Efficacy of the Simeox® Airway Clearance Technology in the Homecare Treatment of Children with Clinically Stable Cystic Fibrosis: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
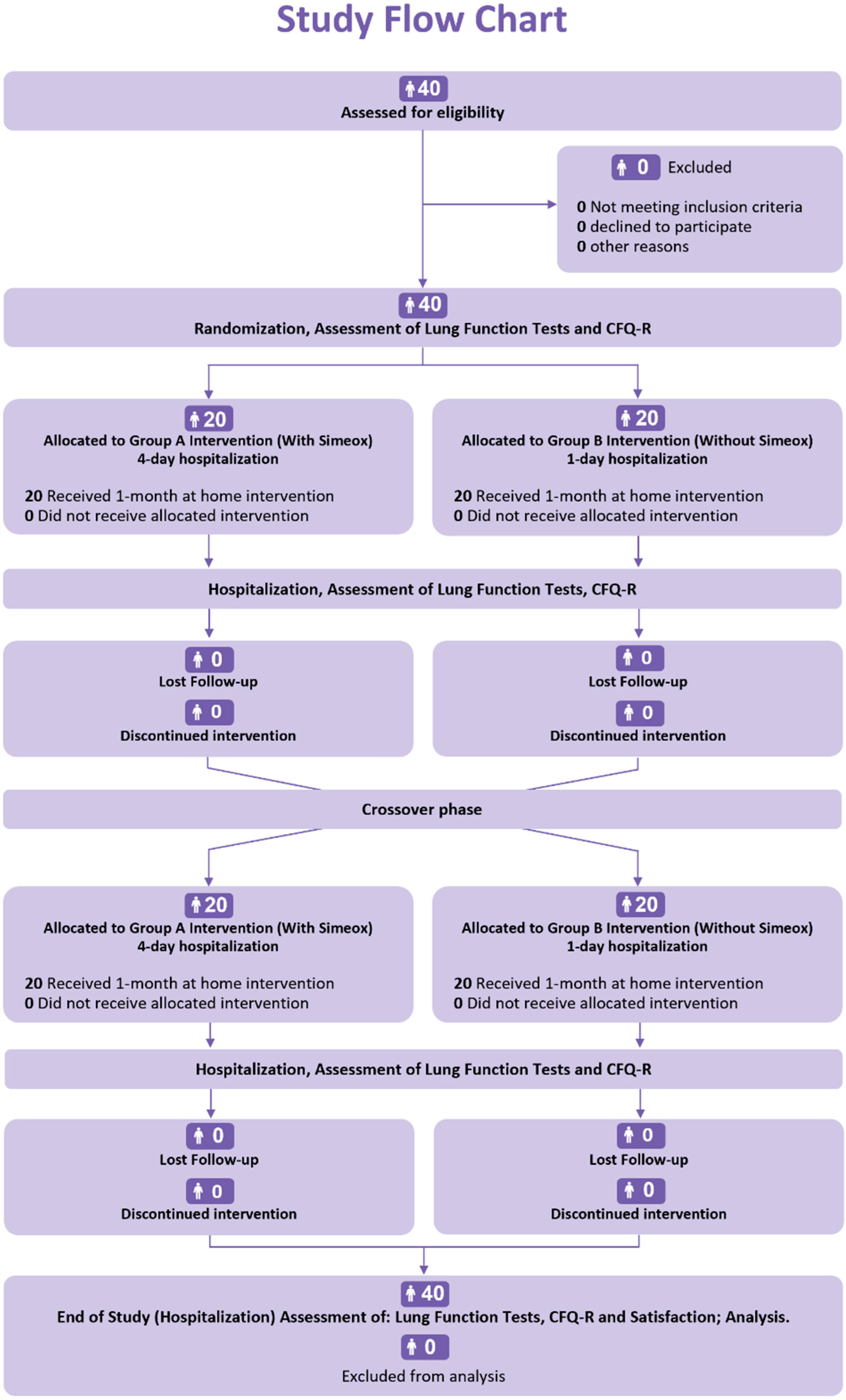
2.2. Patients
2.3. Interventions
2.4. Assessments
2.5. Statistical Analyses
2.6. Comparison of Treatments
2.7. Comparisons of Treatments Versus Baseline
3. Results
3.1. Baseline Characteristics of the Study Group
3.2. Pulmonary Function Test Results
3.3. Mixed-Model Analysis Results
3.4. Simeox® Treatment Satisfactory Questionnaire
3.5. Adverse Events
4. Discussion
4.1. Spirometry, PFT, IOS, Multiple-Breath Washout, CFQ-R
4.2. Safety Results Discussion
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volsko, T.A. Airway clearance therapy: Finding the evidence. Respir. Care 2013, 58, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; Robinson, K.A.; O’Sullivan, B.P.; Finder, J.D.; Vender, R.L.; Willey-Courand, D.B.; White, T.B.; Marshall, B.C.; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: Airway clearance therapies. Respir. Care 2009, 54, 522–537. [Google Scholar] [PubMed]
- Turcios, N.L. Cystic Fibrosis Lung Disease: An Overview. Respir. Care 2020, 65, 233–251. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, J. Standards of Care and Good Clinical Practice for the Physiotherapy Management of Cystic Fibrosis: CF Trust Physiotherapy Guidelines 2011; Cystic Fibrosis Trust: London, UK, 2011. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Daniels, T. Physiotherapeutic management strategies for the treatment of cystic fibrosis in adults. J. Multidiscip. Healthc. 2010, 3, 201–212. [Google Scholar] [CrossRef] [Green Version]
- van der Giessen, L.J.; de Jongste, J.C.; Gosselink, R.; Hop, W.C.J.; Tiddens, H.A. RhDNase before airway clearance therapy improves airway patency in children with CF. Pediatr. Pulmonol. 2007, 42, 624–630. [Google Scholar] [CrossRef]
- Rand, S.; Hill, L.; Prasad, S.A. Physiotherapy in cystic fibrosis: Optimising techniques to improve outcomes. Paediatr. Respir. Rev. 2013, 14, 263–269. [Google Scholar] [CrossRef]
- Wilson, L.M.; Morrison, L.; Robinson, K.A. Airway clearance techniques for cystic fibrosis: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2019, 1, CD011231. [Google Scholar] [CrossRef]
- Belli, S.; Prince, I.; Savio, G.; Paracchini, E.; Cattaneo, D.; Bianchi, M.; Masocco, F.; Bellanti, M.T.; Balbi, B. Airway Clearance Techniques: The Right Choice for the Right Patient. Front. Med. 2021, 8, 544826. [Google Scholar] [CrossRef]
- Bradley, J.M.; Moran, F.M.; Elborn, J.S. Evidence for physical therapies (airway clearance and physical training) in cystic fibrosis: An overview of five Cochrane systematic reviews. Respir. Med. 2006, 100, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Morrison, L.; Innes, S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database Syst. Rev. 2017, 5, CD006842. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.; Burnham, P.; Southern, K.W. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst. Rev. 2017, 10, CD009595. [Google Scholar] [CrossRef] [PubMed]
- McIlwaine, M.; Button, B.; Nevitt, S.J. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, CD003147. [Google Scholar] [CrossRef] [PubMed]
- Main, E.; Prasad, A.; Schans, C. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database Syst. Rev. 2005, 2005, CD002011. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Hall, D.O.; Clayton, C.B.; Nelson, R. Chest physiotherapy in cystic fibrosis: A comparative study of autogenic drainage and the active cycle of breathing techniques with postural drainage. Thorax 1995, 50, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Walicka-Serzysko, K.; Postek, M.; Jeneralska, N.; Cichocka, A.; Milczewska, J.; Sands, D. The effects of the addition of a new airway clearance device to chest physiotherapy in children with cystic fibrosis pulmonary exacerbations. J. Mother Child 2021, 24, 16–24. [Google Scholar] [CrossRef]
- De Boeck, K.; Wilschanski, M.; Castellani, C.; Taylor, C.; Cuppens, H.; Dodge, J.; Sinaasappel, M.; Group, D.W. Cystic fibrosis: Terminology and diagnostic algorithms. Thorax 2006, 61, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Farrell, P.M.; Rosenstein, B.J.; White, T.B.; Accurso, F.J.; Castellani, C.; Cutting, G.R.; Durie, P.R.; Legrys, V.A.; Massie, J.; Parad, R.B.; et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J. Pediatr. 2008, 153, S4–S14. [Google Scholar] [CrossRef] [Green Version]
- Sands, D.; Borawska-Kowalczyk, U. Polska adaptacja Kwestionariusza Jakości Życia przeznaczonego dla dzieci i dorosłych chorych na mukowiscydozę oraz ich rodziców (CFQ-R). Pediatr. Pol. 2009, 84, 165–172. [Google Scholar] [CrossRef]
- Beydon, N.; Davis, S.D.; Lombardi, E.; Allen, J.L.; Arets, H.G.; Aurora, P.; Bisgaard, H.; Davis, G.M.; Ducharme, F.M.; Eigen, H.; et al. An official American Thoracic Society/European Respiratory Society Statement: Pulmonary Function Testing in Preschool Children. Am. J. Respir. Crit. Care Med. 2007, 175, 1304–1345. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Horsley, A. Lung clearance index in the assessment of airways disease. Respir. Med. 2009, 103, 793–799. [Google Scholar] [CrossRef]
- Robinson, P.D.; Latzin, P.; Verbanck, S.; Hall, G.L.; Horsley, A.; Gappa, M.; Thamrin, C.; Arets, H.G.; Aurora, P.; Fuchs, S.I.; et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur. Respir. J. 2013, 41, 507–522. [Google Scholar] [CrossRef] [Green Version]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; de Melo, P.L.; Dellacà, R.L.; Farré, R.; Hall, G.L.; Ioan, I.; Irvin, C.G.; et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef] [Green Version]
- König, P.; Ner, Z.; Acton, J.D.; Ge, B.; Hewett, J. Is an FEV1 of 80% predicted a normal spirometry in cystic fibrosis children and adults? Clin. Respir. J. 2018, 12, 2397–2403. [Google Scholar] [CrossRef]
- Amin, R.; Subbarao, P.; Lou, W.; Jabar, A.; Balkovec, S.; Jensen, R.; Kerrigan, S.; Gustafsson, P.; Ratjen, F. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur. Respir. J. 2011, 37, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, P.M.; Aurora, P.; Lindblad, A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur. Respir. J. 2003, 22, 972–979. [Google Scholar] [CrossRef]
- Rodriguez Hortal, M.C.; Nygren-Bonnier, M.; Hjelte, L. Non-Invasive Ventilation as Airway Clearance Technique in Cystic Fibrosis. Physiother. Res. Int. 2017, 22, e1667. [Google Scholar] [CrossRef] [Green Version]
- Eltorai, A.E.M.; Szabo, A.L.; Antoci, V.; Ventetuolo, C.E.; Elias, J.A.; Daniels, A.H.; Hess, D.R. Clinical Effectiveness of Incentive Spirometry for the Prevention of Postoperative Pulmonary Complications. Respir. Care 2018, 63, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Hemkens, L.G. How Routinely Collected Data for Randomized Trials Provide Long-Term Randomized Real-World Evidence. JAMA Netw. Open 2018, 1, e186014. [Google Scholar] [CrossRef]

| Group Using the Simeox® Technique | Group Using the CPT Technique | |
|---|---|---|
| Morning | MDI or DPI bronchodilator | MDI or DPI bronchodilator |
| Nebulization with physiological or hypertonic saline solution | Nebulization with physiological or hypertonic saline solution | |
| Autogenic drainage, drainage with Simeox® technique for 20 min. | Autogenic drainage for 20 min. | |
| Afternoon | Physical activity | Physical activity |
| Autogenic drainage, drainage with Simeox® technique for 20 min | Autogenic drainage for 20 min | |
| Nebulization with dornase alfa. | Nebulization with dornase alfa. | |
| Evening | MDI or DPI bronchodilatorNebulization with physiological or hypertonic saline solutionDrainage with O-PEP (Aerobika, Flutter, Acapella) for 20 min. | MDI or DPI bronchodilator |
| Nebulization with physiological or hypertonic saline solution | ||
| Drainage with O-PEP (Aerobika, Flutter, Acapella) for 20 min. |
| Demographic Characteristics | All Patients n = 40 | Group A n = 20 | Group B n = 20 |
|---|---|---|---|
| Female, n | 22 | 12 | 10 |
| Male, n | 18 | 8 | 10 |
| Age of patient (years), Mean ± SD | 13.02 ± 2.80 | 13.03 ± 3.05 | 13.01 ± 2.61 |
| Height (cm), Mean ± SD | 154.43 ± 13.87 | 152.40 ± 14.07 | 156.45 ± 13.72 |
| Weight (kg), Mean ± SD | 45.16 ± 12.52 | 42.89 ± 12.07 | 47.42 ± 12.86 |
| BMI (kg/cm²), Mean ± SD | 18.53 ± 2.47 | 18.06 ± 2.42 | 19.00 ± 2.49 |
| Comorbidities | |||
| Pancreatic insufficiency, n (%) | 36 (90.0) | 19 (95.0) | 17 (85.0) |
| Diabetes, n (%) | 15 (37.5) | 9 (45.0) | 6 (30.0) |
| Sinus polyposis, n (%) | 26 (65.0) | 14 (70.0) | 12 (60.0) |
| Cirrhosis, n (%) | 1 (2.5) | 1 (5.0) | 0 (0) |
| Chronic pseudomonas aeruginosa, n (%) | 8 (20.0) | 6 (30.0) | 2 (10.0) |
| Criteria | Simeox® | Conventional | Simeox®—Conventional | p-Value |
|---|---|---|---|---|
| Estimated adjusted Means ± SE or Medians (Q1; Q3) | Estimated Treatment Effect [CI95%] | Uncorrected/Corrected § | ||
| Impulse Oscillometry | ||||
| R 5 Hz | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.01 [−0.01; 0.03] | 0.457/0.810 |
| R 5–R 20 Hz | 0.06 (0.03; 0.10) | 0.06 (0.03; 0.09) | 0.00 | 0.217/0.651 |
| R 20 Hz | 0.38 ± 0.01 | 0.38 ± 0.01 | −0.00 [−0.02; 0.02] | 0.721/0.824 |
| X 5 Hz ** | 0.00 ± 0.01 | 0.03 ± 0.01 | −0.03 [−0.06; 0.00] | 0.060/0.394 |
| AX | 0.50 (0.34; 0.860) | 0.45 (0.35; 0.76) | 0.05 | 0.393/0.810 |
| Spirometry | ||||
| FEV1 z-score | −0.57 (−1.25; 0.27) | −0.82 (−1.37; 0.37) | 0.25 | 0.746/0.824 |
| FVC z-score | −0.31 ± 0.08 | −0.26 ± 0.08 | −0.04 [−0.21; 0.19] | 0.586/0.810 |
| MEF25 z-score | −1.23 ± 0.13 | −1.26 ± 0.13 | 0.03 [−0.23; 0.30] | 0.785/0.825 |
| MEF50 z-score | −0.53 (−1.38; 0.40) | −0.49 (−1.25; 1.12) | 0.04 | 0.094/0.394 |
| MEF75 z-score | 5.31 ± 1.04 | 4.95 ± 1.04 | 0.36 [0.02; 0.13] | 0.008 */0.159 |
| Lung-clearance index | ||||
| LCI 2.5 ** | −0.37 ± 0.34 | 0.54 ± 0.34 | −0.91 [−1.93; 0.11] | 0.079/0.394 |
| LCI 2.5 z-score ** | −0.31 ± 0.57 | 0.93 ± 0.57 | −1.24 [−2.97; 0.50] | 0.156/0.545 |
| Body plethysmography | ||||
| RV z-score | 0.55 ± 0.26 | 0.39 ± 0.26 | 0.16 [−0.46; 0.77] | 0.603/0.810 |
| TLC z-score | −0.20 ± 0.17 | −0.10 ± 0.17 | −0.10 [−0.49; 0.29] | 0.617/0.810 |
| RV/TLC z-score ** | 0.09 ± 0.50 | −0.48 ± 0.51 | 0.57 [−0.97; 2.10] | 0.460/0.810 |
| FRC z-score | 0.64 (−0.66; 1.84) | 0.32 (−0.85; 1.88) | 0.32 | 0.519/0.810 |
| Reff z-score | 0.86 (0.01; 2.06) | 0.79 (−0.39; 2.11) | 0.07 | 0.524/0.810 |
| sReff z-score | 1.58 (0.87; 3.01) | 1.55 (0.45; 2.90) | 0.03 | 0.519/0.810 |
| Rtot z-score | 1.74 ± 0.09 (in log scale) | 1.72 ± 0.09 (in log scale) | 0.02 [−0.12; 0.17] | 0.743/0.824 |
| Criteria | Simeox® | Conventional | Simeox®—Conventional | p-Value |
|---|---|---|---|---|
| Estimated Adjusted Means ± SE or Medians (Q1; Q3) | Estimated Treatment Effect [CI95%] | Uncorrected/Corrected | ||
| Cystic Fibrosis questionnaire (CFQ-R) | ||||
| Physical score (patient) | 90.4 ± 1.61 | 87.0 ± 1.61 | 3.37 [0.56; 6.17] | 0.015 */0.159 |
| Respiratory score (patient) | 82.7 ± 1.63 | 82.5 ± 1.63 | 0.21 [−3.04; 3.46] | 0.897/0.897 |
| Criteria | Baseline ** | Simeox® Treatment | Conventional Treatment | ||
|---|---|---|---|---|---|
| After Treatment | Baseline–Treatment | After Treatment | Baseline–Treatment | ||
| Adjusted Means ± SE or Medians (Q1; Q3) | Adjusted Means ± SE or Medians (Q1; Q3) | Treatment Effect [CI95%] Uncorrected/Corrected § p-Value | Adjusted Means ± SE or Medians (Q1; Q3) | Treatment Effect [CI95%] Uncorrected/Corrected § p-Value | |
| Impulse Oscillometry | |||||
| R 5 Hz | 0.48 ± 0.03 | 0.45 ± 0.02 | 0.03 [0.00; 0.05] -/0.067 | 0.44 ± 0.02 | 0.03 [0.00; 0.06] -/0.013 * |
| R 5- R 20 Hz | 0.06 (0.03; 0.10) | 0.06 (0.03; 0.10) | 0.00 0.576/0.576 | 0.06 (0.03; 0.09) | 0.00 0.138/0.275 |
| R 20 Hz | 0.40 ± 0.02 | 0.38 ± 0.02 | 0.02 [0.00; 0.04] -/0.047 * | 0.38 ± 0.02 | 0.02 [0.00; 0.04] -/0.103 |
| X 5 Hz | −0.17 (−0.20; −0.11) | −0.16 (−0.19; −0.12) | 0.01 0.432/0.432 | −0.15 (−0.19; −0.12) | 0.02 0.038 */0.077 |
| AX | 0.54 (0.30; 0.89) | 0.50 (0.34; 0.86) | 0.04 0.673/0.106 | 0.45 (0.35; 0.76) | 0.09 0.053/0.135 |
| Spirometry | |||||
| FEV1 z-score | Group A **: −1.16 (−2.66; −0.40) | −0.79 (−1.93; −0.24) | 0.37 0.779/0.779 | −0.55 (−0.91; 0.39) | −0.26 0.092/0.186 |
| Group B **: −0.29 (−0.45; 0.40) | |||||
| FVC z-score | −0.24 ± 0.19 | −0.29 ± 0.20 | 0.05 [−0.20; 0.30] -/0.871 | −0.33 ± 0.20 | 0.09 [−0.16; 0.34] -/0.657 |
| MEF25 z-score | −1.08 ± 0.24 | −1.23 ± 0.24 | 0.15 [−0.17; 0.46] -/0.503 | −1.26 ± 0.24 | 0.18 [−0.13; 0.50] -/0.351 |
| MEF50 z-score | −0.21 ± 0.28 | −0.55 ± 0.28 | 0.34 [0.00; 0.67] -/0.048 * | −0.37 ± 0.28 | 0.16 [−0.177; 0.492] -/0.503 |
| MEF75 z-score | −0.13 (−1.45; 0.42) | 0.12 (−1.11; 0.82) | −0.24 0.134/0.267 | −0.23 (−1.37; 0.38) | 0.10 0.572/0.572 |
| Lung-clearance index | |||||
| LCI 2.5 | Group A **: 12.20 ± 0.89 | 11.60 ± 0.89 | 0.51 [−0.27; 1.29] 0.169/0.169 | 9.87 ± 0.61 | −0.69 [−1.27; −0.11] 0.014 */0.027 * |
| Group B **: 9.19 ± 0.61 | |||||
| LCI 2.5 z-score | Group A **: 10.1 (7.7; 13.4) | 9.40 (6.65; 12.60) | 0.07 0.365/0.365 | 7.10 (4.35; 9.35) | −0.80 0.007 */0.014 * |
| Group B **: 6.3 (2.62; 8.9) | |||||
| Body plethysmography | |||||
| RV z-score | Group A **: 1.64 (0.31; 2.55) | 1.42 (0.14; 2.58) | 0.22 0.126/0.252 | 0.17 (−0.46; 1.14) | −0.05 0.587/0.587 |
| Group B **: 0.12 (−0.99; 0.86) | |||||
| TLC z-score | 0.25 (−1.46; 0.77) | 0.05 (−0.83; 1.06) | 0.20 0.620/1.000 | −0.26 (−0.98; 0.88) | 0.51 0.558/1.000 |
| RV/TLC z-score | 1.10 ± 0.37 | 1.21 ± 0.51 | −0.11 [−1.46; 2.12] -/0.979 | 0.33 ± 0.51 | 0.77 [−0.58; 0.50] -/0.356 |
| FRC z-score | 0.55 ± 0.34 | 0.38 ± 0.34 | 0.17 [−0.41; 0.75] -/0.753 | 0.50 ± 0.34 | 0.05 [−0.53; 0.63] -/0.974 |
| Reff z-score | 1.04 (−0.38; 3.16) | 0.86 (0.01; 2.06) | 0.19 0.715/0.779 | 0.79 (−0.39; 2.11) | 0.26 0.390/0.779 |
| sReff z-score | 2.78 ± 0.65 | 2.62 ± 0.65 | 0.16 [−0.66; 0.97] -/0.890 | 2.61 ± 0.65 | 0.16 [−0.65; 0.98] -/0.880 |
| Rtot z-score | 1.31 ± 0.09 (in log scale) | 1.33 ± 0.09 (in log scale) | −0.01 [−0.18; 0.16] -/0.979 | 1.30 ± 0.09 (in log scale) | 0.01 [−0.16; 0.18] -/0.991 |
| Cystic Fibrosis Questionnaire (CFQ-R) | Baseline ** | Simeox® Treatment | Conventional Treatment | ||
|---|---|---|---|---|---|
| After Treatment | Baseline–Treatment | After Treatment | Baseline–Treatment | ||
| Adjusted Means ± SE or Medians (Q1; Q3) | Adjusted Means ± SE or Medians (Q1; Q3) | Treatment Effect [CI95%] Uncorrected/Corrected § p-Value | Adjusted Means ± SE or Medians (Q1; Q3) | Treatment Effect [CI95%] Uncorrected/Corrected § p-Value | |
| Physical score (patient) | 85.3 ± 2.1 | 90.4 ± 2.1 | −5.10 [−9.09; −1.12] -/0.009 * | 87.0 ± 2.1 | −1.74 [−5.73; 2.25] -/0.554 |
| Respiratory score (patient) | 80.4 ± 2.01 | 82.7 ± 2.01 | −2.29 [−6.52; 1.93] -/0.402 | 82.5 ± 2.01 | −2.08 [−6.31; 2.14] -/0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sands, D.; Walicka-Serzysko, K.; Milczewska, J.; Postek, M.; Jeneralska, N.; Cichocka, A.; Siedlecka, E.; Borawska-Kowalczyk, U.; Morin, L. Efficacy of the Simeox® Airway Clearance Technology in the Homecare Treatment of Children with Clinically Stable Cystic Fibrosis: A Randomized Controlled Trial. Children 2023, 10, 204. https://doi.org/10.3390/children10020204
Sands D, Walicka-Serzysko K, Milczewska J, Postek M, Jeneralska N, Cichocka A, Siedlecka E, Borawska-Kowalczyk U, Morin L. Efficacy of the Simeox® Airway Clearance Technology in the Homecare Treatment of Children with Clinically Stable Cystic Fibrosis: A Randomized Controlled Trial. Children. 2023; 10(2):204. https://doi.org/10.3390/children10020204
Chicago/Turabian StyleSands, Dorota, Katarzyna Walicka-Serzysko, Justyna Milczewska, Magdalena Postek, Natalia Jeneralska, Aleksandra Cichocka, Ewa Siedlecka, Urszula Borawska-Kowalczyk, and Laurent Morin. 2023. "Efficacy of the Simeox® Airway Clearance Technology in the Homecare Treatment of Children with Clinically Stable Cystic Fibrosis: A Randomized Controlled Trial" Children 10, no. 2: 204. https://doi.org/10.3390/children10020204





