Identification of Biomarkers Related to Metabolically Unhealthy Obesity in Korean Obese Adolescents: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anthropometric Parameters
2.3. Biochemical Analysis
2.4. MetS Diagnosis
- WC ≥ 90th percentile or adult cut-off if lower
- SBP of 130 mmHg or DBP of 85 mmHg or treatment with anti-hypertensive medication
- TG ≥ 150 mg/dL
- HDL-cholesterol < 40 mg/dL
- Fasting plasma glucose ≥ 100 mg/dL or known type 2 (T2) DM
2.5. Assessment of Nutrition Intake
2.6. Metabolite Measurements
2.7. The Calculation of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Index and Triglyceride-Glucose (TyG) Index
2.8. Data Processing and Statistical Analysis
3. Results
3.1. Participants
3.2. Anthropometric Parameters, Characteristics, and Laboratory Measurements of MUO and MHO in Adolescents
3.3. Plasma Lipid Profiles of MUO and MHO in Adolescents
3.4. IR Assessment Index of MUO and MHO in Adolescents
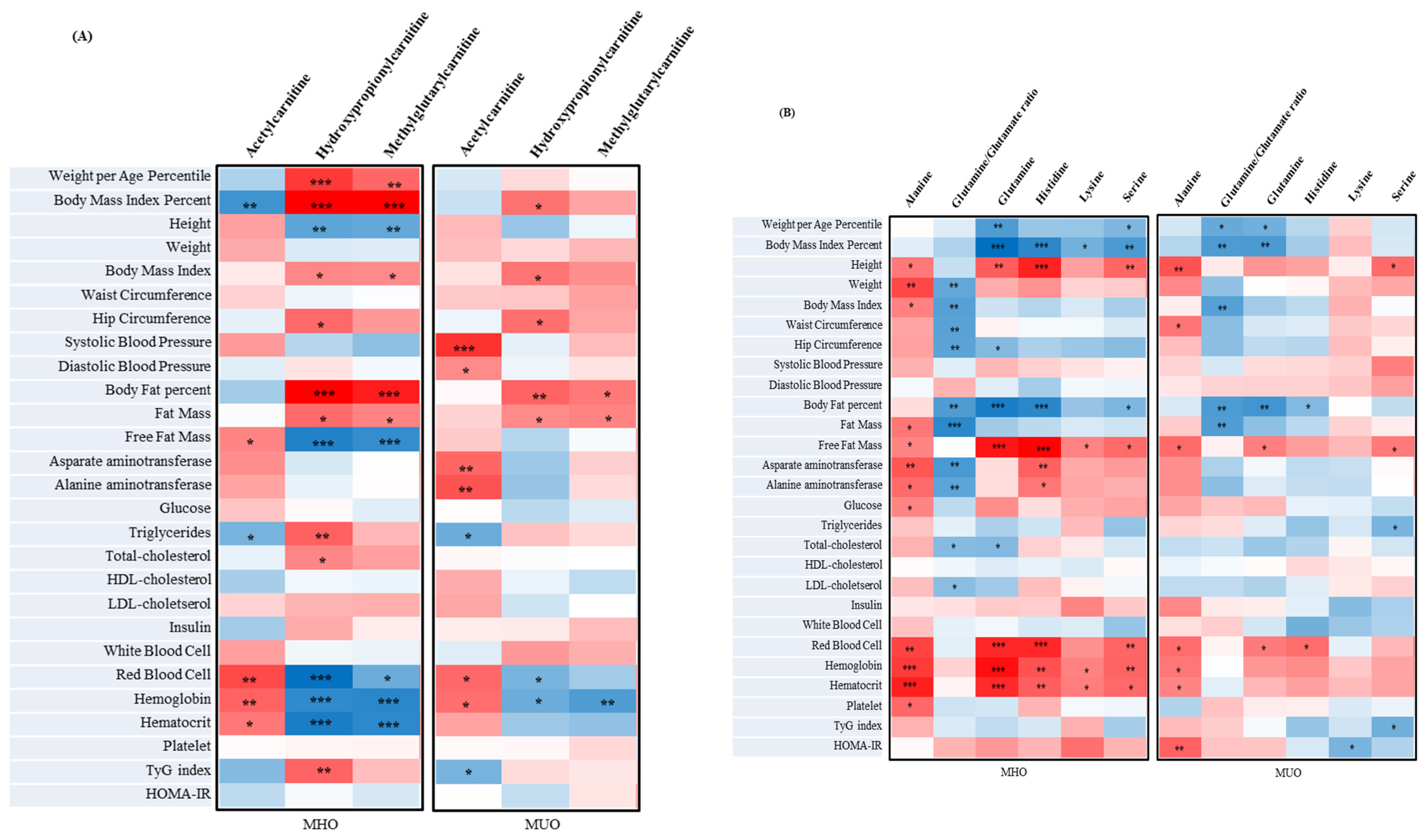
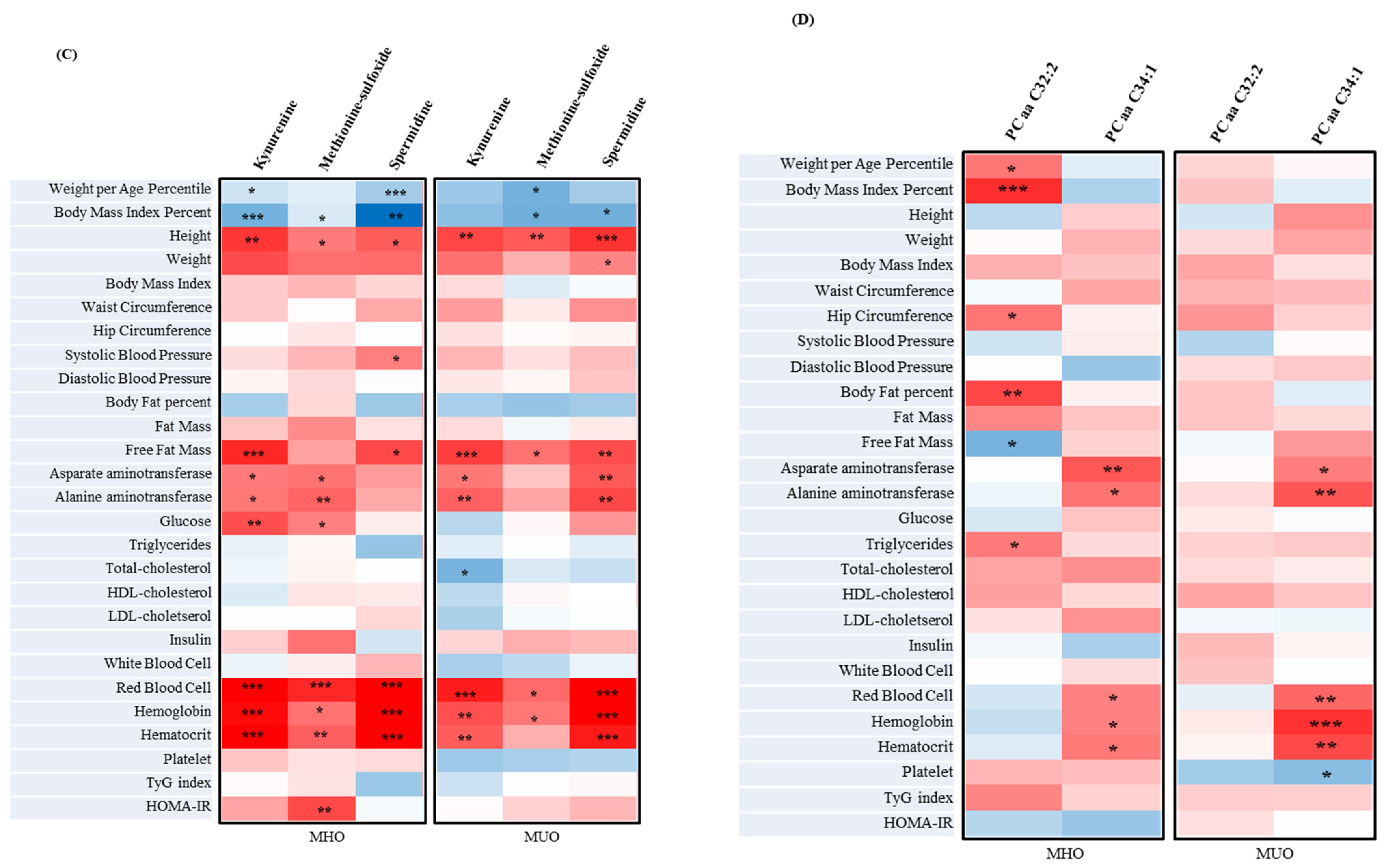
3.5. The Metabolites of MUO and MHO in Adolescents
3.6. Predictors of the MUO Adolescents’ Prevalence ORs on Significantly Different Metabolites
3.7. The Nutrition Intakes of MUO and MHO in Adolescents
3.8. The Correlation between Metabolites Associated with Clinical Parameters and IR Assessment Index of MUO and MHO in Adolescents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AA | Amino acid |
| AC | Acylcarnitine |
| Ala | Alanine |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BA | Biogenic amine |
| BMR | Basal Metabolic Rate |
| BF% | Body fat percentage |
| BMI | Body mass index |
| C2 | Acetylcarnitine |
| C3-OH | Hydroxy propionyl carnitine |
| C5-M-DC | Methyl glutaryl carnitine |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| FFM | Free fat mass |
| Gln | Glutamine |
| Glu | Glutamate |
| GPL | Glycerophospholipid |
| HC | Hip circumference |
| Hct | Hematocrit |
| HDL-cholesterol | High-density lipoprotein cholesterol |
| Hb | Hemoglobin |
| His | Histidine |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| HTN | Hypertension |
| IDF | International Diabetes Foundation |
| IR | Insulin resistance |
| IRB | Institutional Review Board |
| KNIH | Korea National Institute of Health |
| LDL-cholesterol | Low-density lipoprotein cholesterol |
| Lys | Lysine |
| MetS | Metabolic syndrome |
| Met-SO | Methionine-sulfoxide |
| MHO | Metabolically healthy obesity |
| MUO | Metabolically unhealthy obesity |
| OR | Odds ratio |
| PCaa | Phosphatidylcholine diacyl |
| Plt | Platelet |
| RBC | Red blood cell |
| SAS | Statistical Analysis System |
| SBP | Systolic blood pressure |
| SE | Standard errors |
| Ser | Serine |
| SPL | Sphingolipid |
| T2DM | Type 2 diabetes mellitus |
| T-cholesterol | Total cholesterol |
| TG | Triglyceride |
| TyG | Triglycerid-glucose |
| WC | Waist circumference |
| weightth | Weight per age percentile |
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Division of Health and Nutrition Survey; Center for Disease Prevention; KCDC. The 2017 Korean National Growth Charts for Children and Adolescents; KCDC: Chungcheongbuk-do, Korea, 2017; pp. 1–78. [Google Scholar]
- Nikolopoulou, A.; Kadoglou, N.P. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2012, 10, 933–939. [Google Scholar] [CrossRef]
- Guasch, A.; Bulló, M.; Rabassa, A.; Bonada, A.; Del Castillo, D.; Sabench, F.; Salas-Salvadó, J. Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: A cross-sectional study. Cardiovasc. Diabetol. 2012, 11, 149. [Google Scholar]
- Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M.P.; Haffner, S.M. The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care 2003, 26, 3153–3159. [Google Scholar]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann. Hepatol. 2020, 19, 359–366. [Google Scholar]
- American college of obstetricians and gynecologists. ACOG committee opinion No. 714: Obesity in adolescents. Obstet. Gynecol. 2017, 130, e127–e140. [Google Scholar]
- Oh, A.C.Y.; Lee, D. 2013–2017 Obesity Fact Sheet; Korea Health Promotion Institute: Seoul, Republic of Korea, 2018; pp. 1–53. [Google Scholar]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 1–24. [Google Scholar] [CrossRef]
- Kaur, J. Assessment and screening of the risk factors in metabolic syndrome. Med. Sci. 2014, 2, 140–152. [Google Scholar]
- Socha, P.; Hellmuth, C.; Gruszfeld, D.; Demmelmair, H.; Rzehak, P.; Grote, V.; Weber, M.; Escribano, J.; Closa-Monasterolo, R.; Dain, E. Endocrine and metabolic biomarkers predicting early childhood obesity risk. Prev. Asp. Early Nutr. 2016, 85, 81–88. [Google Scholar]
- de Rocha, A.R.F.; de Morais, N.S.; Priore, S.E.; do CC Franceschini, S. Inflammatory Biomarkers and Components of Metabolic Syndrome in Adolescents: A Systematic Review. Inflammation 2021, 45, 14–30. [Google Scholar]
- Balagopal, P.; De Ferranti, S.D.; Cook, S.; Daniels, S.R.; Gidding, S.S.; Hayman, L.L.; McCrindle, B.W.; Mietus-Snyder, M.L.; Steinberger, J. Nontraditional risk factors and biomarkers for cardiovascular disease: Mechanistic, research, and clinical considerations for youth: A scientific statement from the American Heart Association. Circulation 2011, 123, 2749–2769. [Google Scholar]
- Silverstein, J.; Klingensmith, G.; Copeland, K.; Plotnick, L.; Kaufman, F.; Laffel, L.; Deeb, L.; Grey, M.; Anderson, B.; Holzmeister, L.A. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care 2005, 28, 186–212. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Fantauzzi, C.B.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta 2012, 750, 82–97. [Google Scholar]
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397. [Google Scholar] [CrossRef]
- Valainathan, S.; Boukris, A.; Arapis, K.; Schoch, N.; Goujon, G.; Konstantinou, D.; Bécheur, H.; Pelletier, A. Energy expenditure in acute pancreatitis evaluated by the Harris–Benedict equation compared with indirect calorimetry. Clin. Nutr. ESPEN 2019, 33, 57–59. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar]
- Nor, N.S.M.; Lee, S.; Bacha, F.; Tfayli, H.; Arslanian, S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: Comparison with the hyperinsulinemic–euglycemic clamp. Pediatr. Diabetes 2016, 17, 458–465. [Google Scholar]
- Sagun, G.; Oguz, A.; Karagoz, E.; Filizer, A.T.; Tamer, G.; Mesci, B. Application of alternative anthropometric measurements to predict metabolic syndrome. Clinics 2014, 69, 347–353. [Google Scholar]
- Ong, J.P.; Elariny, H.; Collantes, R.; Younoszai, A.; Chandhoke, V.; Reines, H.D.; Goodman, Z.; Younossi, Z.M. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes. Surg. 2005, 15, 310–315. [Google Scholar]
- Kunutsor, S.K.; Bakker, S.J.; Kootstra-Ros, J.E.; Blokzijl, H.; Gansevoort, R.T.; Dullaart, R.P. Inverse linear associations between liver aminotransferases and incident cardiovascular disease risk: The PREVEND study. Atherosclerosis 2015, 243, 138–147. [Google Scholar] [CrossRef]
- Watanabe, M.; Suliman, M.E.; Qureshi, A.R.; Garcia-Lopez, E.; Bárány, P.; Heimbürger, O.; Stenvinkel, P.; Lindholm, B. Consequences of low plasma histidine in chronic kidney disease patients: Associations with inflammation, oxidative stress, and mortality. Am. J. Clin. Nutr. 2008, 87, 1860–1866. [Google Scholar] [CrossRef]
- Feng, R.N.; Niu, Y.C.; Sun, X.W.; Li, Q.; Zhao, C.; Wang, C.; Guo, F.C.; Sun, C.H.; Li, Y. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: A randomised controlled trial. Diabetologia 2013, 56, 985–994. [Google Scholar]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernaández-Gonzaález, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar]
- Navarro-González, D.; Sánchez-Íñigo, L.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martinez, J.A. TyG index change is more determinant for forecasting type 2 diabetes onset than weight gain. Medicine 2016, 95, e3646. [Google Scholar]
- Müllner, E.; Röhnisch, H.E.; Von Brömssen, C.; Moazzami, A.A. Metabolomics analysis reveals altered metabolites in lean compared with obese adolescents and additional metabolic shifts associated with hyperinsulinaemia and insulin resistance in obese adolescents: A cross-sectional study. Metabolomics 2021, 17, 1–13. [Google Scholar]
- Cao, B.; Wang, D.; Pan, Z.; Brietzke, E.; McIntyre, R.S.; Musial, N.; Mansur, R.B.; Subramanieapillai, M.; Zeng, J.; Huang, N.; et al. Characterizing acyl-carnitine biosignatures for schizophrenia: A longitudinal pre-and post-treatment study. Transl. Psychiatry 2019, 9, 1–13. [Google Scholar]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; Delany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef]
- Mey, J.T.; Hari, A.; Axelrod, C.L.; Fealy, C.E.; Erickson, M.L.; Kirwan, J.P.; Dweik, R.A.; Heresi, G.A. Lipids and ketones dominate metabolism at the expense of glucose control in pulmonary arterial hypertension: A hyperglycaemic clamp and metabolomics study. Eur. Respir. J. 2020, 55, 1901700. [Google Scholar]
- Devi, S.; Nongkhlaw, B.; Limesh, M.; Pasanna, R.M.; Thomas, T.; Kuriyan, R.; Kurpad, A.V.; Mukhopadhyay, A. Acyl ethanolamides in Diabetes and Diabetic Nephropathy: Novel targets from untargeted plasma metabolomic profiles of South Asian Indian men. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Ismaeel, A.; Franco, M.E.; Lavado, R.; Papoutsi, E.; Casale, G.P.; Fuglestad, M.; Mietus, C.J.; Haynatzki, G.R.; Smith, R.S.; Bohannon, W.T.; et al. Altered metabolomic profile in patients with peripheral artery disease. J. Clin. Med. 2019, 8, 1463. [Google Scholar] [CrossRef]
- Altmaier, E.; Ramsay, S.L.; Graber, A.; Mewes, H.-W.; Weinberger, K.M.; Suhre, K. Bioinformatics analysis of targeted metabolomics—Uncovering old and new tales of diabetic mice under medication. Endocrinology 2008, 149, 3478–3489. [Google Scholar]
- Haase, D.; Bäz, L.; Bekfani, T.; Neugebauer, S.; Kiehntopf, M.; Möbius-Winkler, S.; Franz, M.; Schulze, P.C. Metabolomic profiling of patients with high gradient aortic stenosis undergoing transcatheter aortic valve replacement. Clin. Res. Cardiol. 2021, 110, 399–410. [Google Scholar]
- Bene, J.; Márton, M.; Mohás, M.; Bagosi, Z.; Bujtor, Z.; Oroszlán, T.; Gasztonyi, B.; Wittmann, I.; Melegh, B. Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann. Nutr. Metab. 2013, 62, 80–85. [Google Scholar]
- Ma, Y.; Sun, L.; Li, J.; Hu, Y.; Gan, Z.; Zong, G.; Zheng, H.; Jin, Q.; Li, H.; Hu, F.B. Erythrocyte PUFAs, circulating acylcarnitines, and metabolic syndrome risk: A prospective study in Chinese. J. Lipid Res. 2019, 60, 421–429. [Google Scholar]
- Lee, A.; Jang, H.B.; Ra, M.; Choi, Y.; Lee, H.-J.; Park, J.Y.; Kang, J.H.; Park, K.-H.; Park, S.I.; Song, J. Prediction of future risk of insulin resistance and metabolic syndrome based on Korean boy’s metabolite profiling. Obes. Res. Clin. Pract. 2015, 9, 336–345. [Google Scholar] [CrossRef]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Roberts-Thomson, K.M.; Russell, R.D. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Holm, L.J.; Buschard, K. L-serine: A neglected amino acid with a potential therapeutic role in diabetes. APMIS 2019, 127, 655–659. [Google Scholar] [CrossRef]
- Holm, L.J.; Krogvold, L.; Hasselby, J.P.; Kaur, S.; Claessens, L.A.; Russell, M.A.; Mathews, C.E.; Hanssen, K.F.; Morgan, N.G.; Koeleman, B.P.C.; et al. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia 2018, 61, 1650–1661. [Google Scholar] [CrossRef]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes 2019, 68, 1353–1358. [Google Scholar]
- Reddy, P.; Leong, J.; Jialal, I. Amino acid levels in nascent metabolic syndrome: A contributor to the pro-inflammatory burden. J. Diabetes Complicat. 2018, 32, 465–469. [Google Scholar]
- Patel, A.; Abdelmalek, L.; Thompson, A.; Jialal, I. Decreased homoserine levels in metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 555–559. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef]
- Papandreou, C.; Hernández-Alonso, P.; Bulló, M.; Ruiz-Canela, M.; Li, J.; Guasch-Ferré, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. High Plasma Glutamate and a Low Glutamine-to-Glutamate Ratio Are Associated with Increased Risk of Heart Failure but Not Atrial Fibrillation in the Prevención con Dieta Mediterránea (PREDIMED) Study. J. Nutr. 2020, 150, 2882–2889. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Guasch-Ferré, M.; Ruiz-Canela, M.; Toledo, E.; Clish, C.; Liang, L.; Razquin, C.; Corella, D.; Estruch, R.; et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: Case-cohort study within the PREDIMED trial. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1040–1049. [Google Scholar]
- Zhang, Y.; Zhang, H.; Rong, S.; Bian, C.; Yang, Y.; Pan, H. NMR spectroscopy based metabolomics confirms the aggravation of metabolic disorder in metabolic syndrome combined with hyperuricemia. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2449–2457. [Google Scholar]
- Mangge, H.; Summers, K.L.; Meinitzer, A.; Zelzer, S.; Almer, G.; Prassl, R.; Schnedl, W.J.; Reininghaus, E.; Paulmichl, K.; Weghuber, D.; et al. Obesity-related dysregulation of the Tryptophan–Kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity 2014, 22, 195–201. [Google Scholar]
- Nagy, B.M.; Nagaraj, C.; Meinitzer, A.; Sharma, N.; Papp, R.; Foris, V.; Ghanim, B.; Kwapiszewska, G.; Kovacs, G.; Klepetko, W. Importance of kynurenine in pulmonary hypertension. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2017, 313, L741–L751. [Google Scholar]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan–kynurenine and kynurenine–nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar]
- Derkach, A.; Sampson, J.; Joseph, J.; Playdon, M.C.; Stolzenberg-Solomon, R.Z. Effects of dietary sodium on metabolites: The Dietary Approaches to Stop Hypertension (DASH)–Sodium Feeding Study. Am. J. Clin. Nutr. 2017, 106, 1131–1141. [Google Scholar]
- Brock, J.W.; Jenkins, A.J.; Lyons, T.J.; Klein, R.L.; Yim, E.; Lopes-Virella, M.; Carter, R.E.; Thorpe, S.R.; Baynes, J.W. Increased methionine sulfoxide content of apoA-I in type 1 diabetes. J. Lipid Res. 2008, 49, 847–855. [Google Scholar]
- Choksomngam, Y.; Pattanakuhar, S.; Chattipakorn, N.; Chattipakorn, S.C. The metabolic role of spermidine in obesity: Evidence from cells to community. Obes. Res. Clin. Pract. 2021, 15, 315–326. [Google Scholar]
- Eisenberg, T.; Abdellatif, M.; Zimmermann, A.; Schroeder, S.; Pendl, T.; Harger, A.; Stekovic, S.; Schipke, J.; Magnes, C.; Schmidt, A.; et al. Dietary spermidine for lowering high blood pressure. Autophagy 2017, 13, 767–769. [Google Scholar]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Jové, M.; Naudí, A.; Portero-Otin, M.; Cabré, R.; Rovira-Llopis, S.; Bañuls, C.; Rocha, M.; Hernández-Mijares, A.; Victor, V.M.; Pamplona, R. Plasma lipidomics discloses metabolic syndrome with a specific HDL phenotype. FASEB J. 2014, 28, 5163–5171. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumari, D.; Gupta, S.K.; Sharma, V.; Mukhi, S.; Kamboj, P.; Sasibhushan, V.; Rai, R.K.; Jatavallabhula, S.L.; Mahajan, D.; et al. Saroglitazar and Hepano treatment offers protection against high fat high fructose diet induced obesity, insulin resistance and steatosis by modulating various class of hepatic and circulating lipids. Biomed. Pharmacother. 2021, 144, 112357. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Y.; Gao, C.; Xiang, X.; Zhang, X.; Wang, Z. Effects of brown rice on metabolomics related to glucose and lipid in normal rats. Wei Sheng Yan Jiu J. Hyg. Res. 2021, 50, 600–608. [Google Scholar]
- Knebel, B.; Strassburger, K.; Szendroedi, J.; Kotzka, J.; Scheer, M.; Nowotny, B.; Müssig, K.; Lehr, S.; Pacini, G.; Finner, H.; et al. Specific metabolic profiles and their relationship to insulin resistance in recent-onset type 1 and type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2130–2140. [Google Scholar]
- Blasko, I.; Defrancesco, M.; Oberacher, H.; Loacker, L.; Kemmler, G.; Marksteiner, J.; Humpel, C. Plasma phosphatidylcholines and vitamin B12/folate levels are possible prognostic biomarkers for progression of Alzheimer’s disease. Exp. Gerontol. 2021, 147, 111264. [Google Scholar]
- Sanyaolu, A.; Okorie, C.; Qi, X.; Locke, J.; Rehman, S. Childhood and adolescent obesity in the United States: A public health concern. Glob. Pediatr. Health 2019, 6, 2333794X19891305. [Google Scholar]
- Ahmad, Q.I.; Ahmad, C.B.; Ahmad, S.M. Childhood obesity. Indian J. Endocrinol. Metab. 2010, 14, 19. [Google Scholar]
- Dubey, P.; Reddy, S.Y.; Singh, V.; Shi, T.; Coltharp, M.; Clegg, D.; Dwivedi, A.K. Association of Exposure to Phthalate Metabolites with Sex Hormones, Obesity, and Metabolic Syndrome in US Women. JAMA Network Open 2022, 5, e2233088. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.; Duffield, T.; Capel, M.; Godden, S.; Overton, M.; Santos, J.; LeBlanc, S. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011, 94, 4897–4903. [Google Scholar] [CrossRef] [Green Version]
| Total Participants (n = 148) | ||||||
|---|---|---|---|---|---|---|
| MHO (n = 74) | MUO (n = 74) | p | ||||
| Anthropometric and Characteristics∮ | ||||||
| Male/Female | n, (%) | 82 (55.41)/66 (44.59) | ||||
| 36 (48.65)/38 (51.35) | 46 (62.16)/28 (37.84) | 0.0982 | ||||
| Age | 14.08 | ±0.097 | 13.93 | ±0.089 | 0.2625 | |
| Height | cm | 163.7 | ±0.809 | 166.9 | ±0.944 | 0.0148 |
| Weight | kg | 91.12 | ±1.267 | 95.19 | ±1.816 | 0.0983 |
| Body Mass Index | kg/m2 | 33.92 | ±0.329 | 34.06 | ±0.453 | 0.9531 |
| Waist Circumference | cm | 102.3 | ±0.912 | 104.6 | ±1.113 | 0.1402 |
| Hip Circumference | cm | 112.6 | ±0.616 | 113.2 | ±0.925 | 0.6343 |
| Systolic Blood Pressure | mmHg | 118.2 | ±1.168 | 133.7 | ±1.453 | <0.0001 |
| Diastolic Blood Pressure | mmHg | 75.34 | ±0.772 | 84.19 | ±1.050 | <0.0001 |
| Body fat | % | 45.42 | ±0.626 | 44.22 | ±0.741 | 0.1739 |
| Fat Mass | kg | 41.56 | ±0.966 | 42.43 | ±1.239 | 0.8330 |
| Free Fat Mass | kg | 49.57 | ±0.760 | 52.76 | ±1.014 | 0.0169 |
| BMI percentage | % | 99.59 | ±0.039 | 99.23 | ±0.146 | 0.0164 |
| Weight per age percentile | nth | 99.97 | ±0.004 | 99.77 | ±0.073 | 0.0073 |
| Basal Metabolic Rate | Kcal | 1169.3 | ±13.28 | 1214.8 | ±18.52 | 0.0480 |
| Laboratory measurements∮ | ||||||
| Asparate aminotransferase | IU/L | 27.68 | ±2.089 | 29.57 | ±1.866 | 0.4439 |
| Alanine aminotransferase | IU/L | 36.81 | ±4.257 | 43.28 | ±4.336 | 0.1794 |
| White Blood Cell | ×103/mm3 | 7.013 | ±0.202 | 7.485 | ±0.164 | 0.0490 |
| Red Blood Cell | ×103/mm3 | 4.949 | ±0.036 | 5.043 | ±0.034 | 0.1841 |
| Hemoglobin | g/dL | 14.19 | ±0.115 | 14.38 | ±0.116 | 0.6055 |
| Hematocrit | g/dL | 43.59 | ±0.309 | 44.20 | ±0.314 | 0.3160 |
| Platele | ×103/mm3 | 311.9 | ±7.332 | 326.6 | ±5.733 | 0.0714 |
| Insulin | ng/mL | 27.06 | ±2.697 | 29.53 | ±1.944 | 0.2221 |
| Glucose | mg/dL | 92.50 | ±0.744 | 98.65 | ±2.439 | 0.0146 |
| Insulin resistance (IR) assessment index∮ | ||||||
| Homeostasis model assessment of insulin resistance (HOMA-IR) | 6.218 | ±0.630 | 7.389 | ±0.616 | 0.2705 | |
| Triglyceride-Glucose Index (TyG index) | 8.417 | ±0.052 | 8.792 | ±0.059 | <0.0001 | |
| Plasma lipid profiles∮ | ||||||
| Triglycerides | mg/dL | 107.1 | ±5.358 | 150.4 | ±8.202 | <0.0001 |
| Total cholesterol | mg/dL | 173.5 | ±3.119 | 180.5 | ±3.416 | 0.0878 |
| LDL-cholesterol | mg/dL | 106.2 | ±2.834 | 105.9 | ±3.071 | 0.9564 |
| HDL-cholesterol | mg/dL | 45.84 | ±0.657 | 44.51 | ±1.167 | 0.1281 |
| Total Participants (n = 148) | ||||||
|---|---|---|---|---|---|---|
| MHO (n = 74) | MUO (n = 74) | p | ||||
| Acylcarnitines∮ | ||||||
| Acetylcarnitine | C2 | 8.486 | ±0.371 | 9.796 | ±0.362 | 0.0184 |
| Hydroxypropionylcarnitine | C3-OH | 0.072 | ±0.004 | 0.077 | ±0.003 | <0.0001 |
| Methylglutarylcarnitine | C5-M-DC | 0.033 | ±0.001 | 0.035 | ±0.001 | 0.0273 |
| Amino Acids∮ | ||||||
| Alanine | 421.3 | ±10.45 | 462.3 | ±80.65 | 0.0125 | |
| Glutamine | 569.4 | ±13.19 | 545.1 | ±13.38 | 0.0041 | |
| Glutamate | 108.3 | ±4.889 | 120.0 | ±4.877 | 0.1244 | |
| Glutamine/Glutamate ratio | 5.926 | ±0.252 | 5.098 | ±0.230 | 0.0179 | |
| Histidine | 94.64 | ±1.645 | 92.43 | ±1.539 | 0.0322 | |
| Lysine | 229.7 | ±4.593 | 218.9 | ±4.463 | 0.0371 | |
| Serine | 134.6 | ±2.921 | 126.9 | ±2.714 | 0.0061 | |
| Biogenic Amines∮ | ||||||
| Kynurenine | 2.168 | ±0.073 | 2.612 | ±0.086 | 0.0003 | |
| Methionine-sulfoxide | 0.657 | ±0.028 | 0.761 | ±0.026 | 0.0194 | |
| Spermidine | 0.202 | ±0.015 | 0.236 | ±0.012 | 0.0346 | |
| Glycerophospholipids∮ | ||||||
| PC aa C32:2 | 2.901 | ±0.109 | 3.180 | ±0.117 | 0.0397 | |
| PC aa C34:1 | 163.1 | ±4.379 | 179.3 | ±4.703 | 0.0402 | |
| Variables | Total Subjects (n = 148) | p | |
|---|---|---|---|
| ORs (95% CI) For MUO Adolescents | |||
| Quartile of Acylcarnitines | |||
| Acetylcarnitine | C2 | 1.606 (1.191–2.165) | 0.0014 |
| Hydroxypropionylcarnitine | C3-OH | 1.114 (0.835–1.488) | 0.4620 |
| Methylglutarylcarnitine | C5-M-DC | 1.114 (0.835–1.488) | 0.4620 |
| Quartile of amino acids | |||
| Alanine | 1.621 (1.194–2.200) | 0.0014 | |
| Glutamine | 0.833 (0.624–1.112) | 0.2131 | |
| Glutamate | 1.364 (1.024–1.818) | 0.0316 | |
| Glutamine/Glutamate ratio | 0.735 (0.547–0.988) | 0.0388 | |
| Histidine | 0.948 (0.712–1.263) | 0.7146 | |
| Lysine | 0.839 (0.626–1.123) | 0.2363 | |
| Serine | 0.796 (0.599–1.058) | 0.1142 | |
| Quartile of biogenic amines | |||
| Kynurenine | 1.661 (1.222–2.259) | 0.0008 | |
| Methionine-sulfoxide | 1.983 (1.371–2.867) | 0.0001 | |
| Spermidine | 1.304 (0.971–1.751) | 0.0752 | |
| Quartile of glycerophospholipids | |||
| PC aa C32:2 | 1.258 (0.939–1.685) | 0.1214 | |
| PC aa C34:1 | 1.424 (1.057–1.917) | 0.0180 | |
| Quartile of insulin resistance assessment index | |||
| Homeostasis model assessment of insulin resistance (HOMA-IR) | 1.360 (1.012–1.827) | 0.0388 | |
| TyG index | 2.046 (1.476–2.835) | <0.0001 | |
| Total Participants (n = 148) | ||||||
|---|---|---|---|---|---|---|
| MHO (n = 74) | MUO (n = 74) | p | ||||
| Nutrition Intakes∮ | ||||||
| Energy | Kcal | 1424.7 | ±45.72 | 1523.0 | ±40.64 | 0.0600 |
| Carbohydrate | g | 211.3 | ±6.588 | 229.1 | ±6.650 | 0.0827 |
| Protein | g | 56.44 | ±2.117 | 60.16 | ±1.751 | 0.0698 |
| Fat | g | 36.93 | ±1.801 | 38.24 | ±1.309 | 0.2151 |
| Total fiber | g | 9.163 | ±0.459 | 9.922 | ±0.459 | 0.2348 |
| Soluble fiber | g | 1.798 | ±0.129 | 2.020 | ±0.119 | 0.1700 |
| Non-soluble fiber | g | 6.703 | ±0.352 | 7.406 | ±0.353 | 0.1336 |
| Cholesterol | mg | 174.5 | ±10.41 | 186.0 | ±11.75 | 0.3985 |
| Calcium | mg | 366.7 | ±19.89 | 404.1 | ±17.10 | 0.0677 |
| Potassium | mg | 1615.1 | ±57.48 | 1776.7 | ±61.32 | 0.0579 |
| Sodium | mg | 2529.6 | ±100.3 | 2692.9 | ±111.4 | 0.3018 |
| Total amino acids | mg | 31,383.4 | ±1340.8 | 32,567.8 | ±1561.1 | 0.5081 |
| Essencial amino acids | mg | 14,294.0 | ±627.4 | 14,950.5 | ±744.5 | 0.4711 |
| Non-essencial amino acids | mg | 17,089.4 | ±823.5 | 17,617.3 | ±822.1 | 0.5450 |
| Isoleucine | mg | 1333.5 | ±59.16 | 1378.2 | ±67.69 | 0.4235 |
| Leucine | mg | 2533.9 | ±110.7 | 2661.2 | ±133.3 | 0.5552 |
| Valine | mg | 1554.5 | ±71.39 | 1621.5 | ±77.19 | 0.4277 |
| Glutamic acid | mg | 6212.1 | ±279.1 | 6319.9 | ±289.8 | 0.5778 |
| Total fatty acids | g | 28.75 | ±1.504 | 28.75 | ±1.227 | 0.6784 |
| Total trans-fatty acids | g | 0.368 | ±0.026 | 0.360 | ±0.018 | 0.4523 |
| Total essential fatty acids | g | 7.836 | ±0.350 | 8.425 | ±0.436 | 0.4208 |
| Total saturated fatty acids | g | 9.922 | ±0.669 | 9.702 | ±0.438 | 0.5272 |
| Total mono-unsaturated fatty aicds | g | 10.201 | ±0.628 | 9.902 | ±0.455 | 0.8554 |
| Total poly-unsaturated fatty acids | g | 8.260 | ±0.363 | 8.788 | ±0.451 | 0.5087 |
| n-3 fatty acids | g | 0.861 | ±0.044 | 0.952 | ±0.057 | 0.2989 |
| n-6 fatty acids | g | 7.161 | ±0.314 | 7.666 | ±0.388 | 0.4401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Jang, H.-B.; Kim, H.-J.; Lee, H.-J. Identification of Biomarkers Related to Metabolically Unhealthy Obesity in Korean Obese Adolescents: A Cross-Sectional Study. Children 2023, 10, 322. https://doi.org/10.3390/children10020322
Jeong S, Jang H-B, Kim H-J, Lee H-J. Identification of Biomarkers Related to Metabolically Unhealthy Obesity in Korean Obese Adolescents: A Cross-Sectional Study. Children. 2023; 10(2):322. https://doi.org/10.3390/children10020322
Chicago/Turabian StyleJeong, Sarang, Han-Byul Jang, Hyo-Jin Kim, and Hye-Ja Lee. 2023. "Identification of Biomarkers Related to Metabolically Unhealthy Obesity in Korean Obese Adolescents: A Cross-Sectional Study" Children 10, no. 2: 322. https://doi.org/10.3390/children10020322






