrate metabolism abnormalities | 2020 | Prentice et al. | CGM effectively identifies carbohydrate metabolism abnormalities that are prevalent in children under 10 years of age. | [55] |
| CGM | Value of CGM in the diagnosis of CFRD | 2023 | Sebastian-Valles et al. | CGM represents the glycemic response as a continuous variable within a patient’s daily routine. | [56] |
| The increased cost associated with CGM may restrict its routine use. | |||||
| CGM | Continuous glucose monitoring versus self-monitoring of blood glucose in the management of CFRD | 2023 | Vagg et al. | CGM may enhance glycemic control when compared to conventional finger-prick glucose monitoring. | [57] |
| CGM; OGTT | Comparative data on CGM versus the OGTT | 2021 | Elidottir et al. | CGM could serve as an additional parameter to the OGTT in the assessment of glucose abnormalities. | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton-Păduraru, D.-T.; Murgu, A.M.; Donos, M.A.; Trofin, F.; Azoicăi, A.N.; Popovici, P.; Stana, A.B.; Gheorghiescu, I.; Trandafir, L.M. An Update in Cystic Fibrosis-Related Diabetes in Children and Adolescents. Children 2023, 10, 1879. https://doi.org/10.3390/children10121879
Anton-Păduraru D-T, Murgu AM, Donos MA, Trofin F, Azoicăi AN, Popovici P, Stana AB, Gheorghiescu I, Trandafir LM. An Update in Cystic Fibrosis-Related Diabetes in Children and Adolescents. Children. 2023; 10(12):1879. https://doi.org/10.3390/children10121879
Chicago/Turabian StyleAnton-Păduraru, Dana-Teodora, Alina Mariela Murgu, Mădălina Andreea Donos, Felicia Trofin, Alice Nicoleta Azoicăi, Paula Popovici, Aurelian Bogdan Stana, Ionela Gheorghiescu, and Laura Mihaela Trandafir. 2023. "An Update in Cystic Fibrosis-Related Diabetes in Children and Adolescents" Children 10, no. 12: 1879. https://doi.org/10.3390/children10121879
APA StyleAnton-Păduraru, D.-T., Murgu, A. M., Donos, M. A., Trofin, F., Azoicăi, A. N., Popovici, P., Stana, A. B., Gheorghiescu, I., & Trandafir, L. M. (2023). An Update in Cystic Fibrosis-Related Diabetes in Children and Adolescents. Children, 10(12), 1879. https://doi.org/10.3390/children10121879