The Single and Combined Effects of Prenatal Nonchemical Stressors and Lead Exposure on Neurodevelopmental Outcomes in Toddlers: Results from the CCREOH Environmental Epidemiologic Study in Suriname
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
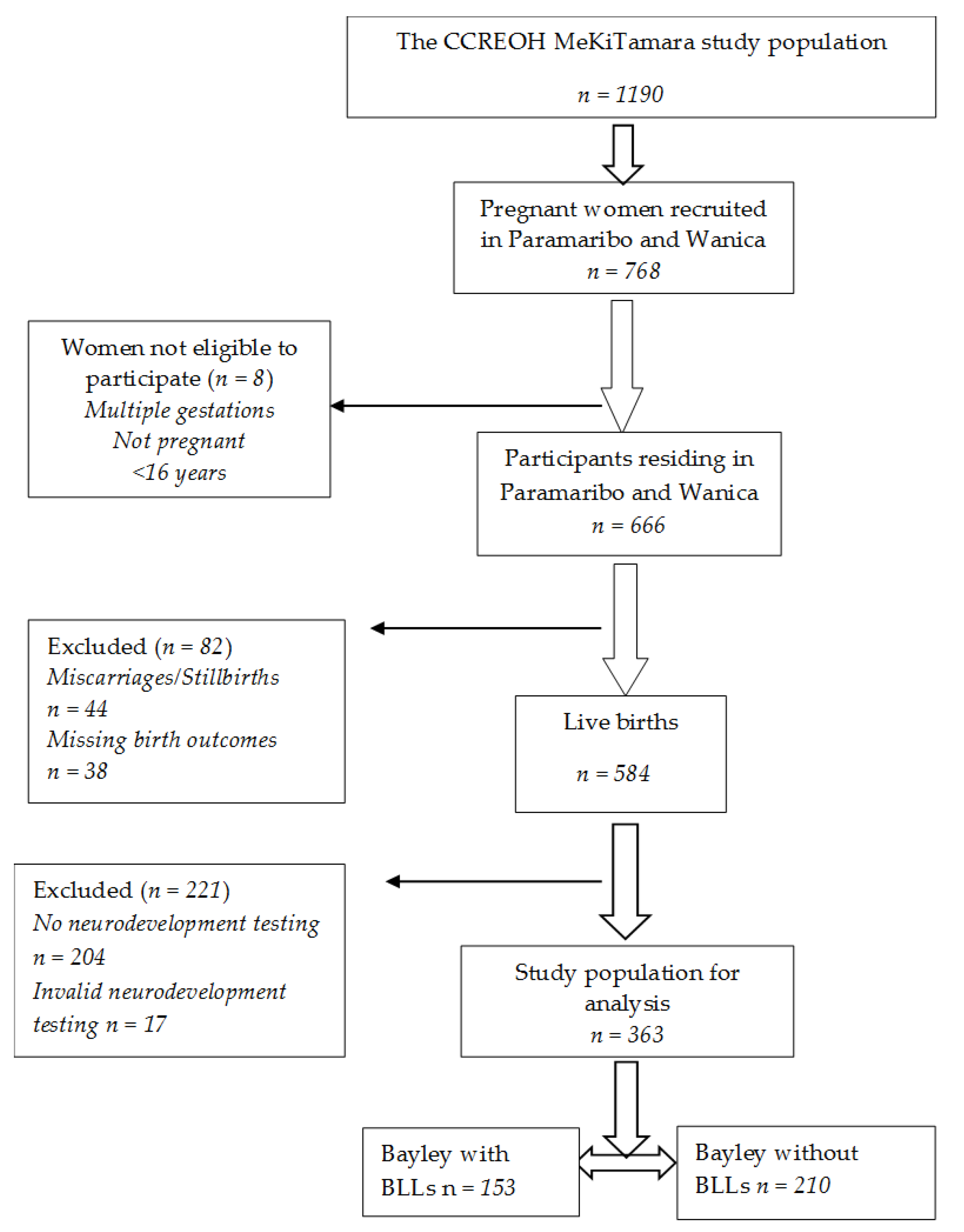
2.2. Study Population and Procedures
2.3. Maternal Perceived Stress
2.4. Maternal Probable Depression
2.5. Prenatal Blood Lead Levels
2.6. Maternal Sociodemographics
2.7. Toddlers’ Sex
2.8. Neurodevelopment Outcomes
2.9. Statistical Analyses
3. Results
4. Discussion
4.1. Limitations
4.2. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Lambertini, L.; Birnbaum, L.S. A Research Strategy to Discover the Environmental Causes of Autism and Neurodevelopmental Disabilities. Environ. Health Perspect. 2012, 120, a258–a260. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Davis, A.C.; Wertlieb, D.; Boo, N.-Y.; Nair, M.K.C.; Halpern, R.; Kuper, H.; Breinbauer, C.; De Vries, P.J.; Gladstone, M.; et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob. Health 2018, 6, e1100–e1121. [Google Scholar] [CrossRef]
- Bitta, M.; Kariuki, S.; Abubakar, A.; Newton, C.R. Burden of neurodevelopmental disorders in low and middle-income countries: A systematic review and meta-analysis. Wellcome Open Res. 2018, 2, 121. [Google Scholar] [CrossRef]
- Gould, E. Childhood Lead Poisoning: Conservative Estimates of the Social and Economic Benefits of Lead Hazard Control. Environ. Health Perspect. 2009, 117, 1162–1167. [Google Scholar] [CrossRef]
- Trasande, L.; Liu, Y. Reducing the Staggering Costs of Environmental Disease in Children, Estimated At $76.6 Billion In 2008. Health Aff. 2011, 30, 863–870. [Google Scholar] [CrossRef]
- Bhang, S.-Y.; Ha, E.; Park, H.; Ha, M.; Hong, Y.-C.; Kim, B.-N.; Lee, S.-J.; Lee, K.Y.; Kim, J.H.; Jeong, J.; et al. Maternal Stress and Depressive Symptoms and Infant Development at Six Months: The Mothers and Children’s Environmental Health (MOCEH) Prospective Study. J. Korean Med. Sci. 2016, 31, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Payne-Sturges, D.C.; Scammell, M.K.; Levy, J.I.; Cory-Slechta, D.A.; Symanski, E.; Shmool, J.L.C.; Laumbach, R.; Linder, S.; Clougherty, J.E. Methods for Evaluating the Combined Effects of Chemical and Nonchemical Exposures for Cumulative Environmental Health Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 2797. [Google Scholar] [CrossRef] [PubMed]
- Huizink, A.C.; De Medina, P.G.R.; Mulder, E.; Visser, G.H.A.; Buitelaar, J.K. Coping in normal pregnancy. Ann. Behav. Med. 2002, 24, 132–140. [Google Scholar] [CrossRef]
- Emmanuel, E.; John, W.S. Maternal distress: A concept analysis. J. Adv. Nurs. 2010, 66, 2104–2115. [Google Scholar] [CrossRef] [Green Version]
- Seimyr, L.; Welles-Nyström, B.; Nissen, E. A history of mental health problems may predict maternal distress in women postpartum. Midwifery 2013, 29, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bergman, K.; Sarkar, P.; O’Connor, T.G.; Modi, N.; Glover, V. Maternal Stress During Pregnancy Predicts Cognitive Ability and Fearfulness in Infancy. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1454–1463. [Google Scholar] [CrossRef]
- Zhu, P.; Sun, M.-S.; Hao, J.-H.; Chen, Y.-J.; Jiang, X.-M.; Tao, R.-X.; Huang, K.; Tao, F.-B. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev. Med. Child Neurol. 2014, 56, 283–289. [Google Scholar] [CrossRef]
- Ortiz, M.T.Y.; Téllez-Rojo, M.M.; Trejo-Valdivia, B.; Schnaas, L.; Osorio-Valencia, E.; Coull, B.; Bellinger, D.; Wright, R.J.; Wright, R.O. Maternal stress modifies the effect of exposure to lead during pregnancy and 24-month old children’s neurodevelopment. Environ. Int. 2016, 98, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Vigil, E.; Thomas, T.; Bellinger, D.C.; Ramthal, A.; Kurpad, A.V.; Duggan, C.P.; Srinivasan, K. Antenatal Depressive Symptoms and Neurodevelopment Outcomes in Children at 30 Months. A Study from South India. Front. Psychiatry 2020, 11, 486175. [Google Scholar] [CrossRef]
- WHO. Childhood Lead Poisoning. 2010. Available online: https://www.who.int/ceh/publications/leadguidance.pdf (accessed on 31 January 2022).
- CDC. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2010. Available online: http://www.cdc.gov/nceh/lead/publications/LeadandPregnancy2010.pdf (accessed on 15 December 2021).
- ACOG. Lead Screening during Pregnancy and Lactation. Committee Opinion. Number 533. Reaffirmed 2019. Available online: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/08/lead-screening-during-pregnancy-and-lactation (accessed on 17 December 2021).
- Ruckart, P.Z.; Jones, R.L.; Courtney, J.G.; LeBlanc, T.T.; Jackson, W.; Karwowski, M.P.; Cheng, P.-Y.; Allwood, P.; Svendsen, E.R.; Breysse, P.N. Update of the Blood Lead Reference Value—United States, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1509–1512. [Google Scholar] [CrossRef]
- Muanya, C. Lead Poisoning Associated with Intellectual Disabilities in Children. 2016. Available online: https://guardian.ng/features/lead-poisoning-associated-with-intellectual-disabilities-in-children/ (accessed on 17 July 2022).
- CDC. Childhood Lead Poisoning Prevention. 2021. Available online: https://www.cdc.gov/nceh/lead/prevention/sources.htm (accessed on 15 December 2021).
- Hu, H.; Téllez-Rojo, M.M.; Bellinger, D.; Smith, D.; Ettinger, A.S.; Lamadrid-Figueroa, H.; Schwartz, J.; Schnaas, L.; Mercado-García, A.; Hernández-Avila, M. Fetal Lead Exposure at Each Stage of Pregnancy as a Predictor of Infant Mental Development. Environ. Health Perspect. 2006, 114, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Perera, F.P.; Jankowski, J.; Mrożek-Budzyn, D.; Mroz, E.; Flak, E.; Edwards, S.; Skarupa, A.; Lisowska-Miszczyk, I. Very Low Prenatal Exposure to Lead and Mental Development of Children in Infancy and Early Childhood: Krakow prospective cohort study. Neuroepidemiology 2009, 32, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Shah-Kulkarni, S.; Ha, M.; Kim, B.-M.; Kim, E.; Hong, Y.-C.; Park, H.; Kim, Y.; Kim, B.-N.; Chang, N.; Oh, S.-Y.; et al. Neurodevelopment in Early Childhood Affected by Prenatal Lead Exposure and Iron Intake. Medicine 2016, 96, e2508. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Virgolini, M.B.; Rossi-George, A.; Thiruchelvam, M.; Lisek, R.; Weston, D. Lifetime Consequences of Combined Maternal Lead and Stress. Basic Clin. Pharmacol. Toxicol. 2008, 102, 218–227. [Google Scholar] [CrossRef]
- Clougherty, J.E.; Shmool, J.L.C.; Kubzansky, L.D. The Role of Non-Chemical Stressors in Mediating Socioeconomic Susceptibility to Environmental Chemicals. Curr. Environ. Health Rep. 2014, 1, 302–313. [Google Scholar] [CrossRef]
- Egan, K.B.; Cornwell, C.R.; Courtney, J.G.; Ettinger, A.S. Blood Lead Levels in U.S. Children Ages 1–11 Years, 1976–2016. Environ. Health Perspect. 2021, 129, 37003. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, M.; Conrad, K.; Marvin, E.; Allen, J.L.; Cory-Slechta, D.A. Endocrine active metals, prenatal stress and enhanced neurobehavioral disruption. Horm. Behav. 2018, 101, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, M.; Varma, G.; Adams, B.; Anderson, D.W.; Schneider, J.S.; A Cory-Slechta, D. Developmental Lead Exposure and Prenatal Stress Result in Sex-Specific Reprograming of Adult Stress Physiology and Epigenetic Profiles in Brain. Toxicol. Sci. 2018, 163, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Surkan, P.J.; Schnaas, L.; Wright, R.J.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Hu, H.; Hernández-Avila, E.M.; Bellinger, D.C.; Schwartz, J.; Perroni, E.; et al. Maternal self-esteem, exposure to lead, and child neurodevelopment. Neurotoxicology 2008, 29, 278–285. [Google Scholar] [CrossRef]
- ABS (General Bureau of Statistics Suriname). Suriname Census Volume 1. Resultaten Achtste (8e) Volks—En Woningtelling in Suriname (Volume 1). Demografische en Sociale Karakteristieken en Migratie. Suriname in Figures nr. 294/2013-05; General Bureau of Statistics: Paramaribo, Suriname, 2013; Available online: https://docslib.org/doc/12100683/algemeen-bureau-voor-de-statistiek-resultaten-achtste (accessed on 12 December 2020).
- Hindori-Mohangoo, A.; Hindori, M. Innovatieve Zorg Rond Zwangerschap en Geboorte in Suriname, 1st ed.; Stichting Perisur: Paramaribo, Suriname, 2017; Available online: https://perisur.org/publicaties/ (accessed on 12 December 2020).
- Koendjbiharie, A.P.; Hindori-Mohangoo, A.D.; Zijlmans, W.C.W.R.; Shankar, A.; Wahid, F.Z.A.; Covert, H.H.; Lichtveld, M.Y.; Drury, S.S. Sociodemographic Influences on Perceived Stress during Pregnancy: Results from the CCREOH Environmental Epidemiologic Study, Suriname. Women 2022, 2, 121–134. [Google Scholar] [CrossRef]
- Gokoel, A.R.; Wahid, F.A.; Zijlmans, W.C.W.R.; Shankar, A.; Hindori-Mohangoo, A.D.; Covert, H.H.; MacDonald-Ottevanger, M.-S.; Lichtveld, M.Y.; Harville, E.W. Influence of perceived stress on prenatal depression in Surinamese women enrolled in the CCREOH study. Reprod. Health 2021, 18, 136. [Google Scholar] [CrossRef]
- Zijlmans, W.; Wickliffe, J.; Hindori-Mohangoo, A.; MacDonald-Ottevanger, S.; Ouboter, P.; Landburg, G.; Codrington, J.; Roosblad, J.; Baldewsingh, G.; Ramjatan, R.; et al. Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Cohort Study: Influences of complex environmental exposures on maternal and child health in Suriname. BMJ Open 2020, 10, e034702. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Wissart, J.; Parshad, O.; Kulkarni, S. Prevalence of pre- and postpartum depression in Jamaican women. BMC Pregnancy Childbirth 2005, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Kessler, R.C.; Gordon, L.U. Measuring Stress: A Guide for Health and Social Scientists; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Solivan, A.E.; Xiong, X.; Harville, E.; Buekens, P. Measurement of Perceived Stress Among Pregnant Women: A Comparison of Two Different Instruments. Matern. Child Health J. 2015, 19, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Melo, E.F.; Cecatti, J.G.; Pacagnella, R.C.; Leite, D.F.; Vulcani, D.E.; Makuch, M.Y. The prevalence of perinatal depression and its associated factors in two different settings in Brazil. J. Affect. Disord. 2012, 136, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- McCabe-Beane, J.E.; Segre, L.S.; Perkhounkova, Y.; Stuart, S.; O’Hara, M.W. The identification of severity ranges for the Edinburgh Postnatal Depression Scale. J. Reprod. Infant Psychol. 2016, 34, 293–303. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Rowland, A.S.; Young, B.N.; Cano, S.; Phelan, S.T.; Artyushkova, K.; Rayburn, W.F.; Lewis, J. Sources of Potential Lead Exposure Among Pregnant Women in New Mexico. Matern. Child Health J. 2012, 17, 172–179. [Google Scholar] [CrossRef]
- Albers, C.A.; Grieve, A.J. Test review: Bayley N. Bayley Scales of Infant and Toddler Development–Third Edition. 2007. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 2007, 25, 180–190. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant and Toddler Development, 3rd ed.; Technical Manual; NCS Pearson, Inc.: Bloomington, MN, USA, 2006. [Google Scholar]
- McLester-Davis, L.; Shankar, A.; Kataria, L.; Hidalgo, A.; van Eer, E.; Koendjbiharie, A.; Ramjatan, R.; Hatch, V.; Middleton, M.; Zijlmans, C.; et al. Validity, reliability, and transcultural adaptations of the Bayley Scales of Infant and Toddler Development (BSID-III-NL) for children in Suriname. Early Hum. Dev. 2021, 160, 105416. [Google Scholar] [CrossRef] [PubMed]
- Engidaw, N.A.; Mekonnen, A.G.; Amogne, F.K. Perceived stress and its associated factors among pregnant women in Bale zone Hospitals, Southeast Ethiopia: A cross-sectional study. BMC Res. Notes 2019, 12, 356. [Google Scholar] [CrossRef]
- Pantha, S.; Pantha, E.; Hayes, B.; Yadav, B.K.; Sharma, P.; Shrestha, A.; Gartoulla, P. Prevalence of Stress among Pregnant Women Attending Antenatal Care in a Tertiary Maternity Hospital in Kathmandu. J. Womens Health Care 2014, 3, 1000183. [Google Scholar] [CrossRef]
- Boakye-Yiadom, A.; Shittu, S.; Dutt, J.; Dapare, P.; Alhassan, A. Perceived stress and anxiety among Ghanaian pregnant women. J. Med. Biomed. Sci. 2015, 4, 29. [Google Scholar] [CrossRef]
- Dadi, A.F.; Miller, E.R.; Mwanri, L. Antenatal depression and its association with adverse birth outcomes in low and middle-income countries: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0227323. [Google Scholar] [CrossRef]
- Donald, K.A.; Wedderburn, C.J.; Barnett, W.; Nhapi, R.T.; Rehman, A.M.; Stadler, J.A.M.; Hoffman, N.; Koen, N.; Zar, H.J.; Stein, D.J. Risk and protective factors for child development: An observational South African birth cohort. PLoS Med. 2019, 16, e1002920. [Google Scholar] [CrossRef] [PubMed]
- Krogh, M.T.; Væver, M.S. Does gender affect Bayley-III scores and test-taking behavior? Infant Behav. Dev. 2019, 57, 101352. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Jordan, C.L.; Breedlove, S.M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.-M.; Swaab, D.F. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Front. Neuroendocr. 2011, 32, 214–226. [Google Scholar] [CrossRef]
- Rimbaud, D.; Restrepo, M.; Louison, A.; Boukhari, R.; Ardillon, V.; Carles, G.; Lambert, V.; Jolivet, A. Blood lead levels and risk factors for lead exposure among pregnant women in western French Guiana: The role of manioc consumption. J. Toxicol. Environ. Health Part A 2017, 80, 382–393. [Google Scholar] [CrossRef]
- Forde, M.S.; Dewailly, E.; Robertson, L.; Sidi, E.A.L.; Côté, S.; Sandy, L.; Dumas, P.; Ayotte, P. Mercury and lead blood concentrations in pregnant women from 10 caribbean countries. Environ. Sci. Process. Impacts 2014, 16, 2184–2190. [Google Scholar] [CrossRef]
- Koutra, K.; Chatzi, L.; Bagkeris, M.; Vassilaki, M.; Bitsios, P.; Kogevinas, M. Antenatal and postnatal maternal mental health as determinants of infant neurodevelopment at 18 months of age in a mother–child cohort (Rhea Study) in Crete, Greece. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 48, 1335–1345. [Google Scholar] [CrossRef]
- Field, T. Prenatal Depression Risk Factors, Developmental Effects and Interventions: A Review. J. Pregnancy Child Health 2017, 4, 301. [Google Scholar] [CrossRef]
- Servili, C.; Medhin, G.; Hanlon, C.; Tomlinson, M.; Worku, B.; Baheretibeb, Y.; Dewey, M.; Alem, A.; Prince, M. Maternal common mental disorders and infant development in Ethiopia: The P-MaMiE Birth Cohort. BMC Public Health 2010, 10, 693. [Google Scholar] [CrossRef]
- Tuovinen, S.; Lahti-Pulkkinen, M.; Girchenko, P.; Lipsanen, J.; Lahti, J.; Heinonen, K.; Reynolds, R.M.; Hämäläinen, E.; Kajantie, E.; Laivuori, H.; et al. Maternal depressive symptoms during and after pregnancy and child developmental milestones. Depression Anxiety 2018, 35, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Milgrom, J.; Gemmill, A.W.; Bilszta, J.L.; Hayes, B.; Barnett, B.; Brooks, J.; Ericksen, J.; Ellwood, D.; Buist, A. Antenatal risk factors for postnatal depression: A large prospective study. J. Affect. Disord. 2008, 108, 147–157. [Google Scholar] [CrossRef]
- Ghaedrahmati, M.; Kazemi, A.; Kheirabadi, G.; Ebrahimi, A.; Bahrami, M. Postpartum depression risk factors: A narrative review. J. Educ. Health Promot. 2017, 6, 60. [Google Scholar] [CrossRef]
- Piccinini, C.A.; Frizzo, G.B.; Brys, I.; Lopes, R.D.C.S. Parenthood in the context of maternal depression at the end of the infant’s first year of life. Estud. Psicol. 2014, 31, 203–214. [Google Scholar] [CrossRef]
- Hamadani, J.D.; Tofail, F.; Hilaly, A.; Huda, S.N.; Engle, P.; Grantham-McGregor, S.M. Use of Family Care Indicators and Their Relationship with Child Development in Bangladesh. J. Health Popul. Nutr. 2010, 28, 23–33. [Google Scholar] [CrossRef]
- Black, M.M.; Baqui, A.H.; Zaman, K.; McNary, S.W.; Le, K.; El Arifeen, S.; Hamadani, J.D.; Parveen, M.; Yunus; Black, R.E. Depressive symptoms among rural Bangladeshi mothers: Implications for infant development. J. Child Psychol. Psychiatry 2007, 48, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.S.; Mahmud, S.; Khan, A.; Ali, B.S. Impact of postpartum anxiety and depression on child’s mental development from two peri-urban communities of Karachi, Pakistan: A quasi-experimental study. BMC Psychiatry 2013, 13, 274. [Google Scholar] [CrossRef]
- Hadley, C.; Tegegn, A.; Tessema, F.; Asefa, M.; Galea, S. Parental symptoms of common mental disorders and children’s social, motor, and language development in sub-Saharan Africa. Ann. Hum. Biol. 2008, 35, 259–275. [Google Scholar] [CrossRef]
- Patra, K.; Greene, M.M.; Patel, A.L.; Meier, P. Maternal Education Level Predicts Cognitive, Language, and Motor Outcome in Preterm Infants in the Second Year of Life. Am. J. Perinatol. 2016, 33, 738–744. [Google Scholar] [CrossRef]
- Ko, G.; Shah, P.; Lee, S.K.; Asztalos, E. Impact of Maternal Education on Cognitive and Language Scores at 18 to 24 Months among Extremely Preterm Neonates. Am. J. Perinatol. 2013, 30, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Yan, S.; Huang, K.; Mao, L.; Ge, X.; Weng, T.; Zuo, A.; Tao, X.; Tao, F. Maternal intelligence quotient and motor development in early childhood: The mediating role of mother’s education. J. Paediatr. Child Health 2018, 55, 87–94. [Google Scholar] [CrossRef]
- Corsi, C.; Santos, M.M.; Marques, L.A.; Rocha, N.A. Impact of extrinsic factors on fine motor performance of children attending day care. Repercussões de fatores extrínsecos no desempenho motor fino de crianças frequentadoras de creches. Rev. Paul. Pediatr. 2016, 34, 439–446. [Google Scholar] [CrossRef]
- Santos, D.; Tolocka, R.; Carvalho, J.; Heringer, L.; Almeida, C.; Miquelote, A. Desempenho motor grosso e sua associação com fatores neonatais, familiares e de exposição à creche em crianças até três anos de idade. Braz. J. Phys. Ther. 2009, 13, 173–179. [Google Scholar] [CrossRef]
- Ravenscroft, E.F.; Harris, S.R. Is Maternal Education Related to Infant Motor Development? Pediatr. Phys. Ther. 2007, 19, 56–61. [Google Scholar] [CrossRef]
- Murray, G.; Veijola, J.; Moilanen, K.; Miettunen, J.; Glahn, D.; Cannon, T.; Jones, P.; Isohanni, M. Infant motor development is associated with adult cognitive categorisation in a longitudinal birth cohort study. J. Child Psychol. Psychiatry 2005, 47, 25–29. [Google Scholar] [CrossRef]
- ABS (General Bureau of Statistics, Suriname). Crude Birth Rate ’ Infant Mortality Rate-Suriname—Under 5 Mortality Rate-Suriname & DISTRICTS. Available online: https://statistics-suriname.org/population-statistics-2/ (accessed on 20 November 2022).
- Hertz-Picciotto, I.; Schramm, M.; Watt-Morse, M.; Chantala, K.; Anderson, J.; Osterloh, J. Patterns and Determinants of Blood Lead During Pregnancy. Am. J. Epidemiol. 2000, 152, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Heard, M.; Chamberlain, A. Effect of Minerals and Food on Uptake of Lead from the Gastrointestinal Tract in Humans. Hum. Toxicol. 1982, 1, 411–415. [Google Scholar] [CrossRef]
- Gulson, B.L.; Mizon, K.J.; Korsch, M.J.; Palmer, J.M.; Donnelly, J.B. Mobilization of lead from human bone tissue during pregnancy and lactation—A summary of long-term research. Sci. Total. Environ. 2002, 303, 79–104. [Google Scholar] [CrossRef]
- Gulson, B.L.; Mizon, K.J.; Palmer, J.M.; Korsch, M.J.; Taylor, A.J.; Mahaffey, K.R. Blood Lead Changes during Pregnancy and Postpartum with Calcium Supplementation. Environ. Health Perspect. 2004, 112, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Avila, M.; Sanin, L.H.; Romieu, I.; E.Palazuelosde, E.; Conyerf, R.; Olaiz, G.; Rojas, R.; Navarrete, J. Higher Milk Intake during Pregnancy Is Associated with Lower Maternal and Umbilical Cord Lead Levels in Postpartum Women. Environ. Res. 1997, 74, 116–121. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Lamadrid-Figueroa, H.; Téllez-Rojo, M.M.; Mercado-García, A.; Peterson, K.E.; Schwartz, J.; Hu, H.; Hernández-Avila, M. Effect of Calcium Supplementation on Blood Lead Levels in Pregnancy: A Randomized Placebo-Controlled Trial. Environ. Health Perspect. 2009, 117, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, W.T.; Friello, P.; Semba, R.D. Interactions between iron deficiency and lead poisoning: Epidemiology and pathogenesis. Sci. Total Environ. 2004, 330, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Willett, W.C.; Schwartz, J.; Sparrow, D.; Weiss, S.; Hu, H. Relation of nutrition to bone lead and blood lead levels in middle-aged to elderly men. The Normative Aging Study. Am. J. Epidemiol. 1998, 147, 1162–1174. [Google Scholar] [CrossRef]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef] [PubMed]
- Janbek, J.; Sarki, M.; Specht, I.O.; Heitmann, B.L. A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur. J. Clin. Nutr. 2019, 73, 1561–1578. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Cory-Slechta, D.A. Epigenetic Mechanisms of Adverse Neurodevelopment in Response to Lead Exposure and Prenatal Stress and the Combination: The Road Ahead. In Epigenetics, the Environment, and Children’s Health across Lifespans; Springer International Publishing: Cham, Switzerland, 2016; pp. 251–277. [Google Scholar]
| Variables | n | % | % Missing |
|---|---|---|---|
| Toddlers’ characteristics | |||
| Sex | 0.0% | ||
| Boys | 183 | 50.4% | |
| Girls | 180 | 49.6% | |
| Age (in months) | 16 (15–20) | ||
| Median (IQR) | |||
| BSID III scaled scores. Median (IQR) | 0.0% | ||
| Cognitive | 11 (9–13) | ||
| Fine motor | 11 (9–13) | ||
| Gross motor | 10 (9–12) | ||
| Receptive | 9 (8–11) | ||
| Expressive | 9 (8–11) | ||
| Social-emotional | 7 (7–9) | ||
| Maternal characteristics | |||
| Blood lead levels | 57.9% | ||
| Median (IQR) | 1.7 (1.1–2.6) | ||
| <3.5 µg/dL | 136 | 88.9% | |
| ≥3.5 µg/dL | 17 | 11.1% | |
| Cohen’s Perceived Stress Scores | 6.1% | ||
| Median (IQR) | 16 (12–19) | ||
| 0–19 low–normal | 261 | 75.4% | |
| 20–40 high | 85 | 24.6% | |
| Edinburgh Depression Scores | 6.3% | ||
| Median (IQR) | 7 (4–10) | ||
| 0–6 no or minimal | 157 | 46.2% | |
| 7–13 mild | 142 | 41.8% | |
| 14–19 moderate | 30 | 8.8% | |
| 20–30 severe | 11 | 3.2% | |
| Age (in years) | 0.0% | ||
| Median (IQR) | 28.4 (24.7–33.0) | ||
| 16–19 | 32 | 8.8% | |
| 20–24 | 65 | 17.9% | |
| 25–29 | 117 | 32.2% | |
| 30–34 | 82 | 22.6% | |
| 35+ | 67 | 18.5% | |
| Parity | 0.3% | ||
| 0 previous live births | 125 | 34.5% | |
| 1 previous live birth | 113 | 31.2% | |
| 2 previous live births | 60 | 16.6% | |
| 3 previous live births | 31 | 8.6% | |
| 4+ previous live births | 33 | 9.1% | |
| Ethnic background | 0.0% | ||
| Creole | 125 | 34.4% | |
| Hindustani | 64 | 17.6% | |
| Indigenous | 5 | 1.4% | |
| Javanese | 14 | 3.9% | |
| Tribal | 68 | 18.7% | |
| Mixed | 87 | 24.0% | |
| Educational level | 0.0% | ||
| primary or not educated | 37 | 10.2% | |
| lower secondary/vocational | 124 | 34.2% | |
| upper secondary/vocational | 120 | 33.1% | |
| tertiary | 82 | 22.6% | |
| Household income (in SRD) | 4.7% | ||
| <800 | 23 | 6.6% | |
| 800–1499 | 60 | 17.3% | |
| 1500–2999 | 113 | 32.7% | |
| 3000+ | 150 | 43.4% | |
| Household size | 0.3% | ||
| <3 persons | 39 | 10.7% | |
| 3+ persons | 324 | 89.3% | |
| Marital status | 0.0% | ||
| Married/living together | 295 | 81.3% | |
| Unmarried/single | 68 | 18.7% | |
| BSID III Scale Scores (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|
| Cognitive | Fine Motor | Gross Motor | Receptive Communication | Expressive Communication | Social-Emotional | |
| Sex of infant | ||||||
| Boys (n = 183) | 10.9 ± 2.6 | 10.6 ± 2.5 | 10.5 ± 2.6 | 8.9 ± 2.2 | 8.9 ± 2.1 | 8.3 ± 2.7 |
| Girls (n = 180) | 10.9 ± 2.7 | 11.1 ± 2.4 | 10.2 ± 2.7 | 9.6 ± 2.1 | 9.5 ± 2.1 | 8.0 ± 2.6 |
| p-value | 0.989 | 0.033 | 0.302 | 0.002 | 0.004 | 0.283 |
| Blood lead levels | ||||||
| <3.5 µg/dL (n = 136) | 11.0 ± 2.8 | 11.0 ± 2.6 | 10.2 ± 2.6 | 9.2 ± 2.0 | 9.4 ± 2.0 | 8.5 ± 2.6 |
| ≥3.5 µg/dL (n = 17) | 10.5 ± 2.8 | 10.9 ± 2.6 | 9.9 ± 1.8 | 7.8 ± 2.0 | 8.8 ± 2.0 | 8.0 ± 2.9 |
| p-value | 0.453 | 0.885 | 0.617 | 0.008 | 0.006 | 0.416 |
| Cohen’s Perceived Stress Scores | ||||||
| 0–19 low–normal (n = 261) | 11.1 ± 2.7 | 10.9 ± 2.4 | 10.5 ± 2.6 | 9.4 ± 2.2 | 9.2 ± 2.1 | 8.0 ± 2.3 |
| 20–40 high (n = 85) | 10.3 ± 2.6 | 10.6 ± 2.6 | 10.0 ± 2.7 | 8.8 ± 2.1 | 9.3 ± 2.2 | 8.7 ± 3.4 |
| p-value | 0.025 | 0.249 | 0.122 | 0.058 | 0.668 | 0.019 |
| Edinburgh Depression Scores | ||||||
| 0–6 no or minimal (n = 157) | 11.2 ± 2.7 | 11.2 ± 2.4 | 10.8 ± 2.8 | 9.4 ± 2.3 | 9.2 ± 2.1 | 8.1 ± 2.3 |
| 7–13 mild (n = 142) | 10.7 ± 2.7 | 10.5 ± 2.5 | 10.2 ± 2.5 | 9.1 ± 2.1 | 9.1 ± 2.3 | 8.0 ± 2.5 |
| 14–30 moderate and severe (n = 41) | 10.3 ± 2.7 | 10.3 ± 2.5 | 9.5 ± 2.5 | 9.0 ± 2.1 | 9.3 ± 1.9 | 8.8 ± 4.0 |
| p-value | 0.114 | 0.009 | 0.009 | 0.383 | 0.816 | 0.207 |
| Age (in years) | ||||||
| 16–19 (n = 32) | 10.9 ± 2.4 | 10.8 ± 2.5 | 10.5 ± 2.6 | 9.5 ± 2.5 | 9.0 ± 2.1 | 8.1 ± 3.3 |
| 20–24 (n = 65) | 10.6 ± 2.4 | 10.5 ± 2.4 | 11.0 ± 3.2 | 9.7 ± 2.1 | 9.5 ± 2.2 | 8.2 ± 2.5 |
| 25–29 (n = 117) | 11.2 ± 3.0 | 11.1 ± 2.3 | 10.2 ± 2.5 | 9.0 ± 2.2 | 9.0 ± 2.0 | 8.1 ± 2.6 |
| 30–34 (n = 82) | 10.9 ± 2.5 | 10.7 ± 2.4 | 10.2 ± 2.3 | 9.1 ± 2.2 | 9.5 ± 2.5 | 8.1 ± 2.8 |
| 35+ (n = 67) | 10.8 ± 2.5 | 11.0 ± 2.6 | 10.3 ± 2.6 | 9.3 ± 2.0 | 9.0 ± 1.8 | 8.1 ± 2.2 |
| p-value | 0.725 | 0.486 | 0.310 | 0.250 | 0.370 | 0.998 |
| Parity | ||||||
| 0–3 previous live births (n = 329) | 11.0 ± 2.6 | 10.9 ± 2.5 | 10.4 ± 2.7 | 9.3 ± 2.1 | 9.2 ± 2.1 | 8.1 ± 2.6 |
| 4+ previous live births (n = 33) | 10.4 ± 3.0 | 10.5 ± 2.2 | 10.0 ± 1.7 | 8.7 ± 2.2 | 8.9 ± 2.2 | 8.7 ± 3.0 |
| p-value | 0.225 | 0.392 | 0.427 | 0.150 | 0.439 | 0.182 |
| Ethnic background | ||||||
| Creole (n = 125) | 11.2 ± 2.5 | 11.2 ± 2.3 | 10.7 ± 2.7 | 9.3 ± 2.1 | 9.2 ± 2.1 | 7.9 ± 2.5 |
| Hindustani (n = 64) | 10.9 ± 2.8 | 10.9 ± 2.4 | 9.9 ± 2.7 | 9.6 ± 2.1 | 9.6 ± 2.3 | 8.3 ± 2.5 |
| Indigenous (n = 5) | 10.0 ± 1.6 | 12.8 ± 2.0 | 10.4 ± 1.5 | 9.2 ± 1.3 | 9.0 ± 1.2 | 7.2 ± 3.1 |
| Javanese (n = 14) | 10.4 ± 2.3 | 10.7 ± 2.3 | 9.9 ± 3.0 | 9.8 ± 1.9 | 9.7 ± 2.0 | 7.7 ± 2.1 |
| Tribal (n = 68) | 10.4 ± 2.7 | 10.1 ± 2.3 | 10.2 ± 2.2 | 8.7 ± 2.1 | 8.8 ± 1.9 | 8.4 ± 3.5 |
| Mixed (n = 87) | 10.9 ± 2.7 | 10.8 ± 2.7 | 10.5 ± 2.9 | 9.3 ± 2.3 | 9.2 ± 2.3 | 8.3 ± 2.0 |
| p-value | 0.353 | 0.028 | 0.441 | 0.267 | 0.391 | 0.652 |
| Educational level | ||||||
| lower (n = 161) | 10.3 ± 2.7 | 10.5 ± 2.4 | 10.4 ± 2.5 | 8.9 ± 2.1 | 9.0 ± 2.0 | 8.2 ± 3.1 |
| higher (n = 202) | 11.4 ± 2.5 | 11.2 ± 2.4 | 10.6 ± 2.8 | 9.5 ± 2.2 | 9.4 ± 2.2 | 8.1 ± 2.2 |
| p-value | < 0.001 | 0.006 | 0.140 | 0.002 | 0.110 | 0.650 |
| Household income (in SRD) | ||||||
| <800 (n = 23) | 11.4 ± 3.1 | 11.2 ± 2.3 | 10.3 ± 1.6 | 9.3 ± 1.7 | 9.2 ± 1.9 | 8.5 ± 3.4 |
| 800–1499 (n = 60) | 10.2 ± 2.7 | 10.1 ± 2.2 | 9.5 ± 2.3 | 8.8 ± 1.8 | 8.9 ± 2.2 | 8.4 ± 3.3 |
| 1500–2999 (n = 113) | 10.7 ± 2.7 | 10.8 ± 2.6 | 10.6 ± 3.0 | 9.4 ± 2.3 | 9.1 ± 1.9 | 7.7 ± 2.4 |
| 3000+ (n = 150) | 11.4 ± 2.5 | 11.3 ± 2.4 | 10.6 ± 2.6 | 9.4 ± 2.1 | 9.5 ± 2.3 | 8.3 ± 2.3 |
| p-value | 0.019 | 0.010 | 0.050 | 0.223 | 0.154 | 0.209 |
| Household size | ||||||
| <3 persons (n = 39) | 11.0 ± 2.6 | 11.2 ± 2.6 | 10.1 ± 2.5 | 9.1 ± 2.3 | 9.2 ± 2.0 | 7.7 ± 2.0 |
| 3+ persons (n = 324) | 10.9 ± 2.7 | 10.8 ± 2.4 | 10.4 ± 2.7 | 9.3 ± 2.1 | 9.2 ± 2.1 | 8.2 ± 2.7 |
| p-value | 0.860 | 0.425 | 0.542 | 0.681 | 0.856 | 0.356 |
| Marital status | ||||||
| Married/living together (n = 295) | 11.0 ± 2.7 | 10.9 ± 2.4 | 10.3 ± 2.7 | 9.2 ± 2.2 | 9.2 ± 2.2 | 8.0 ± 2.5 |
| Unmarried/single (n = 68) | 10.7 ± 2.6 | 10.8 ± 2.4 | 10.7± 2.6 | 9.2 ± 2.2 | 9.1 ± 2.0 | 8.4 ± 3.0 |
| p-value | 0.433 | 0.681 | 0.227 | 0.896 | 0.732 | 0.317 |
| β [95% CI: LB-UB] for BSID III Scaled Scores | ||||||
|---|---|---|---|---|---|---|
| Cognitive | Fine Motor | Gross Motor | Receptive Communication | Expressive Communication | Social-Emotional | |
| Model 1: | ||||||
| Single lead exposure | −0.08 [-0.40–0.24] | −0.08 [−0.37–0.20] | 0.00 [−0.30–0.29] | −0.26 [−0.49–−0.02] | −0.19 [−0.42–0.04] | −0.12 [−0.42–0.19] |
| Single stress exposure | −0.01 [−0.12–0.10] | −0.04 [−0.14–0.05] | 0.04 [−0.06–0.14] | 0.00 [−0.08–0.08] | 0.02 [−0.06–0.10] | 0.06 [−0.04–0.17] |
| Single probable depression exposure | −0.08 [−0.21–0.04] | −0.08 [−0.19–0.02] | −0.13 [−0.24–−0.02] | −0.05 [−0.14–0.04] | −0.02 [−0.11–0.07] | 0.00 [−0.12–0.12] |
| Model 2: | ||||||
| Cumulative exposure risk index # | −0.44 [−0.95–0.06] | −0.74 [−1.41–−0.01] | −0.55 [−1.23–0.12] | −0.33 [−0.81–0.14] | −0.30 [−0.84–0.25] | 0.30 [−0.34–0.94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koendjbiharie, A.P.; Hindori-Mohangoo, A.D.; Zijlmans, W.C.W.R.; Wickliffe, J.K.; Shankar, A.; Covert, H.H.; Lichtveld, M.Y.; Grünberg, A.W.; Drury, S.S. The Single and Combined Effects of Prenatal Nonchemical Stressors and Lead Exposure on Neurodevelopmental Outcomes in Toddlers: Results from the CCREOH Environmental Epidemiologic Study in Suriname. Children 2023, 10, 287. https://doi.org/10.3390/children10020287
Koendjbiharie AP, Hindori-Mohangoo AD, Zijlmans WCWR, Wickliffe JK, Shankar A, Covert HH, Lichtveld MY, Grünberg AW, Drury SS. The Single and Combined Effects of Prenatal Nonchemical Stressors and Lead Exposure on Neurodevelopmental Outcomes in Toddlers: Results from the CCREOH Environmental Epidemiologic Study in Suriname. Children. 2023; 10(2):287. https://doi.org/10.3390/children10020287
Chicago/Turabian StyleKoendjbiharie, Aloysius Ph., Ashna D. Hindori-Mohangoo, Wilco C. W. R. Zijlmans, Jeffrey K. Wickliffe, Arti Shankar, Hannah H. Covert, Maureen Y. Lichtveld, Antoon W. Grünberg, and Stacy S. Drury. 2023. "The Single and Combined Effects of Prenatal Nonchemical Stressors and Lead Exposure on Neurodevelopmental Outcomes in Toddlers: Results from the CCREOH Environmental Epidemiologic Study in Suriname" Children 10, no. 2: 287. https://doi.org/10.3390/children10020287





