Subcutaneous Granuloma Annulare vs. Subcutaneous Vascular Malformations in Children: A Diagnostic Challenge
Abstract
:1. Introduction
2. Patients and Methods
3. Results
3.1. Patients with Subcutaneous Granuloma Annulare
3.1.1. Clinical Presentation
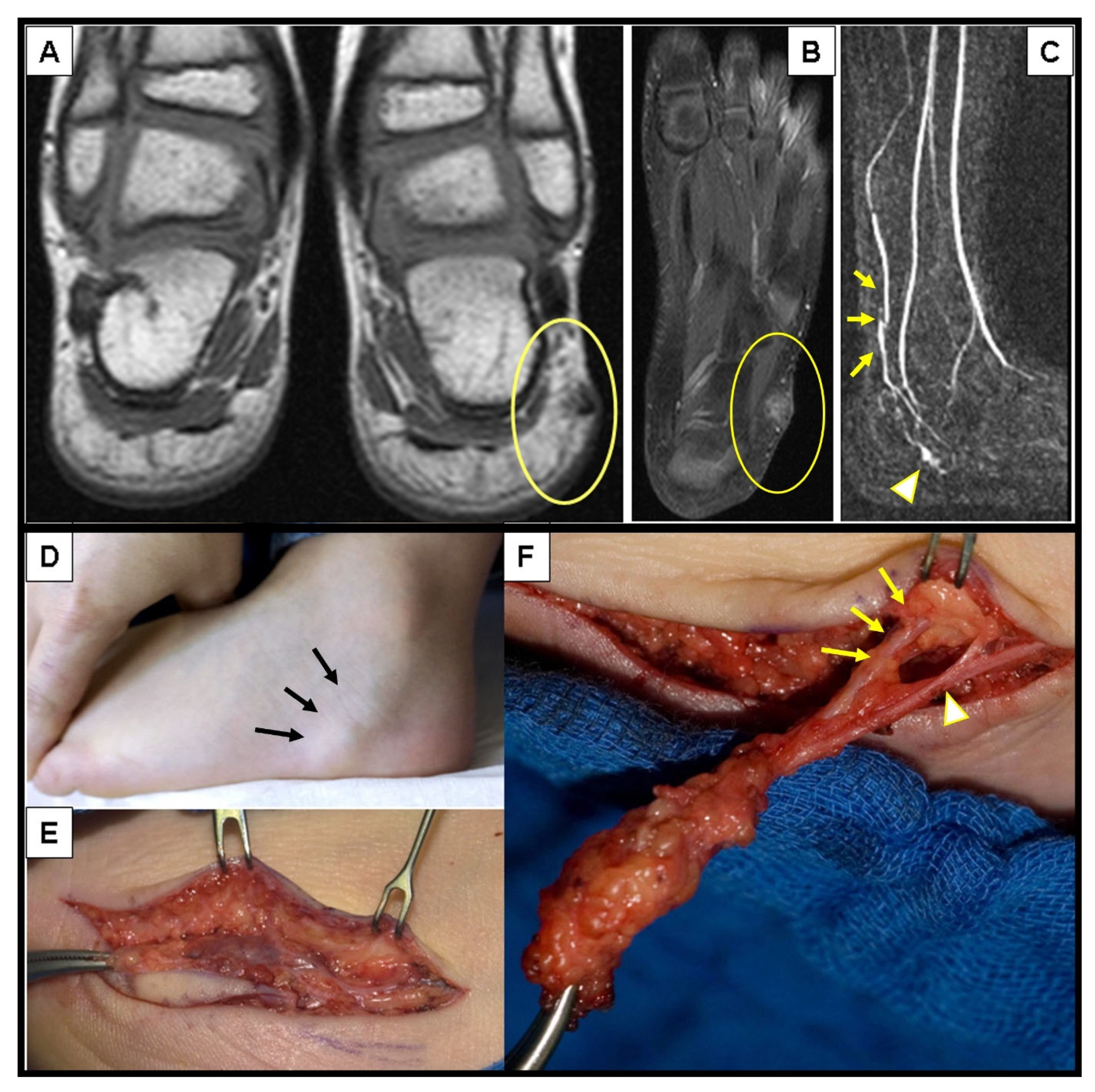
3.1.2. Presentation on X-ray
3.1.3. Presentation on Ultrasound
3.1.4. Presentation on MRI
3.1.5. Invasive Diagnostic Procedures
3.2. Patients with Low-Flow Subcutaneous Vascular Malformations
3.2.1. Clinical Presentation
3.2.2. Presentation on Ultrasound
3.2.3. Presentation on MRI
3.2.4. Invasive Diagnostic Procedures
3.3. Retrospective Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patrizi, A.; Gurioli, C.; Neri, I. Childhood granuloma annulare: A review. G. Ital. Dermatol. Venereol. 2014, 149, 663–674. [Google Scholar]
- Requena, L.; Fernández-Figueras, M.T. Subcutaneous granuloma annulare. Semin. Cutan. Med. Surg. 2007, 26, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Keimig, E.L. Granuloma Annulare. Dermatol. Clin. 2015, 33, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Thornsberry, L.A.; English, J.C. Etiology, Diagnosis, and Therapeutic Management of Granuloma Annulare: An Update. Am. J. Clin. Dermatol. 2013, 14, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, F.; Paloni, G.; Berti, I. Subcutaneous Granuloma Annulare: A Diagnostic Conundrum-Learning from Mistakes. Pediatr. Emerg. Care 2017, 33, e30–e31. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.N.; Hansmann, S.; Kümmerle-Deschner, J.; Schäfer, J.; Horger, M. Das subkutane Granuloma annulare. RöFo Fortschr. Auf Dem Geb. Röntgenstrahlen Und Bildgeb. Verfahr. 2007, 179, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.R.; Mamet, F.; Kokta, V.; Coulombe, J. Subcutaneous nodules in children: Don’t forget deep granuloma annulare: A Case Report. SAGE Open Med. Case Rep. 2020, 8, 2050313X20935713. [Google Scholar]
- Vogel, S.A.; Hess, C.P.; Dowd, C.F.; Hoffman, W.Y.; Kane, A.J.; Rajaii, R.; Frieden, I.J. Early Versus Later Presentations of Venous Malformations: Where and Why? Pediatr. Dermatol. 2013, 30, 534–540. [Google Scholar] [CrossRef]
- Haxhija, E.Q.; Spendel, S.; Höllwarth, M.E. Chirurgische Therapie vaskulärer Malformationen bei Kindern und Jugendlichen. Handchir. Mikrochir. Plast. Chir. 2009, 41, 100–106. [Google Scholar] [CrossRef]
- ISSVA Classification for Vascular Anomalies©. Homepage of International Society for the Study of Vascular Anomalies. Available online: https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf (accessed on 7 February 2023).
- White, C.L.; Olivieri, B.; Restrepo, R.; McKeon, B.; Karakas, S.P.; Lee, E.Y. Low-Flow Vascular Malformation Pitfalls: From Clinical Examination to Practical Imaging Evaluation—Part 1, Lymphatic Malformation Mimickers. Am. J. Roentgenol. 2016, 206, 940–951. [Google Scholar] [CrossRef]
- Endo, Y.; Sekiguchi, A.; Motegi, S.; Ishikawa, O. Subcutaneous granuloma annulare on the heel: A case report and review of the Japanese published work. J. Dermatol. 2020, 47, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Sabuncuoğlu, H.; Oge, K.; Söylemezoğlu, F.; Sağlam, A. Subcutaneous granuloma annulare of the scalp in childhood: A case report and review of the literature. Turk. Neurosurg. 2007, 17, 19–22. [Google Scholar]
- Jankowski, P.P.; Krishna, P.H.; Rutledge, J.C.; Waldhausen, J.; Avellino, A.M. Surgical Management and Outcome of Scalp Subcutaneous Granuloma Annulare in Children. Neurosurgery 2008, 63, E1002. [Google Scholar] [CrossRef] [PubMed]
- Grogg, K.L.; Nascimento, A.G. Subcutaneous Granuloma Annulare in Childhood: Clinicopathologic Features in 34 Cases. Pediatrics 2001, 107, e42. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Blessing, K.; Gray, E.S. Pseudorheumatoid Nodule (Deep Granuloma Annulare) of Childhood: Clinicopathologic Features of Twenty Patients. Pediatr. Dermatol. 1994, 11, 6–9. [Google Scholar] [CrossRef]
- Lega, S.; Rabusin, M.; Pederiva, F. Acute-onset pretibial swelling. Indian Pediatr. 2014, 51, 334. [Google Scholar] [CrossRef]
- Beqo, B.; Tschauner, S.; Haxhija, E.Q. Subcutaneous “Epifascial Cap” as the Pathognomonic Imaging Sign of Subcutaneous Granuloma Annulare in Childhood—First Description! Eur. Surg. 2022, 54 (Suppl. S1), 75. [Google Scholar]
- Vázquez-Osorio, I.; Quevedo, A.; Rodríguez-Vidal, A.; Rodríguez-Díaz, E. Usefulness of ultrasonography in the diagnosis of subcutaneous granuloma annulare. Pediatr. Dermatol. 2018, 35, e200–e201. [Google Scholar] [CrossRef]
- Pesce, V.; Notarnicola, A.; Moretti, B. Diagnostic dilemma of a subcutaneous nodule following a trauma in a child: Immunohistochemical examination put the final diagnosis of deep granuloma annulare. Musculoskelet. Surg. 2010, 94, 49–51. [Google Scholar] [CrossRef]
- Li, W.-Y.; Reinisch, J.F. Cysts, pits, and tumors. Plast. Reconstr. Surg. 2009, 124 (Suppl. S1), 106e–116e. [Google Scholar] [CrossRef]
- Knight, P.J.; Reiner, C.B. Superficial lumps in children: What, when, and why? Pediatrics 1983, 72, 147–153. [Google Scholar] [CrossRef]
- Letts, M.; Carpenter, B.; Soucy, P.; Davidson, D. Subcutaneous granuloma annulare of the extremities in children. Can. J. Surg. 2000, 43, 425–430. [Google Scholar]
- Riebel, T.; Scheer, I. Subkutanes Granuloma annulare: Eine nicht zu vergessende Differenzialdiagnose bei expansiven (Weichteil-) Läsionen an den unteren Extremitäten von jungen Kindern. Ultraschall der Med. Eur. J. Ultrasound 2010, 32, 604–607. [Google Scholar] [CrossRef]
- Stenzel, M.; Voß, U.; Mutze, S.; Hesse, V. A Pretibial Lump in a Toddler—Sonographic Findings in Subcutaneous Granuloma Annulare. Ultraschall der Med. Eur. J. Ultrasound 2008, 31, 68–70. [Google Scholar] [CrossRef]
- Putnam, T.C. Lumps and bumps in children. Pediatr. Rev. 1992, 13, 371–378. [Google Scholar] [CrossRef]
- Navarro, O.M.; Laffan, E.E.; Ngan, B.-Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics 2009, 29, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Bartlett, E.; Lee, E.I. Vascular Malformations: A Review. Semin. Plast. Surg. 2014, 28, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Nosher, J.L.; Murillo, P.G.; Liszewski, M.; Gendel, V.; Gribbin, C.E. Vascular anomalies: A pictorial review of nomenclature, diagnosis and treatment. World J. Radiol. 2014, 6, 677–692. [Google Scholar] [CrossRef]
- Pappas, D.C.; Persky, M.S.; Berenstein, A. Evaluation and treatment of head and neck venous vascular malformations. Ear Nose Throat J. 1998, 77, 914–916, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Navarro, O.M. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 2: Vascular malformations. Pediatr. Radiol. 2017, 47, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Ferrara, D.; di Serafino, M.; Diplomatico, M.; Vezzali, N.; Giugliano, A.M.; Colafati, G.S.; Zeccolini, M.; Tomà, P. Classification and ultrasound findings of vascular anomalies in pediatric age: The essential. J. Ultrasound 2019, 22, 13–25. [Google Scholar] [CrossRef]
- Kollipara, R.; Odhav, A.; Rentas, K.E.; Rivard, D.C.; Lowe, L.H.; Dinneen, L. Vascular anomalies in pediatric patients: Updated classification, imaging, and therapy. Radiol. Clin. N. Am. 2013, 51, 659–672. [Google Scholar] [CrossRef]
- Dubois, J.; Alison, M. Vascular anomalies: What a radiologist needs to know. Pediatr. Radiol. 2010, 40, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Colletti, G.; Valassina, D.; Bertossi, D.; Melchiorre, F.; Vercellio, G.; Brusati, R. Contemporary Management of Vascular Malformations. J. Oral Maxillofac. Surg. 2014, 72, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Callahan, M.J. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: A 5-year retrospective review. Pediatr. Radiol. 2013, 43 (Suppl. S1), 23–40. [Google Scholar] [CrossRef]
- Hwang, E.J.; Yoon, H.-S.; Cho, S.; Park, H.S. The diagnostic value of ultrasonography with 5-15-MHz probes in benign subcutaneous lesions. Int. J. Dermatol. 2015, 54, e469–e475. [Google Scholar] [CrossRef]
- Paltiel, H.J.; Burrows, P.E.; Kozakewich, H.P.W.; Zurakowski, D.; Mulliken, J.B. Soft-Tissue Vascular Anomalies: Utility of US for Diagnosis. Radiology 2000, 214, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Trop, I.; Dubois, J.; Guibaud, L.; Grignon, A.; Patriquin, H.; McCuaig, C.; Garel, L.A. Soft-tissue venous malformations in pediatric and young adult patients: Diagnosis with Doppler US. Radiology 1999, 212, 841–845. [Google Scholar] [CrossRef]
- Morrow, M.S.; Oliveira, A.M. Imaging of Lumps and Bumps in Pediatric Patients: An Algorithm for Appropriate Imaging and Pictorial Review. Semin. Ultrasound CT MRI 2014, 35, 415–429. [Google Scholar] [CrossRef]
- Bansal, A.G.; Rosenberg, H.K. Sonography of pediatric superficial lumps and bumps: Illustrative examples from head to toe. Pediatr. Radiol. 2017, 47, 1171–1183. [Google Scholar] [CrossRef]
- Hyodoh, H.; Hori, M.; Akiba, H.; Tamakawa, M.; Hyodoh, K.; Hareyama, M. Peripheral vascular malformations: Imaging, treatment approaches, and therapeutic issues. Radiographics 2005, 25 (Suppl. S1), S159–S171. [Google Scholar] [CrossRef]
- Donnelly, L.F.; Adams, D.M.; Bisset, G.S. Vascular malformations and hemangiomas: A practical approach in a multidisciplinary clinic. AJR Am. J. Roentgenol. 2000, 174, 597–608. [Google Scholar] [CrossRef]
- Burrows, P.E.; Laor, T.; Paltiel, H.; Robertson, R.L. Diagnostic Imaging in the Evaluation of Vascular Birthmarks. Dermatol. Clin. 1998, 16, 455–488. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.M.; Yakes, W.F.; Ray, R.L.; Dreisbach, J.N.; Parker, S.H.; Luethke, J.M.; Stavros, A.T.; Slater, D.D.; Burke, B.J. MR imaging of symptomatic peripheral vascular malformations. AJR Am. J. Roentgenol. 1992, 159, 107–112. [Google Scholar] [CrossRef] [PubMed]
- van Rijswijk, C.S.P.; van der Linden, E.; van der Woude, H.-J.; van Baalen, J.M.; Bloem, J.L. Value of dynamic contrast-enhanced MR imaging in diagnosing and classifying peripheral vascular malformations. AJR Am. J. Roentgenol. 2002, 178, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Hoffer, F.A.; Barnes, P.D.; Mulliken, J.B. Biological classification of soft-tissue vascular anomalies: MR correlation. Am. J. Roentgenol. 1991, 157, 559–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| N | Age in Years—Median (Range) | Girls N (%) | Surgical Intervention N (%) | |
|---|---|---|---|---|
| SGA | 12 | 3.25 (2–5) | 9 (75%) | 12 (100%) |
| SVM | 47 | 5 (0–18) | 19 (40%) | 45 (96%) |
| Total N (%) | Head and Neck N (%) | Upper Extremity N (%) | Lower Extremity N (%) | Trunk N (%) | |
|---|---|---|---|---|---|
| SGA | 12 (100%) | 3 (25%) | 3 (25%) | 6 (50%) | 0 |
| SVM | 47 (100%) | 6 (13%) | 23 (51%) | 8 (19%) | 10 (17%) |
| LM | 27 (57%) | 1 | 16 | 3 | 7 |
| LVM | 8 (17%) | 3 | 3 | 1 | 1 |
| VM | 12 (26%) | 2 | 4 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beqo, B.P.; Gasparella, P.; Flucher, C.; Tschauner, S.; Brcic, I.; Haxhija, E.Q. Subcutaneous Granuloma Annulare vs. Subcutaneous Vascular Malformations in Children: A Diagnostic Challenge. Children 2023, 10, 362. https://doi.org/10.3390/children10020362
Beqo BP, Gasparella P, Flucher C, Tschauner S, Brcic I, Haxhija EQ. Subcutaneous Granuloma Annulare vs. Subcutaneous Vascular Malformations in Children: A Diagnostic Challenge. Children. 2023; 10(2):362. https://doi.org/10.3390/children10020362
Chicago/Turabian StyleBeqo, Besiana P., Paolo Gasparella, Christina Flucher, Sebastian Tschauner, Iva Brcic, and Emir Q. Haxhija. 2023. "Subcutaneous Granuloma Annulare vs. Subcutaneous Vascular Malformations in Children: A Diagnostic Challenge" Children 10, no. 2: 362. https://doi.org/10.3390/children10020362





