Developmental Pathway Choices of Young People Presenting to a Gender Service with Gender Distress: A Prospective Follow-Up Study
Abstract
:| Biological sex: refers to the pattern of findings on chromosomal testing. An XY chromosomal pattern refers to male sex (♂), and an XX chromosomal pattern refers to female sex (♀). All participants in the study had chromosomal testing as part of their medical workups. Cisgender: refers to a gender identity that is aligned (congruent) with biological sex. Desistance in the cohort as a whole: In the cohort as a whole, desistance refers to the resolution/disappearance of the gender-related distress that was the foundation for the young person to present to the service. Desistance in the Gender Dysphoria subgroup: In the subgroup with a formal diagnosis of Gender Dysphoria (DSM-5), desistance refers to discontinuation of the journey to transition to the other gender (transgender pathway). In the gender dysphoria subgroup, the act of desisting from the transgender pathway included cessation of social transition, puberty blockers, or cross-sex hormones or a combination of these elements. Gender: refers to each participant’s subjective experience of identity along the gender spectrum. Gender Dysphoria (GD): refers to a feeling of distress (dysphoria) that meets diagnostic criteria for gender dysphoria as per DSM-5 [9]. Gender-related distress: refers to a feeling of distress (dysphoria) pertaining to gender that may or may not meet DSM-5 criteria for gender dysphoria. Persistence: refers to continuation of the journey to transition to the other gender (transgender pathway). In the current cohort, persistence could include social transition, treatment with puberty blockers, treatment with cross-sex hormones, gender-affirming surgery, or any one element or of a combination of elements. Transgender: refers to a gender identity that is not aligned with biological sex but is instead aligned with the other sex. In the case of the participants from the current cohort, experiencing the self as transgender was the foundation for the subjective experience of gender dysphoria that met the DSM-5 criteria for gender dysphoria. |
1. National Practices and Policies
1.1. Finland
1.2. Sweden
1.3. United Kingdom
- −
- Closure of the Tavistock service (a centralised NHS service) [35,36] and the opening of “Regional centres [that] should be led by experienced providers of tertiary paediatric care to ensure a focus on child health and development, with strong links to mental health services. These will generally be specialist children’s hospitals”.
- −
- Services “should have established academic and education functions to ensure that ongoing research and training is embedded within the service delivery model”.
- −
- Services “should have an appropriate multi-professional workforce to enable them to provide an integrated model of care that manages the holistic needs of this population”.
- −
- “Staff should maintain a broad clinical perspective to embed the care of children and young people with gender uncertainty within a broader child and adolescent health context”. Along these lines, the Cass Review noted that “We also welcome the recognition that this is a heterogenous group and that not all children and young people will want or require a medical pathway, and that the service needs to include the appropriate skill mix to support both those individuals who do require medical intervention and those who do not” (p. 2) [34].
1.4. Australia
- −
- Improved access to gender-affirming treatments and care is therefore a key priority. Almost three-quarters of transgender and gender diverse respondents to our LGBTQ community survey indicated difficulties accessing such services (71%). Barriers to access include the limited number of services in NSW, the high costs of some treatment options such as puberty blockers and surgeries, and the requirement for a diagnosis of ‘gender dysphoria’ by a psychiatrist to access hormone replacement therapy (HRT) (p. 13) [41].
- −
- The strategy should enable a pathway of care for people seeking to affirm their gender. The pathway of care should focus on depathologising and reducing barriers to accessing gender-affirming treatments and care. The pathway of care should centre on the expertise, informed consent, rights and lived experience of transgender and gender diverse adults, adolescents and children (p. 20) [41].
- −
- [Depathologising] refers to moving away from classifying transgender people as having a mental health condition such as ‘gender dysphoria’ and from the requirement of a diagnosis of gender dysphoria before access to gender-affirming treatments and care is permitted (footnote bottom of page 20).
1.5. United States
1.6. Summary
2. Methods
- −
- For all young people who had exited the Gender Service, final follow-up telephone calls with them and their families were attempted between November 2022 and January 2023. No calls were made to the young people and families who had previously requested no further follow-up calls (n = 3). The interviewer (JE) used a script to guide the questions asked during the telephone interview (see Text Box 2).
- −
- For young people who had exited the service and who could not be contacted by telephone during the November 2022–January 2023 period (see above), information was collated from past clinic letters, from letters sent by the clinicians within the adult health system to whom the young person’s care had been transitioned, and from previous follow-up phone calls up to the middle of 2021.
- −
- For young people who were still engaged in the Gender Service—that is, they had ongoing face-to-face visits in the clinic—information from recent clinic letters was used. Telephone follow-up was undertaken to clarify any missing information.
| Hello, my name is Dr JE from the Children’s Hospital at Westmead. Am I speaking to [patient/parent]? I work with psychiatrist Dr KK in Psychological Medicine and was calling in regard to follow-up for the gender study you were enrolled in some time ago. Is it Ok if I ask a few questions? |
| Question 1—Asked only if this information was not known from patient notes or previous follow-up calls Did you ever receive stage 1 therapy, commonly known as puberty blockers? If yes: What age were you when they were started? |
| Question 2—Asked only if this information was not known from patient notes or previous follow-up calls Did you ever receive stage 2 therapy, commonly known as cross-sex hormones or gender reaffirming hormones? If yes: What age were you when they were started? Are you currently still taking them. If not, when were they ceased? |
| Question 3 Do you have any current medical or mental health conditions? If yes: What conditions? Are they being treated? |
| Question 4 Are you currently working or studying? If yes: What type of employment/study? |
| Question 5 Have you undergone any gender-related surgery, or are you considering surgery in the future? If yes: What type of surgery? Age surgery occurred? |
Data Analysis
3. Results
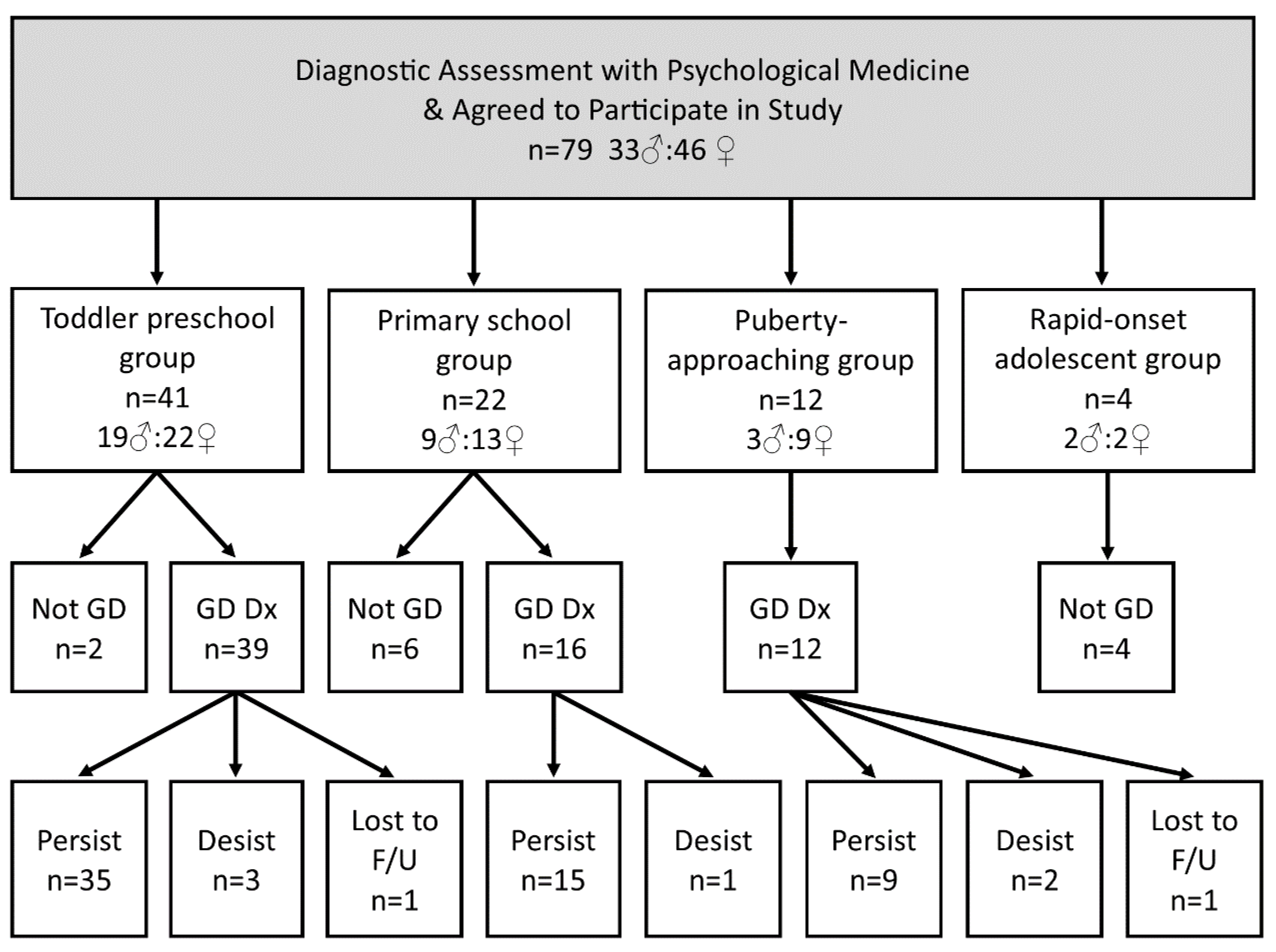
3.1. Demographics
3.2. Information Sources Pertaining to Outcomes
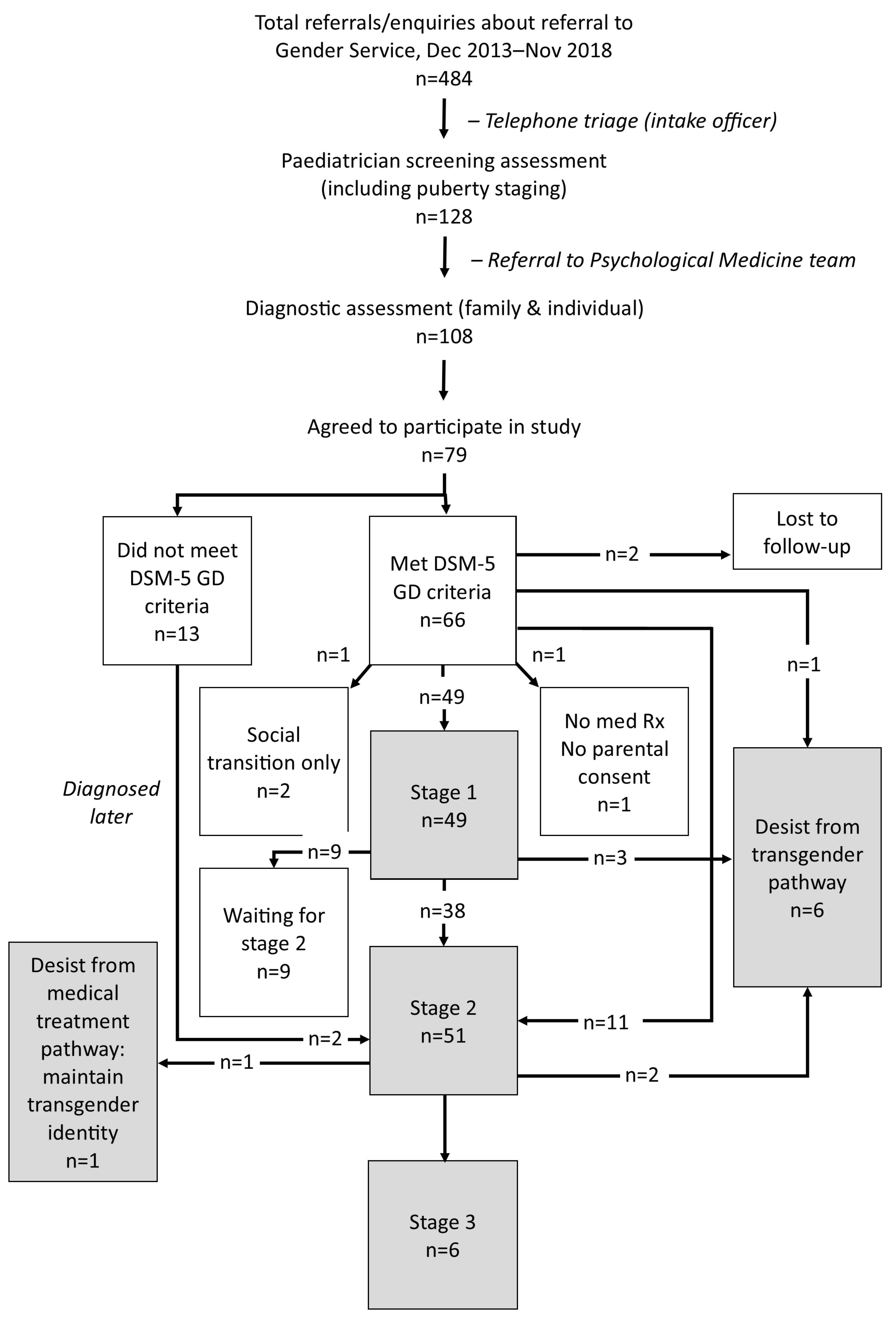
3.3. The Diagnostic Assessment Process
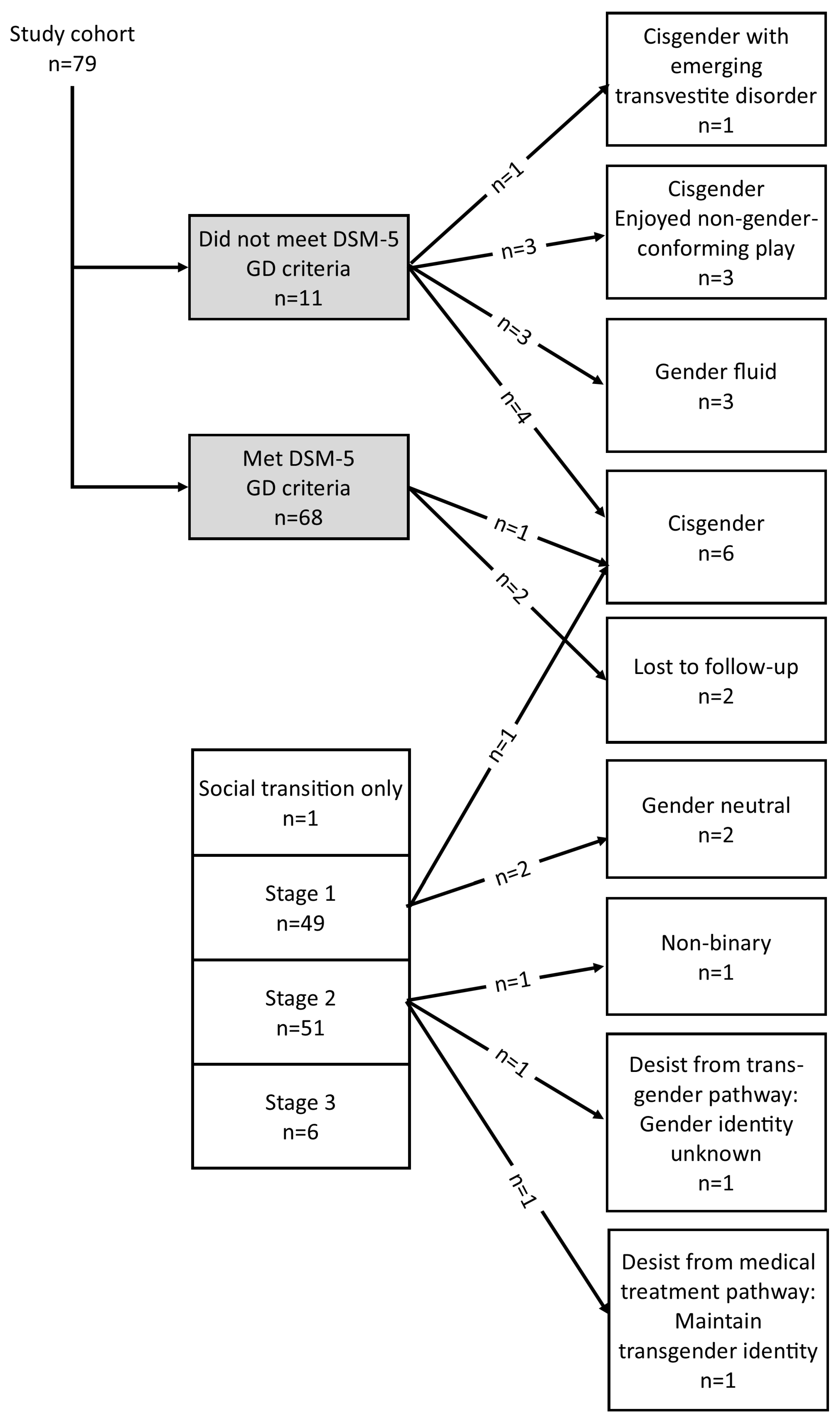
3.4. Developmental Pathway Choices of Study Participants
3.5. Treatment with Gonadotropin-Releasing Hormone Analogues (Puberty Blockers)
3.6. Treatment with Cross-Sex Hormones
3.7. Gender-Affirming Surgery
3.8. Rates of Persistence and Desistance
3.9. Rates of Comorbid Mental Health Concerns on Follow-Up
3.10. Educational/Occupational Outcomes
3.11. Sample Characteristics Viewed through the Lens of the Recent Swedish Guidelines
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, H.; Sasaki, S.; Bradley, S.J.; Singh, D.; Fantus, S.; Owen-Anderson, A.; Di Giacomo, A.; Bain, J.; Zucker, K.J. Patterns of referral to a gender identity service for children and adolescents (1976–2011): Age, sex ratio, and sexual orientation. J. Sex Marital Ther. 2013, 39, 1–6. [Google Scholar] [CrossRef]
- Zucker, K.J. Epidemiology of gender dysphoria and transgender identity. Sex Health 2017, 14, 404–411. [Google Scholar] [CrossRef]
- Zucker, K.J.; Wood, H.; Wasserman, L.; VanderLaan, D.P.; Aitken, M. Increasing Referrals for Gender Dysphoria. J. Adolesc. Health 2016, 58, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Kaltiala-Heino, R.; Bergman, H.; Tyolajarvi, M.; Frisen, L. Gender dysphoria in adolescence: Current perspectives. Adolesc. Health Med. Ther. 2018, 9, 31–41. [Google Scholar] [CrossRef]
- Zucker, K.J. Adolescents with Gender Dysphoria: Reflections on Some Contemporary Clinical and Research Issues. Arch. Sex. Behav. 2019, 48, 1983–1992. [Google Scholar] [CrossRef]
- Johns, M.M.; Lowry, R.; Andrzejewski, J.; Barrios, L.C.; Demissie, Z.; McManus, T.; Rasberry, C.N.; Robin, L.; Underwood, J.M. Transgender Identity and Experiences of Violence Victimization, Substance Use, Suicide Risk, and Sexual Risk Behaviors Among High School Students-19 States and Large Urban School Districts, 2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 67–71. [Google Scholar] [CrossRef]
- GIDS. Gender Identity Development Service, 2020. Referrals to GIDS, Financial Years 2015–2016 to 2019–2020. GIDS. Available online: http://gids.nhs.uk/number-referrals (accessed on 6 January 2023).
- Thompson, L.; Sarovic, D.; Wilson, P.; Sämfjord, A.; Gillberg, C. A PRISMA systematic review of adolescent gender dysphoria literature: 1) Epidemiology. PLoS Glob. Public Health 2022, 2, e0000245. [Google Scholar] [CrossRef]
- AmericanPsychiatricAssociation. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 4th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Kozlowska, K.; McClure, G.; Chudleigh, C.; Maguire, A.M.; Gessler, D.; Scher, S.; Ambler, G.R. Australian children and adolescents with gender dysphoria: Clinical presentations and challenges experienced by a multidisciplinary team and gender service. Hum. Syst. Ther. Cult. Attach. 2021, 1, 70–95. [Google Scholar] [CrossRef]
- Hembree, W.C.; Cohen-Kettenis, P.; Delemarre-van de Waal, H.A.; Gooren, L.J.; Meyer, W.J., 3rd; Spack, N.P.; Tangpricha, V.; Montori, V.M.; Endocrine, S. Endocrine treatment of transsexual persons: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2009, 94, 3132–3154. [Google Scholar] [CrossRef]
- WPATH. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People; World Professional Association for Transgender Health (WPATH): East Dundee, IL, USA, 2011; Available online: https://www.wpath.org (accessed on 20 December 2013).
- Gooren, L.; Delemarre-van de Waal, H. The feasibility of endocrine interventions in juvenile transsexuals. J. Psychol. Hum. Sex. 1996, 8, 69–74. [Google Scholar] [CrossRef]
- van der Loos, M.A.T.C.; Klink, D.T.; Hannema, S.E.; Bruinsma, S.; Steensma, T.D.; Kreukels, B.P.C.; Cohen-Kettenis, P.T.; de Vries, A.L.C.; den Heijer, M.; Wiepjes, C.M. Children and adolescents in the Amsterdam Cohort of Gender Dysphoria: Trends in diagnostic- and treatment trajectories during the first 20 years of the Dutch Protocol. J. Sex. Med. 2023, qdac029. [Google Scholar] [CrossRef]
- Hembree, W.C.; Cohen-Kettenis, P.T.; Gooren, L.; Hannema, S.E.; Meyer, W.J.; Murad, M.H.; Rosenthal, S.M.; Safer, J.D.; Tangpricha, V.; T'Sjoen, G.G. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. Endocr. Pract. 2017, 23, 1437. [Google Scholar] [CrossRef]
- Hembree, W.C.; Cohen-Kettenis, P.T.; Gooren, L.; Hannema, S.E.; Meyer, W.J.; Murad, M.H.; Rosenthal, S.M.; Safer, J.D.; Tangpricha, V.; T'Sjoen, G.G. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 3869–3903. [Google Scholar] [CrossRef]
- Nooteboom, L.A.; Mulder, E.A.; Vermeiren, R.; Eilander, J.; van den Driesschen, S.I.; Kuiper, C.H.Z. Practical Recommendations for Youth Care Professionals to Improve Evaluation and Reflection During Multidisciplinary Team Discussions: An Action Research Project. Int. J. Integr. Care 2022, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, S.; Dubois, V.; Cools, M. Impact of gender-affirming treatment on bone health in transgender and gender diverse youth. Endocr. Connect 2022, 11, e220280. [Google Scholar] [CrossRef] [PubMed]
- Perl, L.; Lee, J.Y.; Rosenthal, S.M. Chapter 7-Medical Side Effects of GnRH Agonists. In Pubertal Suppression in Transgender Youth; Finlayson, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–52. [Google Scholar]
- Schneider, M.A.; Spritzer, P.M.; Soll, B.M.B.; Fontanari, A.M.V.; Carneiro, M.; Tovar-Moll, F.; Costa, A.B.; da Silva, D.C.; Schwarz, K.; Anes, M.; et al. Brain Maturation, Cognition and Voice Pattern in a Gender Dysphoria Case under Pubertal Suppression. Front. Hum. Neurosci. 2017, 11, 528. [Google Scholar] [CrossRef]
- Chen, D.; Strang, J.F.; Kolbuck, V.D.; Rosenthal, S.M.; Wallen, K.; Waber, D.P.; Steinberg, L.; Sisk, C.L.; Ross, J.; Paus, T.; et al. Consensus Parameter: Research Methodologies to Evaluate Neurodevelopmental Effects of Pubertal Suppression in Transgender Youth. Transgender Health 2020, 5, 246–257. [Google Scholar] [CrossRef]
- Kozlowska, K.; Chudleigh, C.; McClure, G.; Maguire, A.M.; Ambler, G.R. Attachment Patterns in Children and Adolescents With Gender Dysphoria. Front. Psychol. 2020, 11, 582688. [Google Scholar] [CrossRef]
- Zucker, K.J.; Bradley, S.J.; Owen-Anderson, A.; Kibblewhite, S.J.; Cantor, J.M. Is gender identity disorder in adolescents coming out of the closet? J. Sex Marital Ther. 2008, 34, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.; Steensma, T.D.; Blanchard, R.; VanderLaan, D.P.; Wood, H.; Fuentes, A.; Spegg, C.; Wasserman, L.; Ames, M.; Fitzsimmons, C.L.; et al. Evidence for an altered sex ratio in clinic-referred adolescents with gender dysphoria. J. Sex. Med. 2015, 12, 756–763. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, N.M.; Carmichael, P.; Steensma, T.D.; Zucker, K.J. Evidence for a Change in the Sex Ratio of Children Referred for Gender Dysphoria: Data From the Gender Identity Development Service in London (2000–2017). J. Sex. Med. 2018, 15, 1381–1383. [Google Scholar] [CrossRef]
- de Graaf, N.M.; Giovanardi, G.; Zitz, C.; Carmichael, P. Sex Ratio in Children and Adolescents Referred to the Gender Identity Development Service in the UK (2009–2016). Arch. Sex. Behav. 2018, 47, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Steensma, T.D.; Cohen-Kettenis, P.T.; Zucker, K.J. Evidence for a Change in the Sex Ratio of Children Referred for Gender Dysphoria: Data from the Center of Expertise on Gender Dysphoria in Amsterdam (1988–2016). J. Sex Marital Ther. 2018, 44, 713–715. [Google Scholar] [CrossRef] [PubMed]
- The_National_Board_of_Health_and_Welfare_(Sweden). The Evolution of the Diagnosis of Gender Dysphoria-Prevalence, Concurrent Psychiatric Diagnoses and Mortality in Suicide. 2020. Available online: https://www.socialstyrelsen.se/globalassets/sharepointdokument/artikelkatalog/ovrigt/2020-2-6600.pdf. (accessed on 20 December 2022).
- Kaltiala-Heino, R.; Sumia, M.; Tyolajarvi, M.; Lindberg, N. Two years of gender identity service for minors: Overrepresentation of natal girls with severe problems in adolescent development. Child Adolesc. Psychiatry Ment. Health 2015, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaltiala, R.; Heino, E.; Tyolajarvi, M.; Suomalainen, L. Adolescent development and psychosocial functioning after starting cross-sex hormones for gender dysphoria. Nord. J. Psychiatry 2020, 74, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Council for Choices in Health Care in Finland_(COHERE Finland) Medical Treatment Methods for Dysphoria Associated with Variations in Gender Identity in Minors–Recommendation (Sukupuolidysforia Alaikäiset—Palveluvalikoima) 2020. Available online: http://www.palveluvalikoima.fi/ (accessed on 16 December 2022).
- Socialstyrensen (The Natioanal Board of Health and Welfare [Sweden]) Uppdaterat Kunskapsstöd för Vård vid Könsdysfori hos Unga (Care of Children and Young People with Gender Dysphoria. National Knowledge Support with Recommendations to the Profession and Decision Makers. 2022. Available online: https://www.socialstyrelsen.se/om-socialstyrelsen/pressrum/press/uppdaterat-kunskapsstod-for-vard-vid-konsdysfori-hos-unga/ (accessed on 6 January 2023).
- NHS_England. Implementing Advice from the Cass Review. Improving and Expanding Services for Children and Young People Experiencing Gender Incongruence and Gender dysphoria. Available online: https://www.england.nhs.uk/commissioning/spec-services/npc-crg/gender-dysphoria-clinical-programme/implementing-advice-from-the-cass-review/ (accessed on 16 December 2022).
- Cass, H. Independent Review of Gender Identity Services for Children and Young People (Cass Review, Interim Report). Available online: https://cass.independent-review.uk/ (accessed on 16 December 2022).
- Dyer, C. NHS gender identity service to close and be replaced by regional centres. BMJ 2022, 378, o1916. [Google Scholar] [CrossRef]
- Hayward, E.; Bannerman, L. Tavistock Gender Clinic Forced to Shut Over Safety Fears. Available online: https://archive.ph/UTXAW (accessed on 28 July 2022).
- Telfer, M.M.; Tollit, M.A.; Pace, C.C.; Pang, K.C. Australian standards of care and treatment guidelines for transgender and gender diverse children and adolescents. Med. J. Aust. 2018, 209, 132–136. [Google Scholar] [CrossRef]
- Telfer, M.M.; Tollit, M.A.; Pace, C.C.; Pang, K.C. Australian Standards of Care and Treatment Guidelines for Trans and Gender Diverse Children and Adolescents, Version 1.3; Royal Children’s Hospital: Melbourne, Australia, 2020; Available online: https://www.rch.org.au/uploadedFiles/Main/Content/adolescent-medicine/australian-standards-of-care-and-treatment-guidelines-for-trans-and-gender-diverse-children-and-adolescents.pdf (accessed on 3 February 2023).
- Hidalgo, M.A.; Ehrensaft, D.; Tishelman, A.C.; Clark, L.F.; Garofalo, R.; Rosenthal, S.M.; Spack, N.P.; Olson, J. The Gender Affirmative Model: What We Know and What We Aim to Learn. Hum. Dev. 2013, 56, 285–290. [Google Scholar] [CrossRef]
- Keo-Meier, C.; Ehrensaft, D. Introduction to the Gender Affirmative Model. In The Gender Affirmative Model: An Interdisciplinary Approach to Supporting Transgender and Gender Expansive Children; Keo-Meier, C., Ehrensaft, D., Eds.; American Psychological Association: Washington, DC, USA, 2018; pp. 3–19. [Google Scholar]
- New South Wales Government. NSW LGBTIQ+ Health Strategy 2022-2027. Summary of Evidence; New South Wales Government: Sydney, Australia, 2022.
- New South Wales Government. NSW LGBTIQ+ Health Strategy 2022-2027. For People of Diverse Sexualities and Genders, and Intersex People, to Achieve Health Outcomes That Matter to Them; New South Wales Government: Sydney, Australia, 2022.
- Coleman, E.; Radix, A.E.; Bouman, W.P.; Brown, G.R.; de Vries, A.L.C.; Deutsch, M.B.; Ettner, R.; Fraser, L.; Goodman, M.; Green, J.; et al. Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. Int. J. Transgend Health 2022, 23, S1–S259. [Google Scholar] [CrossRef]
- Endocrine Society. Transgender Health. Position Statement. Available online: https://www.endocrine.org/-/media/endocrine/files/advocacy/position-statement/position_statement_transgender_health_pes.pdf (accessed on 20 December 2022).
- Brignardello-Petersen, R.; Wierchioch, W. Effects of Gender Affirming Therapies in People with Gender Dysphoria: Evaluation of Best Availble Evidence (Main Document); Florida Agency for Helath Care Administration (Access Currently Blocked): Tallahassee, FL, USA, 2022. [Google Scholar]
- Florida Department of Health. Treatment of Gender Dysphoria for Children and Adolescents; Florida Department of Health: Tallahassee, FL, USA, 2022. Available online: https://www.floridahealth.gov/_documents/newsroom/press-releases/2022/04/20220420-gender-dysphoria-guidance.pdf (accessed on 23 December 2022).
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Ramos, G.G.F.; Mengai, A.C.S.; Daltro, C.A.T.; Cutrim, P.T.; Zlotnik, E.; Beck, A.P.A. Systematic Review: Puberty suppression with GnRH analogues in adolescents with gender incongruity. J. Endocrinol. Investig. 2021, 44, 1151–1158. [Google Scholar] [CrossRef]
- Rew, L.; Young, C.C.; Monge, M.; Bogucka, R. Review: Puberty blockers for transgender and gender diverse youth-a critical review of the literature. Child Adolesc. Ment. Health 2021, 26, 3–14. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Evidence Review: Gonadotrophin Releasing Hormone Analogues for Children and Adolescents with Gender Dysphoria; National Institute for Health and Care Excellence (NICE): London, UK, 2022; Available online: https://cass.independent-review.uk/wp-content/uploads/2022/09/20220726_Evidence-review_GnRH-analogues_For-upload_Final.pdf (accessed on 20 December 2022).
- Gean, E. Topic Brief: Treatments for Gender Dysphoria in Transgender Youth. In This Report Was Developed by the Scientific Resource Center under Contract to the Agency for Healthcare Research and Quality (AHRQ); Agency for Healthcare Research and Quality (AHRQ): Rockville, MA, USA, 2021. Available online: https://effectivehealthcare.ahrq.gov/system/files/docs/topic-brief-gender-dysphoria.pdf (accessed on 20 December 2022).
- Levine, S.B.; Abbruzzese, E.; Mason, J.W. Reconsidering Informed Consent for Trans-Identified Children, Adolescents, and Young Adults. J. Sex Marital Ther. 2022, 48, 706–727. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.B.; Abbruzzese, E.; Mason, J.W. What Are We Doing to These Children? Response to Drescher, Clayton, and Balon Commentaries on Levine et al., 2022. J. Sex Marital. Ther. 2022, 49, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, E.; Levine, S.B.; Mason, J.W. The Myth of “Reliable Research” in Pediatric Gender Medicine: A critical evaluation of the Dutch Studies-and research that has followed. J. Sex Marital. Ther. 2023, 1–27. [Google Scholar] [CrossRef]
- Nahata, L.; Quinn, G.P. ‘Harm threshold’: Capacity for decision-making may be reduced by long-term pubertal suppression. J. Med. Ethics 2020, 46, 759–760. [Google Scholar] [CrossRef]
- d’Abrera, J.C.; D’Angelo, R.; Halasz, G.; Prager, S.; Morris, P. Informed consent and childhood gender dysphoria: Emerging complexities in diagnosis and treatment. Australas. Psychiatry Bull. R. Aust. N. Z. Coll. Psychiatr. 2020, 28, 536–538. [Google Scholar] [CrossRef]
- Latham, A. Puberty Blockers for Children: Can They Consent? New Bioeth 2022, 28, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Pazos Guerra, M.; Gomez Balaguer, M.; Gomes Porras, M.; Hurtado Murillo, F.; Sola Izquierdo, E.; Morillas Arino, C. Transsexuality: Transitions, detransitions, and regrets in Spain. Endocrinol. Diabetes Y Nutrición. 2020, 67, 562–567. [Google Scholar] [CrossRef]
- Bell, K. A Detransitioner’s Viewpoint. Available online: https://www.google.co.uk/amp/s/safeschoolsallianceuk.net/2020/05/12/a-de-transitioners-viewpoint-keira-bell/amp/ (accessed on 1 July 2020).
- Cohen, C. What I wish I’d known when I was 19 and had sex reassignment surgery. Washington Post, 11 April 2022. [Google Scholar]
- Littman, L. Individuals Treated for Gender Dysphoria with Medical and/or Surgical Transition Who Subsequently Detransitioned: A Survey of 100 Detransitioners. Arch. Sex. Behav. 2021, 50, 3353–3369. [Google Scholar] [CrossRef]
- Vandenbussche, E. Detransition-Related Needs and Support: A Cross-Sectional Online Survey. J. Homosex. 2022, 69, 1602–1620. [Google Scholar] [CrossRef]
- Helena. By Any Other Name. The Story of My Transition and Detransition. Available online: https://protect-au.mimecast.com/s/W1z2CzvkyVCMl9VZYT4RH5S?domain=lacroicsz.substack.com (accessed on 1 March 2022).
- Lahl, J. The Detransition Diaries: Saving Our Sisters Film; The Center for Bioethics and Culture Network: Pleasant Hill, CA, USA, 2022. [Google Scholar]
- O’Malley, S.; Ayad, S. Detransitioned & Damaged by the Dutch Protocol: Teiresias. Apple Poscasts. Episode 99. 2022. Available online: https://podcasts.apple.com/us/podcast/gender-a-wider-lens-podcast/id1542655295?i=1000589562973 (accessed on 6 January 2023).
- Boyd, I.L.; Hackett, T.; Bewley, S. Care of Transgender Patients: A General Practice Quality Improvement Approach. Healthcare 2022, 10, 121. [Google Scholar] [CrossRef]
- Clayton, A. The Gender Affirmative Treatment Model for Youth with Gender Dysphoria: A Medical Advance or Dangerous Medicine? Arch. Sex. Behav. 2022, 51, 691–698. [Google Scholar] [CrossRef]
- Balon, R. Commentary on Levine et al: Festina Lente (Rush Slowly). J. Sex Marital Ther. 2022, 48, 775–778. [Google Scholar] [CrossRef]
- Clayton, A. Commentary on Levine et al.: A Tale of Two Informed Consent Processes. J. Sex Marital Ther. 2023, 49, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Drescher, J. Informed Consent or Scare Tactics? A Response to Levine et al.’s “Reconsidering Informed Consent for Trans-Identified Children, Adolescents, and Young Adults”. J. Sex Marital Ther. 2023, 49, 99–107. [Google Scholar] [CrossRef]
- de Vries, A.L.C. Ensuring Care for Transgender Adolescents Who Need It: Response to 'Reconsidering Informed Consent for Trans-Identified Children, Adolescents and Young Adults'. J. Sex Marital Ther. 2023, 49, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ouliaris, C. Consent for treatment of gender dysphoria in minors: Evolving clinical and legal frameworks. Med. J. Aust. 2022, 216, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics (November 2022), Labour Force, Australia, ABS Website. 2022. Available online: https://www.abs.gov.au/statistics/labour/employment-and-unemployment/labour-force-australia/latest-release (accessed on 12 January 2023).
- Biggs, M. The Dutch Protocol for Juvenile Transsexuals: Origins and Evidence. J. Sex Marital Ther. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kuitenbrouwer, J.; Vasterman, P. Ook transzorg moet aan medisch-wetenschappelijke standaarden voldoen (Transcare must also meet medical-scientific standards). NRC 2022. Available online: https://archive.vn/fFLBL (accessed on 6 January 2023).
- Zucker, K.J. Debate: Different strokes for different folks. Child Adolesc. Ment. Health 2020, 25, 36–37. [Google Scholar] [CrossRef]
- Brik, T.; Vrouenraets, L.; de Vries, M.C.; Hannema, S.E. Trajectories of Adolescents Treated with Gonadotropin-Releasing Hormone Analogues for Gender Dysphoria. Arch. Sex. Behav. 2020, 49, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Butler, G.; Carruthers, P.; Carmichael, P. Trajectories of transgender adolescents referred for endocrine intervention in England. Arch. Dis. Child. 2022, 107, 1012–1017. [Google Scholar] [CrossRef]
- McCallion, S.; Smith, S.; Kyle, H.; Shaikh, M.G.; Wilkinson, G.; Kyriakou, A. An appraisal of current service delivery and future models of care for young people with gender dysphoria. Eur. J. Pediatr. 2021, 180, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.L.; Steensma, T.D.; Doreleijers, T.A.; Cohen-Kettenis, P.T. Puberty suppression in adolescents with gender identity disorder: A prospective follow-up study. J. Sex. Med. 2011, 8, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kettenis, P.T.; Steensma, T.D.; de Vries, A.L. Treatment of adolescents with gender dysphoria in the Netherlands. Child Adolesc. Psychiatr. Clin. N. Am. 2011, 20, 689–700. [Google Scholar] [CrossRef]
- de Vries, A.L.; McGuire, J.K.; Steensma, T.D.; Wagenaar, E.C.; Doreleijers, T.A.; Cohen-Kettenis, P.T. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics 2014, 134, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Dunsford, M.; Skagerberg, E.; Holt, V.; Carmichael, P.; Colizzi, M. Psychological Support, Puberty Suppression, and Psychosocial Functioning in Adolescents with Gender Dysphoria. J. Sex. Med. 2015, 12, 2206–2214. [Google Scholar] [CrossRef]
- Costa, R.; Colizzi, M. The effect of cross-sex hormonal treatment on gender dysphoria individuals' mental health: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- AusPATH. AusPATH Statement about the Interim Service Specification for the Specialist Service for Children and Young People with Gender Dysphoria (Phase 1 Providers) by NHS England. 2022. Available online: https://auspath.org.au/2022/11/16/auspath-statement-about-the-interim-service-specification-for-the-specialist-service-for-children-and-young-people-with-gender-dysphoria-phase-1-providers-by-nhs-england/ (accessed on 6 January 2023).
- de Vries, A.L.; Noens, I.L.; Cohen-Kettenis, P.T.; van Berckelaer-Onnes, I.A.; Doreleijers, T.A. Autism spectrum disorders in gender dysphoric children and adolescents. J. Autism Dev. Disord. 2010, 40, 930–936. [Google Scholar] [CrossRef]
- Warrier, V.; Greenberg, D.M.; Weir, E.; Buckingham, C.; Smith, P.; Lai, M.C.; Allison, C.; Baron-Cohen, S. Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals. Nat. Commun. 2020, 11, 3959. [Google Scholar] [CrossRef]
- Reisner, S.L.; Vetters, R.; Leclerc, M.; Zaslow, S.; Wolfrum, S.; Shumer, D.; Mimiaga, M.J. Mental health of transgender youth in care at an adolescent urban community health center: A matched retrospective cohort study. J. Adolesc. Health 2015, 56, 274–279. [Google Scholar] [CrossRef]
- Strauss, P.; Cook, A.; Winter, S.; Watson, V.; Wright Toussaint, D.; Lin, A. Mental Health Issues and Complex Experiences of Abuse Among Trans and Gender Diverse Young People: Findings from Trans Pathways. LGBT Health 2020, 7, 128–136. [Google Scholar] [CrossRef]
- Thompson, L.; Sarovic, D.; Wilson, P.; Sämfjord, A.; Gillberg, C. A PRISMA systematic review of adolescent gender dysphoria literature: 2) mental health. PLoS Glob. Public Health 2022, 2, e0000426. [Google Scholar] [CrossRef]
- Herrmann, L.; Bindt, C.; Schweizer, K.; Micheel, J.; Nieder, T.O.; Haass, J.; Schoettle, D.; Becker-Hebly, I. Autism Spectrum Disorders and Gender Dysphoria Among Children and Adolescents: Systematic Review on the Co-Occurrence. Psychiatr. Prax. 2020, 47, 300–307. [Google Scholar] [CrossRef]
- Glidden, D.; Bouman, W.P.; Jones, B.A.; Arcelus, J. Gender Dysphoria and Autism Spectrum Disorder: A Systematic Review of the Literature. Sex. Med. Rev. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Caldarera, A.; Brustia, P.; Gerino, E.; Dimitrios, L.; Rollè, L. Co-occurrence of gender dysphoria and eating disorders: A systematic review of the literature. Eur. Psychiatry 2016, 33, S188–S189. [Google Scholar] [CrossRef]
- Kallitsounaki, A.; Williams, D.M. Autism Spectrum Disorder and Gender Dysphoria/Incongruence. A systematic Literature Review and Meta-Analysis. J. Autism Dev. Disord. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gosling, M. Gender-Questioning Teenagers: Puberty Blockers and Hormone Treatment vs Placebo. Sex Matters (Not-for-Profit Company Registered by Guarantee). 2022. Available online: https://sex-matters.org/wp-content/uploads/2022/12/Teenagers-medication-vs-placebo.pdf (accessed on 6 January 2023).
- Cohen-Kettenis, P.T.; Schagen, S.E.; Steensma, T.D.; de Vries, A.L.; Delemarre-van de Waal, H.A. Puberty suppression in a gender-dysphoric adolescent: A 22-year follow-up. Arch. Sex. Behav. 2011, 40, 843–847. [Google Scholar] [CrossRef] [Green Version]
| Subset of Young People with Low Bone Density Prior to Commencement of Puberty Blockers | |||
| Participant | Low baseline bone density (low prior to commencement of puberty blockers) | Further decrease in bone density following puberty suppression (low baseline bone density) | Decrease in bone density following puberty suppression (normal baseline bone density) |
| Case 1 | Yes | Yes | -- |
| Case 2 | Yes | Yes | -- |
| Case 4 | Yes | Yes | -- |
| Case 5 | Yes | Yes (small deterioration only) | |
| Subset of Young People with Normal Bone Density Prior to Commencement of Puberty Blockers | |||
| Case 3 | No | -- | Yes |
| Case 6 | No | -- | Yes |
| Case 7 | No | -- | Yes |
| Biological Sex (♂/♀) | Age at Which the Medical Pathway Was Declined | Whilst Considering Puberty Suppression | During Puberty Suppression (Duration of Treatment) | During Cross-Sex Hormone Treatment (Duration of Treatment) | Stated Gender Identity at Time of Declining Medical Pathway |
|---|---|---|---|---|---|
| ♀ | 12 years | √ | Cisgender | ||
| ♀ | 13 years | √ (1.83 years of PS) | Cisgender | ||
| ♀ | 13 years | √ (1.08 years of PS) | Gender neutral | ||
| ♀ | 15 years | √ (1.5 years of PS) | Gender neutral | ||
| ♀ | 16 years | √ (2.33 years of PS and 4 months of CSH) | Transgender with social transition only * | ||
| ♂ | 17 years | √ (4.75 years of PS and 8 months of CSH) | Non-binary | ||
| ♀ | 18 years | √ (3.00 years of CSH) | Not known |
| Number (%) on Clinical Assessment in December 2013–November 2018 (Total n = 79) | Number (%) on Follow-Up (Reported Mental Health Concerns) in November/December 2022 (Total n = 50) | |
|---|---|---|
| Comorbid MH diagnosis | 70 (88.6%) | 44 (88.0%) |
| No MH diagnosis | 9 (11.4%) | 7 (14.0%) |
| Anxiety | 50 (63.3%) | 22 (44%) |
| Depression | 49 (62.0%) | 25 (50%) |
| Any behavioural disorder (including ADHD, ODD) | 28 (35.4%) | 11 (22.0%) |
| Autism * | 11 (13.9%) | 15 (30%) |
| Learning difficulties ** | 8 (11.9%) | 1 (2%) |
| Eating disorder | 2 (2.5%) | 2 (4%) |
| Psychosis | 1 (1.3%) | 0 (0%) |
| Substance abuse | -- | 1 (2%) |
| Intellectual disability | -- | 1 (2%) |
| Chronic fatigue syndrome | -- | 1 (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkadi, J.; Chudleigh, C.; Maguire, A.M.; Ambler, G.R.; Scher, S.; Kozlowska, K. Developmental Pathway Choices of Young People Presenting to a Gender Service with Gender Distress: A Prospective Follow-Up Study. Children 2023, 10, 314. https://doi.org/10.3390/children10020314
Elkadi J, Chudleigh C, Maguire AM, Ambler GR, Scher S, Kozlowska K. Developmental Pathway Choices of Young People Presenting to a Gender Service with Gender Distress: A Prospective Follow-Up Study. Children. 2023; 10(2):314. https://doi.org/10.3390/children10020314
Chicago/Turabian StyleElkadi, Joseph, Catherine Chudleigh, Ann M. Maguire, Geoffrey R. Ambler, Stephen Scher, and Kasia Kozlowska. 2023. "Developmental Pathway Choices of Young People Presenting to a Gender Service with Gender Distress: A Prospective Follow-Up Study" Children 10, no. 2: 314. https://doi.org/10.3390/children10020314
APA StyleElkadi, J., Chudleigh, C., Maguire, A. M., Ambler, G. R., Scher, S., & Kozlowska, K. (2023). Developmental Pathway Choices of Young People Presenting to a Gender Service with Gender Distress: A Prospective Follow-Up Study. Children, 10(2), 314. https://doi.org/10.3390/children10020314






