The Use of Stress Cardiovascular Imaging in Pediatric Population
Abstract
:1. Introduction
2. Stress Echocardiography
2.1. Introduction
2.2. Type of Stress Agents
2.3. Protocols
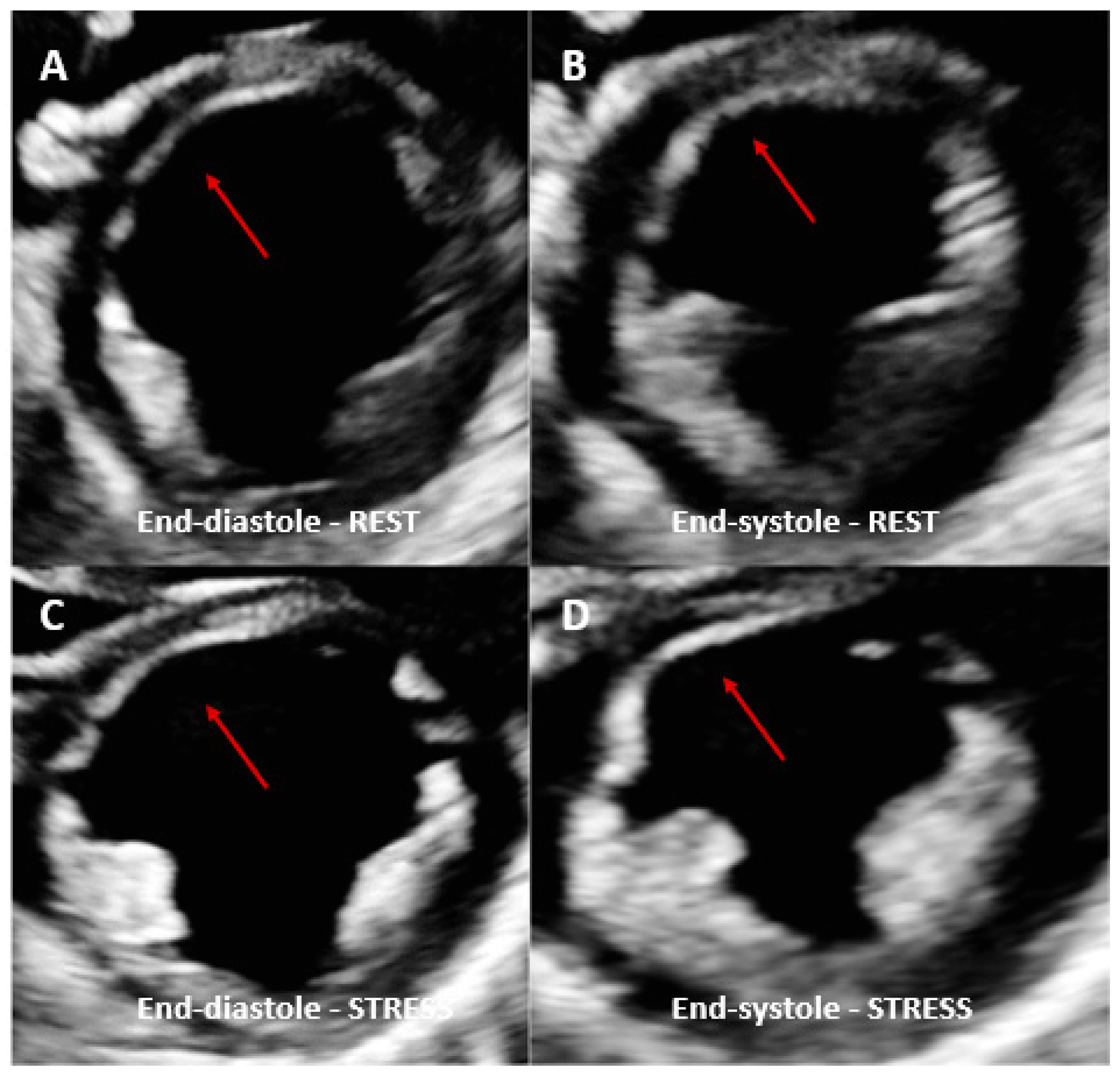
2.4. Limitations
3. Stress Imaging in Pediatric Nuclear Cardiology Stress
3.1. Introduction
3.2. Type of Stress Agents
3.3. Protocol
3.4. Limitations
4. Stress Cardiovascular Magnetic Resonance
4.1. Introduction
4.2. Stress CMR: Adult vs. Pediatric Populations
4.3. Type of Stress Agents
4.4. Protocol
4.5. Limitations
5. Stress Computed Tomography Perfusion Imaging
5.1. Introduction
5.2. Protocol
5.3. Limitations
6. Clinical Applications of Stress Imaging in Children
6.1. Arterial Switch Operation (ASO)
6.2. Heart Transplant
6.3. Kawasaki Disease
6.4. Others Clinical Indications
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ALARA | as low as reasonable achievable |
| ASO | arterial switch operation |
| ATP | adenosine triphosphate |
| AV | atrioventricular |
| CA | coronary artery |
| CAD | coronary artery disease |
| CAV | coronary artery vasculopathy |
| CFR | coronary flow reserve |
| CMD | coronary microvascular dysfunction |
| CMR | cardiovascular magnetic resonance |
| CT | computed tomography |
| CTCA | computed tomography coronary angiography |
| DSE | dobutamine stress echocardiography |
| EANM | European Association of Nuclear Medicine |
| ESE | exercise stress echocardiography |
| FFR | fractional flow reserve |
| GBCA | gadolinium-based contrast agent |
| HR | heart rate |
| ICDs | implantable cardioverter defibrillators |
| IMR | index of microcirculatory resistance |
| KD | Kawasaki disease |
| LGE | late gadolinium enhancement |
| LV | left ventricle |
| MBF | myocardial blood flow |
| MPR | myocardial perfusion reserve |
| MRI | magnetic resonance imaging |
| Ms | milliseconds |
| NSF | nephrogenic systemic fibrosis |
| PET | positron emission tomography |
| QGS | quantitative gated SPECT |
| RCA | right coronary artery |
| ROI | region of interest |
| RV | right ventricle |
| SPECT | single-photon emission computerized tomography |
| TGA | transposition of the great arteries |
References
- Paridon, S.M.; Alpert, B.S.; Boas, S.R.; Cabrera, M.E.; Caldarera, L.L.; Daniels, S.R.; Kimball, T.R.; Knilans, T.K.; Nixon, P.A.; Rhodes, J.; et al. Clinical Stress Testing in the Pediatric Age Group. Circulation 2006, 113, 1905–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toro-Salazar, O.H.; Gillan, E.; O’Loughlin, M.T.; Burke, G.S.; Ferranti, J.; Stainsby, J.; Liang, B.; Mazur, W.; Raman, S.V.; Hor, K.N. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc. Imaging 2013, 6, 873–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahl, E.; Duffy, C.E.; Chaudhry, F.A. The role of stress echocardiography in children. Echocardiography 2000, 17, 507–512. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Perfor-mance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.; Xie, F.; Smith, L.M.; O’Leary, E.; Smith, K.; Olson, J.; Nalty, K.; Hess, R.; Graham, M.; Therrien, S.; et al. Prospective randomized comparison of conventional stress echocardiography and real-time perfusion stress echocardiography in detecting significant coronary artery disease. J. Am. Soc. Echocardiogr. 2012, 25, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Peltier, M.; Vancraeynest, D.; Pasquet, A.; Ay, T.; Roelants, V.; D’hondt, A.M.; Melin, J.A.; Vanoverschelde, J.L.-J. Assessment of the physiologic significance of coronary disease with dipyridamole real-time myocardial contrast echocardiography. Comparison with technetium-99m sestamibi single-photon emission computed tomography and quantitative coronary angiography. J. Am. Coll. Cardiol. 2004, 43, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoni, S.; Zoghbi, W.A.; Xie, F.; Kricsfeld, D.; Iskander, S.; Gobar, L.; Mikati, I.A.; Abukhalil, J.; Verani, M.S.; O’Leary, E.L.; et al. Real-time assessment of myocardial perfusion and wall motion during bicycle and treadmill exercise echocardiography: Comparison with single photon emission computed tomography. J. Am. Coll. Cardiol. 2001, 37, 741–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.; Berlacher, K.; Lindner, J.R.; Mankad, S.V.; Rose, G.A.; Wang, A. COCATS 4 Task Force 5: Training in echocardiography. J. Am. Coll. Cardiol. 2015, 65, 1786–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesely, M.R.; Dilsizian, V. Nuclear Cardiac Stress Testing in the Era of Molecular Medicine. J. Nucl. Med. 2008, 49, 399–413. [Google Scholar] [CrossRef] [Green Version]
- Hamamichi, Y.; Ichida, F.; Tsubata, S.; Hirono, K.; Watanabe, S.; Rui, C.; Yu, X.; Uese, K.-I.; Hashimoto, I.; Simizu, M.; et al. Dobutamine Stress Radionuclide Ventriculography Reveals Silent Myocardial Dysfunction in Kawasaki Disease. Circ. J. 2002, 66, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Milanesi, O.; Stellin, G.; Zucchetta, P. Nuclear Medicine in Pediatric Cardiology. Semin. Nucl. Med. 2017, 47, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Weindling, S.N.; Wernovsky, G.; Colan, S.D.; Parker, J.; Boutin, C.; Mone, S.M.; Costello, J.; Castañeda, A.R.; Treves, S. Myocardial perfusion, function and exercise tolerance after the arterial switch operation. J. Am. Coll. Cardiol. 1994, 23, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.-M. Cardiomyopathy in the pediatric patients. Pediatr. Neonatol. 2018, 59, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, B.; Goudie, B.; Remmert, J.; Gidding, S.S. Usefulness of Myocardial Perfusion Imaging With Exercise Testing in Children. Pediatr. Cardiol. 2012, 33, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Boknik, P.; Eskandar, J.; Hofmann, B.; Zimmermann, N.; Neumann, J.; Gergs, U. Role of Cardiac A2A Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 11, 627838. [Google Scholar] [CrossRef]
- Alzahrani, T.; Khiyani, N.; Zeltser, R. Adenosine SPECT Thallium Imaging. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Henzlova, M.J.; Duvall, W.L.; Einstein, A.J.; Travin, M.I.; Verberne, H.J. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J. Nucl. Cardiol. 2016, 23, 606–639. [Google Scholar] [CrossRef] [Green Version]
- Stern, H.; Sauer, U.; Locher, D.; Bauer, R.; Meisner, H.; Sebening, F.; Bühlmeyer, K. Left ventricular function assessed with echocar-diography and myocardial perfusion assessed with scintigraphy under dipyridamole stress in pediatric patients after repair for anomalous origin of the left coronary artery from the pulmonary artery. J. Thorac. Cardiovasc. Surg. 1993, 106, 723–732. [Google Scholar] [CrossRef]
- Fukuda, T.; Ishibashi, M.; Shinohara, T.; Miyake, T.; Kudoh, T.; Saga, T. Follow-Up Assessment of the Collateral Circulation in Patients with Kawasaki Disease Who Underwent Dipyridamole Stress Technetium-99m Tetrofosmin Scintigraphy. Pediatr. Cardiol. 2005, 26, 558–564. [Google Scholar] [CrossRef]
- Mann, A.; Williams, J. Considerations for Stress Testing Performed in Conjunction with Myocardial Perfusion Imaging. J. Nucl. Med. Technol. 2020, 48, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, C.V.; Krishnamurthy, R.; Masand, P.; Moffett, B.; Schlingmann, T.; Cheong, B.Y.; Krishnamurthy, R. Myocardial Stress Perfusion MRI: Experience in Pediatric and Young-Adult Patients Following Arterial Switch Operation Utilizing Regadenoson. Pediatr. Cardiol. 2018, 39, 1249–1257. [Google Scholar] [CrossRef]
- Doan, T.T.; Wilkinson, J.C.; Loar, R.W.; Pednekar, A.S.; Masand, P.M.; Noel, C.V. Regadenoson Stress Perfusion Cardiac Magnetic Resonance Imaging in Children With Kawasaki Disease and Coronary Artery Disease. Am. J. Cardiol. 2019, 124, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- EANM.org. Available online: https://www.eanm.org/publications/dosage-calculator (accessed on 27 September 2022).
- Dosage Card. Available online: https://www.eanm.org/contenteanm/uploads/2017/01/EANM_Dosage_Card_040214.pdf (accessed on 27 September 2022).
- Dae, M.W. Pediatric Nuclear Cardiology. Semin. Nucl. Med. 2007, 37, 382–390. [Google Scholar] [CrossRef]
- Verberne, H.J.; Acampa, W.; Anagnostopoulos, C.; Ballinger, J.; Bengel, F.; De Bondt, P.; Buechel, R.R.; Cuocolo, A.; van Eck-Smit, B.L.F.; Flotats, A.; et al. EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur. J. Nucl. Med. 2015, 42, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengel, F.M.; Hauser, M.; Duvernoy, C.S.; Kuehn, A.; Ziegler, S.I.; Stollfuss, J.C.; Beckmann, M.; Sauer, U.; Muzik, O.; Schwaiger, M.; et al. Myocardial blood flow and coronary flow reserve late after anatomical correction of transposition of the great arteries. J. Am. Coll. Cardiol. 1998, 32, 1955–1961. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Pampaloni, M.; Allada, V.; Fishbein, M.C.; Schelbert, H.R. Myocardial perfusion and viability by positron emission tomography in infants and children with coronary abnormalities: Correlation with echocardiography, coronary angiography, and histopathology. J. Am. Coll. Cardiol. 2003, 41, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Manabe, O.; Kikuchi, T.; Scholte, A.J.H.A.; Mahdiui, M.E.; Nishii, R.; Zhang, M.R.; Suzuki, E.; Yoshinaga, K. Radiopharmaceutical tracers for cardiac imaging. J. Nucl. Cardiol. 2018, 25, 1204–1236. [Google Scholar] [CrossRef] [Green Version]
- Ford, P.V.; Chatziioannou, S.N.; Moore, W.H.; Dhekne, R.D. Overestimation of the LVEF by quantitative gated SPECT in simulated left ventricles. J. Nucl. Med. 2001, 42, 454–459. [Google Scholar] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Role of cardiovascular magnetic resonance in the guidelines of the Eu-ropean Society of Cardiology. J. Cardiovasc. Magn. Reson. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwitter, J.; Wacker, C.M.; van Rossum, A.C.; Lombardi, M.; Al-Saadi, N.; Ahlstrom, H.; Dill, T.; Larsson, H.B.; Flamm, S.D.; Marquardt, M.; et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J. 2008, 29, 480–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwitter, J.; Wacker, C.M.; Wilke, N.; Al-Saadi, N.; Sauer, E.; Huettle, K.; Schönberg, S.O.; Luchner, A.; Strohm, O.; Ahlstrom, H.; et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: Perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: A comparative multicentre, multivendor trial. Eur. Heart J. 2012, 34, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Car-diovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. CE-MARC 2 Investigators. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scin-tigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Buechel, E.V.; Grosse-Wortmann, L.; Fratz, S.; Eichhorn, J.; Sarikouch, S.; Greil, G.; Beerbaum, P.; Bucciarelli-Ducci, C.; Bonello, B.; Sieverding, L.; et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: An expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 281–297. [Google Scholar] [CrossRef]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echo-cardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the use of cardiovascular magnetic resonance in pediatric congenital and acquired heart disease: Endorsed by The American Heart Association. J. Cardiovasc. Magn. Reson. 2022, 24, 37. [Google Scholar]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef]
- Writing Committee Members; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Cardiovasc. Comput. Tomogr. 2022, 16, 54–122. [Google Scholar]
- Buechel, E.R.; Balmer, C.; Bauersfeld, U.; Kellenberger, C.J.; Schwitter, J. Feasibility of perfusion cardiovascular magnetic resonance in paediatric patients. J. Cardiovasc. Magn. Reson. 2009, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Stagnaro, N.; Moscatelli, S.; Cheli, M.; Bondanza, S.; Marasini, M.; Trocchio, G. Dobutamine Stress Cardiac MRI in Pediatric Patients with Suspected Coronary Artery Disease. Pediatr. Cardiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ntsinjana, H.N.; Tann, O.; Hughes, M.; Derrick, G.; Secinaro, A.; Schievano, S.; Muthurangu, V.; Taylor, A.M. Utility of adenosine stress perfusion CMR to assess paediatric coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 898–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, J.; Wielopolski, P.A.; Meijboom, F.J.; Witsenburg, M.; Bogers, A.J.; Pattynama, P.M.; Helbing, W.A. Diastolic function in repaired tetralogy of Fallot at rest and during stress: Assessment with MR imaging. Radiology 2007, 243, 212–219. [Google Scholar] [CrossRef]
- Robbers-Visser, D.; Harkel, D.J.T.; Kapusta, L.; Strengers, J.L.; Dalinghaus, M.; Meijboom, F.J.; Pattynama, P.M.; Bogers, A.J.; Helbing, W.A. Usefulness of Cardiac Magnetic Resonance Imaging Combined With Low-Dose Dobutamine Stress to Detect an Abnormal Ventricular Stress Response in Children and Young Adults After Fontan Operation at Young Age. Am. J. Cardiol. 2008, 101, 1657–1662. [Google Scholar] [CrossRef]
- Fratz, S.; Hager, A.; Busch, R.; Kaemmerer, H.; Schwaiger, M.; Lange, R.; Hess, J.; Stern, H.C. Patients after atrial switch operation for transposition of the great arteries can not increase stroke volume under dobutamine stress as opposed to patients with congenitally corrected transposition. Circ. J. 2008, 72, 1130–1135. [Google Scholar] [CrossRef] [Green Version]
- Tulevski, I.I.; van der Wall, E.E.; Groenink, M.; Dodge-Khatami, A.; Hirsch, A.; Stoker, J.; Mulder, B.J. Usefulness of magnetic resonance imaging dobutamine stress in asymptomatic and minimally symptomatic patients with decreased cardiac reserve from con-genital heart disease (complete and corrected transposition of the great arteries and subpulmonic obstruction). Am. J. Cardiol. 2002, 89, 1077–1081. [Google Scholar]
- Tulevski, I.I.; Lee, P.L.; Groenink, M.; van der Wall, E.E.; Stoker, J.; Pieper, P.G.; Romkes, H.; Hirsch, A.; Mulder, B.J. Dobutamine-induced increase of right ventricular contractility without increased stroke volume in adolescent patients with transposition of the great arteries: Evaluation with magnetic resonance imaging. Int. J. Card. Imaging. 2000, 16, 471–478. [Google Scholar] [CrossRef]
- Dodge-Khatami, A.; Tulevski, I.I.; Bennink, G.B.; Hitchcock, J.F.; de Mol, B.A.; van der Wall, E.E.; Mulder, B.J. Comparable systemic ventricular function in healthy adults and patients with unoperated congenitally corrected transposition using MRI dobuta-mine stress testing. Ann. Thorac. Surg. 2002, 73, 1759–1764. [Google Scholar] [CrossRef]
- Moscatelli, S.; Borrelli, N.; Sabatino, J.; Leo, I.; Avesani, M.; Montanaro, C.; Di Salvo, G. Role of Cardiovascular Imaging in the Fol-low-Up of Patients with Fontan Circulation. Children 2022, 9, 1875. [Google Scholar] [CrossRef]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef]
- Pezel, T.; Garot, P.; Hovasse, T.; Unterseeh, T.; Champagne, S.; Kinnel, M.; Toupin, S.; Louvard, Y.; Morice, M.C.; Sanguineti, F. Vasodilatation stress cardiovascular magnetic resonance imaging: Feasibility, workflow and safety in a large prospective reg-istry of more than 35,000 patients. Arch. Cardiovasc. Dis. 2021, 114, 490–503. [Google Scholar] [CrossRef]
- Rappaport, B.; Mellon, R.D.; Simone, A.; Woodcock, J. Defining Safe Use of Anesthesia in Children. N. Engl. J. Med. 2011, 364, 1387–1390. [Google Scholar] [CrossRef]
- Shellock, F.G.; Woods, T.O.; Crues, J.V., 3rd. MR Labeling Information for Implants and Devices: Explanation of Terminology. Radiology 2009, 253, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Nazarian, S.; Hansford, R.; Roguin, A.; Goldsher, D.; Zviman, M.M.; Lardo, A.C.; Caffo, B.S.; Frick, K.D.; Kraut, M.A.; Kamel, I.R.; et al. A Prospective Evaluation of a Protocol for Magnetic Resonance Imaging of Patients With Implanted Cardiac Devices. Ann. Intern. Med. 2011, 155, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Nazarian, S.; Beinart, R.; Halperin, H.R. Magnetic Resonance Imaging and Implantable Devices. Circ. Arrhythmia Electrophysiol. 2013, 6, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Bucciarelli-Ducci, C.; Vardas, P. Reconsidering safety and reducing barriers to MRI in patients with cardiac implantable electronic devices. Eur. Heart J. 2022, 43, 2479–2481. [Google Scholar] [CrossRef]
- Bhuva, A.N.; Moralee, R.; Brunker, T.; Lascelles, K.; Cash, L.; Patel, K.P.; Lowe, M.; Sekhri, N.; Alpendurada, F.; Pennell, D.J.; et al. Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices. Eur. Heart J. 2021, 43, 2469–2478. [Google Scholar] [CrossRef]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Malone, L.J.; Morin, C.E.; Browne, L.P. Coronary computed tomography angiography in children. Pediatr. Radiol. 2022, 52, 2498–2509. [Google Scholar] [CrossRef]
- Secinaro, A.; Ait-Ali, L.; Curione, D.; Clemente, A.; Gaeta, A.; Giovagnoni, A.; Alaimo, A.; Esposito, A.; Tchana, B.; Sandrini, C.; et al. Recommendations for cardiovascular magnetic resonance and computed tomography in congenital heart disease: A consensus paper from the CMR/CCT working group of the Italian Society of Pediatric Cardiology (SICP) and the Italian College of Cardiac Radiology endorsed by the Italian Society of Medical and Interventional Radiology (SIRM) Part I. Radiol. Med. 2022, 127, 788–802. [Google Scholar] [CrossRef]
- Leschka, S.; Waelti, S.; Wildermuth, S. Principles of CT Imaging. In Cardiac CT and MR for Adult Congenital Heart Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 77–105. [Google Scholar]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Baggiano, A.; Fazzari, F.; Guglielmo, M.; Muscogiuri, G.; Berzovini, C.M.; Pasquini, A.; Mushtaq, S.; et al. Incremental Diagnostic Value of Stress Computed Tomography Myocardial Perfusion With Whole-Heart Coverage CT Scanner in Intermediate- to High-Risk Symptomatic Patients Suspected of Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 338–349. [Google Scholar] [CrossRef]
- Ho, K.-T.; Chua, K.-C.; Klotz, E.; Panknin, C. Stress and Rest Dynamic Myocardial Perfusion Imaging by Evaluation of Complete Time-Attenuation Curves With Dual-Source CT. JACC Cardiovasc. Imaging 2010, 3, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Bastarrika, G.; Ramos-Duran, L.; Rosenblum, M.A.; Kang, D.K.; Rowe, G.W.; Schoepf, U.J. Adenosine-Stress Dynamic Myocardial CT Perfusion Imaging. Investig. Radiol. 2010, 45, 306–313. [Google Scholar] [CrossRef]
- Buber, J.; Rhodes, J. Exercise Physiology and Testing in Adult Patients with Congenital Heart Disease. Heart Fail. Clin. 2014, 10, 23–33. [Google Scholar] [CrossRef]
- Warnes, C.A. Transposition of the great arteries. Circulation 2006, 114, 2699–2709. [Google Scholar] [CrossRef] [Green Version]
- Pedra, S.R.; Pedra, C.A.; Abizaid, A.A.; Braga, S.L.N.; Staico, R.; Arrieta, R.; Costa, J.R.; Vaz, V.D.; Fontes, V.F.; Sousa, J.E.R. In-tracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. J. Am. Coll Cardiol. 2005, 45, 2061–2068. [Google Scholar] [CrossRef] [Green Version]
- Hui, L.; Chau, A.K.T.; Leung, M.P.; Chiu, C.S.W.; Cheung, Y.F. Assessment of left ventricular function long term after arterial switch operation for transposition of the great arteries by dobutamine stress echocardiography. Heart 2005, 91, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar]
- Sterrett, L.E.; Schamberger, M.S.; Ebenroth, E.S.; Siddiqui, A.R.; Hurwitz, R.A. Myocardial perfusion and exercise capacity 12 years after arterial switch surgery for D-transposition of the great arteries. Pediatr. Cardiol. 2011, 32, 785–791. [Google Scholar] [CrossRef]
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Braga, F.G.M.; Binder, T.; et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 919–948. [Google Scholar] [CrossRef]
- Del Pasqua, A.; Chinali, M.; D’Anna, C.; Ciliberti, P.; Esposito, C.; Gugliotta, M.; Milewski, P.; Perrone, M.A.; Romeo, F.; Carotti, A.; et al. Evidence of impaired longitudinal strain in pre-Fontan palliation in functional single left ventricle. J. Cardiovasc. Med. 2019, 20, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Dedieu, N.; Greil, G.; Wong, J.; Fenton, M.; Burch, M.; Hussain, T. Diagnosis and management of coronary allograft vasculopathy in children and adolescents. World J. Transplant. 2014, 4, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Dipchand, A.I.; Bharat, W.; Manlhiot, C.; Safi, M.; Lobach, N.E.; McCrindle, B.W. A prospective study of dobutamine stress echocardiography for the assessment of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatr. Trans Plant 2008, 12, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Abernathey, E.; Lunze, F.; Colan, S.D.; O’Neill, S.; Bergersen, L.; Geva, T.; Blume, E.D. Utility of exercise stress echo-cardiography in pediatric cardiac transplant recipients: A single-center experience. J. Heart Lung Transpl. 2012, 31, 517–523. [Google Scholar] [CrossRef]
- Soslow, J.H.; Samyn, M.M. Multi-modal imaging of the pediatric heart transplant recipient. Transl. Pediatr. 2019, 8, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Husain, N.; Watanabe, K.; Berhane, H.; Gupta, A.; Markl, M.; Rigsby, C.K.; Robinson, J.D. Multi-parametric cardiovascular magnetic resonance with regadenoson stress perfusion is safe following pediatric heart transplantation and identifies history of rejection and cardiac allograft vasculopathy. J. Cardiovasc. Magn. Reson. 2021, 23, 1–13. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardio-vascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and An-esthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [PubMed]
- Zilberman, M.V.; Goya, G.; Witt, S.A.; Glascock, B.; Kimball, T.R. Dobutamine stress echocardiography in the evaluation of young patients with Kawasaki disease. Pediatr. Cardiol. 2003, 24, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Noto, N.; Kamiyama, H.; Karasawa, K.; Ayusawa, M.; Sumitomo, N.; Okada, T.; Takahashi, S. Long-term prognostic impact of dobutamine stress echocardiography in patients with Kawasaki disease and coronary artery lesions: A 15-year follow-up study. J. Am. Coll Cardiol. 2014, 63, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Pahl, E.; Sehgal, R.; Chrystof, D.; Neches, W.H.; Webb, C.L.; Duffy, C.E.; Shulman, S.T.; Chaudhry, F.A. Feasibility of exercise stress echocardiography for the follow up of children with coronary involvement secondary to Kawasaki disease. Circulation 1995, 91, 122–128. [Google Scholar] [CrossRef]
- Dorfman, A.L.; Geva, T.; Samyn, M.M.; Greil, G.; Krishnamurthy, R.; Messroghli, D.; Festa, P.; Secinaro, A.; Soriano, B.; Taylor, A.; et al. SCMR expert consensus statement for cardiovascular magnetic resonance of acquired and non-structural pediatric heart disease. J. Cardiovasc. Magn. Reson. 2022, 24, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Intorcia, A.; Morgagni, R.; Marchei, M.; Sergi, D.; Pugliese, L.; Ferrante, P.; Chiocchi, M.; Borzi, M.; Romeo, F. Primary cardiac lymphoma: The role of multimodality imaging. J. Cardiovasc. Med. 2018, 19, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.; Cafiero, G.; Perrone, M.A.; Bianco, M.; Salvati, A.; Giordano, U.; Silva Kikina, S.; Guccione, P.; De Zorzi, A.; Galletti, L. The Effects of Physical Inactivity and Exercise at Home in Young Patients with Congenital Heart Disease during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10065. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Coronary artery anomalies: An entity in search of an identity. Circulation 2007, 115, 1296–1305. [Google Scholar] [CrossRef]
- Agrawal, H.; Wilkinson, J.C.; Noel, C.V.; Qureshi, A.M.; Masand, P.M.; Mery, C.M.; Sexson-Tejtel, S.K.; Molossi, S. Impaired Myocardial Perfusion on Stress CMR Correlates With Invasive FFR in Children With Coronary Anomalies. J. Am. Coll. Cardiol. 2021, 33, E45–E51. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Andrade, L.; Cook, S.C. Aortic Coarctation. Cardiol. Clin. 2020, 38, 337–351. [Google Scholar] [CrossRef]
- Banaszak, P.; Szkutnik, M.; Kusa, J.; Banaszak, B.; Bialkowski, J. Utility of the dobutamine stress echocardiography in the evaluation of the effects of a surgical repair of aortic coarctation in children. Cardiol. J. 2009, 16, 20–25. [Google Scholar]
- Santos, R.D.; Gidding, S.S.; Hegele, R.A.; Cuchel, M.A.; Barter, P.J.; Watts, G.F.; Baum, S.J.; Catapano, A.L.; Chapman, M.J.; Defesche, J.C.; et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016, 4, 850–861. [Google Scholar] [CrossRef]
- Citro, R.; Bursi, F.; Bellino, M.; Picano, E. The Role of Stress Echocardiography in Valvular Heart Disease. Curr. Cardiol. Rep. 2022, 24, 1477–1485. [Google Scholar] [CrossRef]
- Shivu, G.N.; Phan, T.T.; Abozguia, K.; Ahmed, I.; Wagenmakers, A.; Henning, A.; Narendran, P.; Stevens, M.; Frenneaux, M. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation 2010, 121, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Leo, I.; Nakou, E.; Artico, J.; Androulakis, E.; Wong, J.; Moon, J.C.; Indolfi, C.; Bucciarelli-Ducci, C. Strengths and weaknesses of alter-native noninvasive imaging approaches for microvascular ischemia. J. Nucl. Cardiol. 2022, 2022, 1–12. [Google Scholar]
- Tonet, E.; Pompei, G.; Faragasso, E.; Cossu, A.; Pavasini, R.; Passarini, G.; Tebaldi, M.; Campo, G. Coronary Microvascular Dys-function: PET, CMR and CT Assessment. J. Clin. Med. 2021, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.L.; Chang, M.C.; Liao, C.Y. Myocardial Dipyridamole-Stress Dynamic SPECT and Cardiac Adenosine-Stress MRI Unmasking the Janus Face of Coronary Microvascular Dysfunction in a 15-Year-Old Boy Incurring Recurrent Angina Pectoris, Myocardial Ischemia, and No Obstructive Coronary Artery Disease: An 11-Year Follow-Up. Acta Cardiol. Sin. 2022, 38, 204–209. [Google Scholar] [PubMed]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Tu, S.; Song, L.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.; Chen, Y.; Pu, J.; et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 2021, 398, 2149–2159. [Google Scholar] [CrossRef]



| Modality | Advantages | Disadvantages |
|---|---|---|
| Echocardiography |
|
|
| Nuclear Imaging |
|
|
| Computed Tomography (CT) |
|
|
| Cardiovascular Magnetic Resonance (CMR) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscatelli, S.; Bianco, F.; Cimini, A.; Panebianco, M.; Leo, I.; Bucciarelli-Ducci, C.; Perrone, M.A. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children 2023, 10, 218. https://doi.org/10.3390/children10020218
Moscatelli S, Bianco F, Cimini A, Panebianco M, Leo I, Bucciarelli-Ducci C, Perrone MA. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children. 2023; 10(2):218. https://doi.org/10.3390/children10020218
Chicago/Turabian StyleMoscatelli, Sara, Francesco Bianco, Andrea Cimini, Mario Panebianco, Isabella Leo, Chiara Bucciarelli-Ducci, and Marco Alfonso Perrone. 2023. "The Use of Stress Cardiovascular Imaging in Pediatric Population" Children 10, no. 2: 218. https://doi.org/10.3390/children10020218
APA StyleMoscatelli, S., Bianco, F., Cimini, A., Panebianco, M., Leo, I., Bucciarelli-Ducci, C., & Perrone, M. A. (2023). The Use of Stress Cardiovascular Imaging in Pediatric Population. Children, 10(2), 218. https://doi.org/10.3390/children10020218









