Glycemic Stress Index: Does It Correlate with the Intensive Care Length of Stay?
Abstract
:1. Introduction
2. Methods
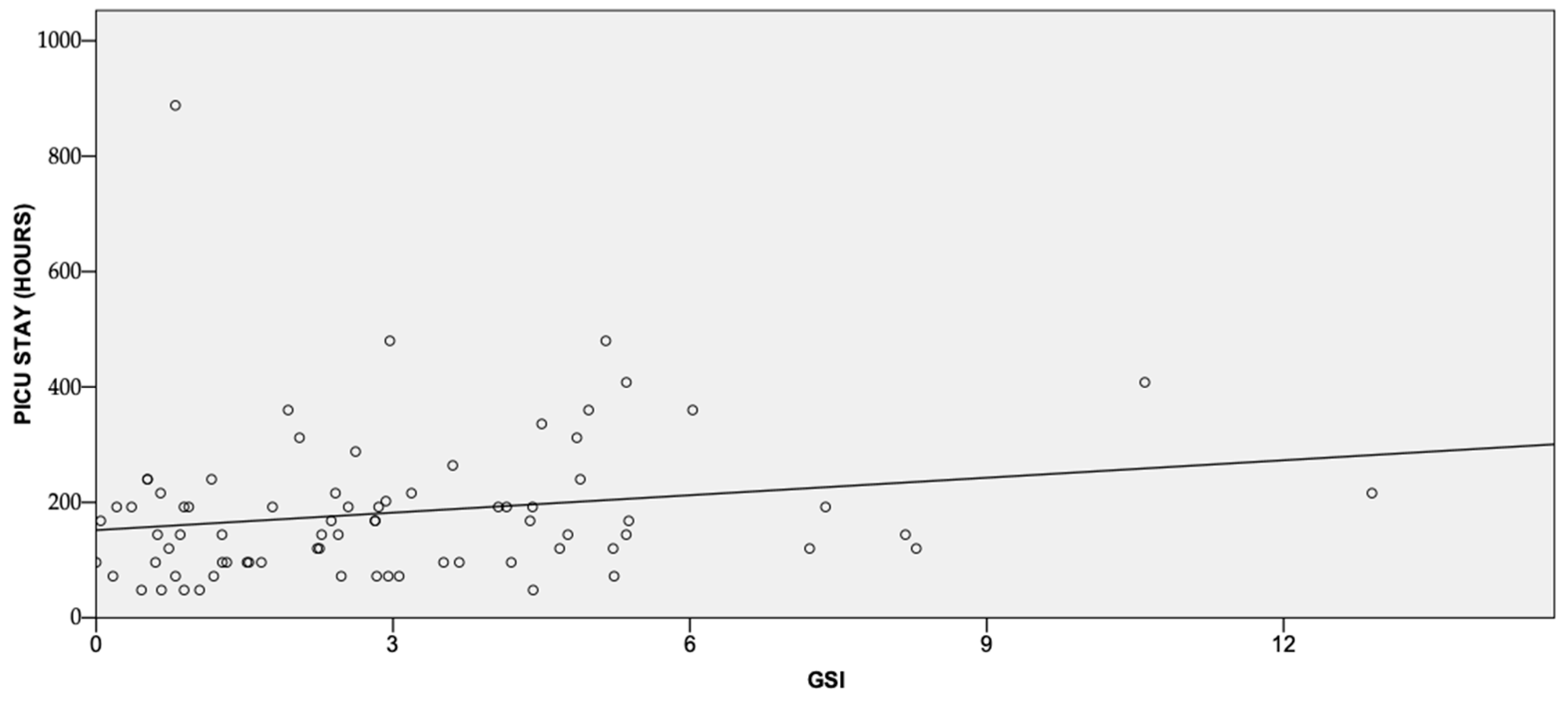
3. Results and Discussion
4. Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schricker, T.; Lattermann, R. Perioperative catabolism. Can. J. Anaesth. 2015, 62, 182–193. [Google Scholar] [CrossRef]
- Ljungqvist, O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract. Res. Clin. Anaesthesiol. 2009, 23, 401–409. [Google Scholar] [CrossRef]
- Wu, Y.; Lai, W.; Pei, J.; Zhao, Y.; Wang, Q.; Xiang, B. Hyperglycemia and its association with clinical outcomes in postsurgical neonates and small infants in the intensive care unit. J. Pediatr. Surg. 2016, 51, 1142–1145. [Google Scholar] [CrossRef]
- Filho, N.O.; Alves, R.L.; Fernandes, A.T.; Castro, F.S.; Melo, J.R.; Modolo, N.S. Association of increased morbidity with the occurrence of hyperglycemia in the immediate postoperative period after elective pediatric neurosurgery. J. Neurosurg. Pediatr. 2016, 17, 625–629. [Google Scholar] [CrossRef]
- Moga, M.A.; Manlhiot, C.; Marwali, E.M.; McCrindle, B.W.; Van Arsdell, G.S.; Schwartz, S.M. Hyperglycemia after pediatric cardiac surgery: Impact of age and residual lesions. Crit. Care Med. 2011, 39, 266–272. [Google Scholar] [CrossRef]
- Bansal, B.; Carvalho, P.; Mehta, Y.; Yadav, J.; Sharma, P.; Mithal, A.; Trehan, N. Prognostic significance of glycemic variability after cardiac surgery. J. Diabetes Complicat. 2016, 30, 613–617. [Google Scholar] [CrossRef]
- Marin-Vivas, R.R.; Saldivar-Muller, C.E.; Sanchez-Banuelos, C.C.; Flores-Lujano, J.; Nunez-Enriquez, J.C. Prognostic factors of postoperative severe hyperglycemia after cardiac surgery in pediatric patients. Rev. Med. Inst. Mex. Seguro. Soc. 2017, 55, 324–329. [Google Scholar]
- Krueger, J.J.; Brotschi, B.; Balmer, C.; Bernet, V.; Latal, B. Postoperative Hyperglycemia and 4-Year Neurodevelopmental Outcome in Children Operated for Congenital Heart Disease. J. Pediatr. 2015, 167, 1253–1258.e1251. [Google Scholar] [CrossRef]
- Palermo, R.A.; Palac, H.L.; Wald, E.L.; Wainwright, M.S.; Costello, J.M.; Eltayeb, O.M.; Backer, C.L.; Epting, C.L. Metabolic Uncoupling Following Cardiopulmonary Bypass. Congenit. Heart Dis. 2015, 10, E250–E257. [Google Scholar] [CrossRef]
- Stephen, A.; Alles, M.; de Graaf, C.; Fleith, M.; Hadjilucas, E.; Isaacs, E.; Maffeis, C.; Zeinstra, G.; Matthys, C.; Gil, A. The role and requirements of digestible dietary carbohydrates in infants and toddlers. Eur. J. Clin. Nutr. 2012, 66, 765–779. [Google Scholar] [CrossRef]
- Smith, M.D.; McCall, J.; Plank, L.; Herbison, G.P.; Soop, M.; Nygren, J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst. Rev. 2014, 8, CD009161. [Google Scholar] [CrossRef]
- Lee, B.; Soh, S.; Shim, J.K.; Kim, H.Y.; Lee, H.; Kwak, Y.L. A randomised trial to evaluate preoperative oral carbohydrate administration on insulin resistance in off-pump coronary artery bypass patients. Eur. J. Anaesthesiol. 2017, 34, 740–747. [Google Scholar] [CrossRef]
- Leeds, I.L.; Boss, E.F.; George, J.A.; Strockbine, V.; Wick, E.C.; Jelin, E.B. Preparing enhanced recovery after surgery for implementation in pediatric populations. J. Pediatr. Surg. 2016, 51, 2126–2129. [Google Scholar] [CrossRef]
- Dennhardt, N.; Beck, C.; Huber, D.; Sander, B.; Boehne, M.; Boethig, D.; Leffler, A.; Sumpelmann, R. Optimized preoperative fasting times decrease ketone body concentration and stabilize mean arterial blood pressure during induction of anesthesia in children younger than 36 months: A prospective observational cohort study. Paediatr. Anaesth. 2016, 26, 838–843. [Google Scholar] [CrossRef]
- Isserman, R.; Elliott, E.; Subramanyam, R.; Kraus, B.; Sutherland, T.; Madu, C.; Stricker, P.A. Quality improvement project to reduce pediatric clear liquid fasting times prior to anesthesia. Paediatr. Anaesth. 2019, 29, 698–704. [Google Scholar] [CrossRef]
- Piastra, M.; Pizza, A.; Tosi, F.; Mensi, S.; Massimi, L.; De Bellis, A.; Biasucci, D.G.; Luca, E.; Conti, G.; De Luca, D. Validation of the Glycemic Stress Index in Pediatric Neurosurgical Intensive Care. Neurocrit. Care 2017, 26, 388–392. [Google Scholar] [CrossRef]
- Pietrini, D.; Di Rocco, C.; Di Bartolomeo, R.; Conti, G.; Ranelletti, F.O.; De Luca, D.; Tosi, F.; Mensi, S.; D’Arrigo, S.; Piastra, M. No-glucose strategy influences posterior cranial fossa tumors’ postoperative course: Introducing the Glycemic Stress Index. J. Neurooncol. 2009, 93, 361–368. [Google Scholar] [CrossRef]
- Floh, A.A.; Manlhiot, C.; Redington, A.N.; McCrindle, B.W.; Clarizia, N.A.; Caldarone, C.A.; Schwartz, S.M. Insulin resistance and inflammation are a cause of hyperglycemia after pediatric cardiopulmonary bypass surgery. J. Thorac. Cardiovasc. Surg. 2015, 150, 498–504 e491. [Google Scholar] [CrossRef]
- Ueno, K.; Seki, S.; Shiokawa, N.; Matsuba, T.; Miyazono, A.; Hazeki, D.; Imoto, Y.; Kawano, Y. Validation of acute kidney injury according to the modified KDIGO criteria in infants after cardiac surgery for congenital heart disease. Nephrology 2018, 24, 294–300. [Google Scholar] [CrossRef]
- Iwase, S.; Nakada, T.A.; Shimada, T.; Oami, T.; Shimazui, T.; Takahashi, N.; Yamabe, J.; Yamao, Y.; Kawakami, E. Prediction algorithm for ICU mortality and length of stay using machine learning. Sci. Rep. 2022, 12, 12912. [Google Scholar] [CrossRef]
- Prince, R.D.; Akhondi-Asl, A.; Mehta, N.M.; Geva, A. A Machine Learning Classifier Improves Mortality Prediction Compared with Pediatric Logistic Organ Dysfunction-2 Score: Model Development and Validation. Crit. Care Explor. 2021, 3, e0426. [Google Scholar] [CrossRef]
- Medeiros, N.B.; Fogliatto, F.S.; Rocha, M.K.; Tortorella, G.L. Forecasting the length-of-stay of pediatric patients in hospitals: A scoping review. BMC Health Serv. Res. 2021, 21, 938. [Google Scholar] [CrossRef]
- Brandi, S.; Troster, E.J.; Cunha, M. Length of stay in pediatric intensive care unit: Prediction model. Einstein 2020, 18, eAO5476. [Google Scholar] [CrossRef]
- Russell, R.A.; Ghanayem, N.S.; Kuhn, E.M.; Jeffries, H.E.; Scanlon, M.C.; Rice, T.B. Relationship between risk-adjustment tools and the pediatric logistic organ dysfunction score. World J. Pediatr. Congenit. Heart Surg. 2014, 5, 16–21. [Google Scholar] [CrossRef]
- Jeffries, H.E.; Soto-Campos, G.; Katch, A.; Gall, C.; Rice, T.B.; Wetzel, R. Pediatric Index of Cardiac Surgical Intensive Care Mortality Risk Score for Pediatric Cardiac Critical Care. Pediatr. Crit. Care Med. 2015, 16, 846–852. [Google Scholar] [CrossRef]
- Parkman, S.E.; Woods, S.L. Infants who have undergone cardiac surgery: What can we learn about lengths of stay in the hospital and presence of complications? J. Pediatr. Nurs. 2005, 20, 430–440. [Google Scholar] [CrossRef]


| Demographic Data (Mean ± SD) | |
|---|---|
| Age (months) | 5.1 (± 0.9) |
| Weight (kg) | 35 (± 1.5) |
| Operation variables (median, 95% CI) | |
| Length of surgery (min) | 226 (217–240) |
| Length of anesthesia (min) | 350 (347–380) |
| Length of bypass (min) | 118 (112–128) |
| Length of cross-clamp (min) | 67 (65–76) |
| Type of CHD (n, %) | |
| Aortic stenosis | 1 (1.2) |
| Atrial septal defect | 1 (1.2) |
| Atrioventricular septal defect | 10 (11.8) |
| Double outlet right ventricle | 7 (8.2) |
| Pulmonary stenosis | 4 (4.7) |
| Pulmonary venous stenosis | 1 (1.2) |
| Tetralogy of Fallot | 21 (24.7) |
| Ventricular septal defect | 40 (47.1) |
| Type of surgery (n, %) | |
| Atrial septal defect closure | 1 (1.2) |
| Aortic valve repair | 1 (1.2) |
| Atrioventricular septal defect repair | 10 (11.8) |
| Double outlet right ventricle repair | 7 (8.2) |
| Pulmonary artery repair | 3 (3.5) |
| Pulmonary venous stenosis repair | 1 (1.2) |
| Pulmonary valve repair | 1 (1.2) |
| Tetralogy of Fallot repair | 21 (24.7) |
| Ventricular septal defect repair | 40 (47.1) |
| Preoperative medications (n, %) | |
| Diuretics | 43 (50.6) |
| Beta blockers | 6 (7.1) |
| Digoxin | 1 (1.2) |
| Others (i.e., H2 antagonist, PPI, iron) | 10 (41.1) |
| Intraoperative medications (n, %) | |
| Epinephrine | 41 (48.2) |
| Dopamine | 60 (70.6) |
| Dobutamine | 7 (8.20) |
| Milrinone | 79 (92.9) |
| Nitroglycerine | 1 (1.2) |
| Nitroprusside | 3 (3.5) |
| Esmolol | 5 (5.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georges, M.; Engelhardt, T.; Ingelmo, P.; Mentegazzi, F.; Bertolizio, G. Glycemic Stress Index: Does It Correlate with the Intensive Care Length of Stay? Children 2023, 10, 328. https://doi.org/10.3390/children10020328
Georges M, Engelhardt T, Ingelmo P, Mentegazzi F, Bertolizio G. Glycemic Stress Index: Does It Correlate with the Intensive Care Length of Stay? Children. 2023; 10(2):328. https://doi.org/10.3390/children10020328
Chicago/Turabian StyleGeorges, Mathieu, Thomas Engelhardt, Pablo Ingelmo, Federico Mentegazzi, and Gianluca Bertolizio. 2023. "Glycemic Stress Index: Does It Correlate with the Intensive Care Length of Stay?" Children 10, no. 2: 328. https://doi.org/10.3390/children10020328
APA StyleGeorges, M., Engelhardt, T., Ingelmo, P., Mentegazzi, F., & Bertolizio, G. (2023). Glycemic Stress Index: Does It Correlate with the Intensive Care Length of Stay? Children, 10(2), 328. https://doi.org/10.3390/children10020328





