Use of Cardio-Pulmonary Ultrasound in the Neonatal Intensive Care Unit
Abstract
:1. Introduction
2. Targeted Neonatal Echocardiography
2.1. Technique and Training
2.2. Patent Ductus Arteriosus Evaluation
2.3. Early and Late Pulmonary Hypertension
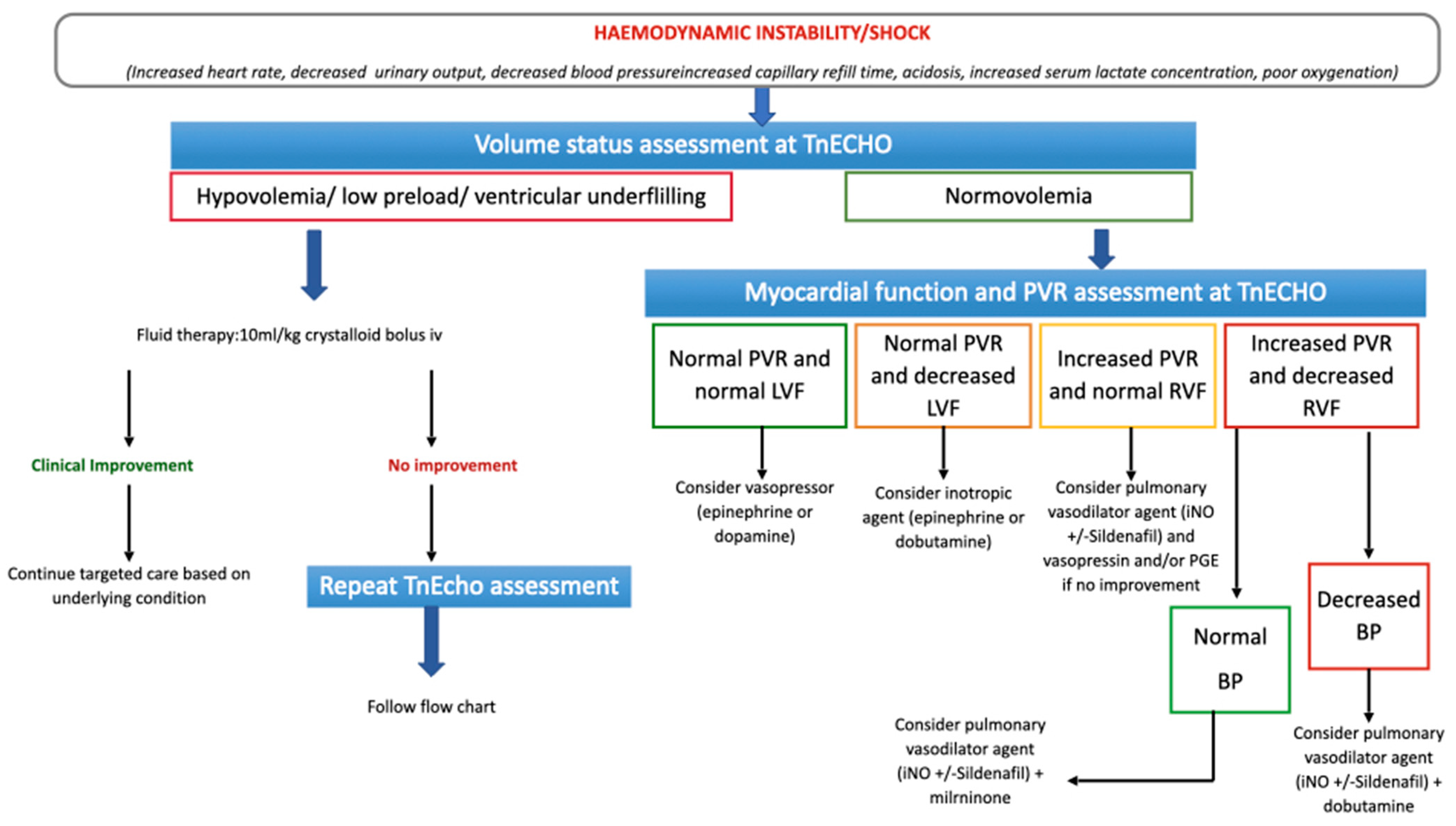
2.4. Targeted Choice of Volume Replacement, Inotropes and Vasopressors during Neonatal Shock
3. Lung Ultrasound
3.1. Lung Ultrasound Technique and Training
3.2. Lung Artifacts
3.2.1. A-Lines
3.2.2. B-Lines
3.3. Lung Ultrasound Patterns
- A-Pattern (Figure 2A), normally aerated lung (aeration score = 0): A-lines, bilateral lung sliding (Video S2). The A-pattern in the absence of lung sliding suggests the presence of pneumothorax. In that case the lung point needs to be searched for. The lung point is point where the pneumothorax ends and the normal contact between the parietal and visceral pleura is restored. The corresponding ultrasound image is a scan where the a normal sliding is detected right aside a static pleura, representing the loss of contact between the parietal and visceral pleura. Therefore after the lung point, the pleural space is filled with air and the pleural sliding is absent (Video S3);
- B1-pattern (Figure 2B), moderate loss of lung aeration (aeration score = 1): three or more B-lines occupying less than 50% of the scanned area;
- B2-pattern (Figure 2C), severe loss of aeration (aeration score = 2): coalescent B-lines, occupying more than 50% of the scanned area;
- White lung (Figure 2D): compact B-lines that cause the acoustic shadow of the ribs to disappear within the entire scanning zone, anteriorly and posteriorly without spared areas;
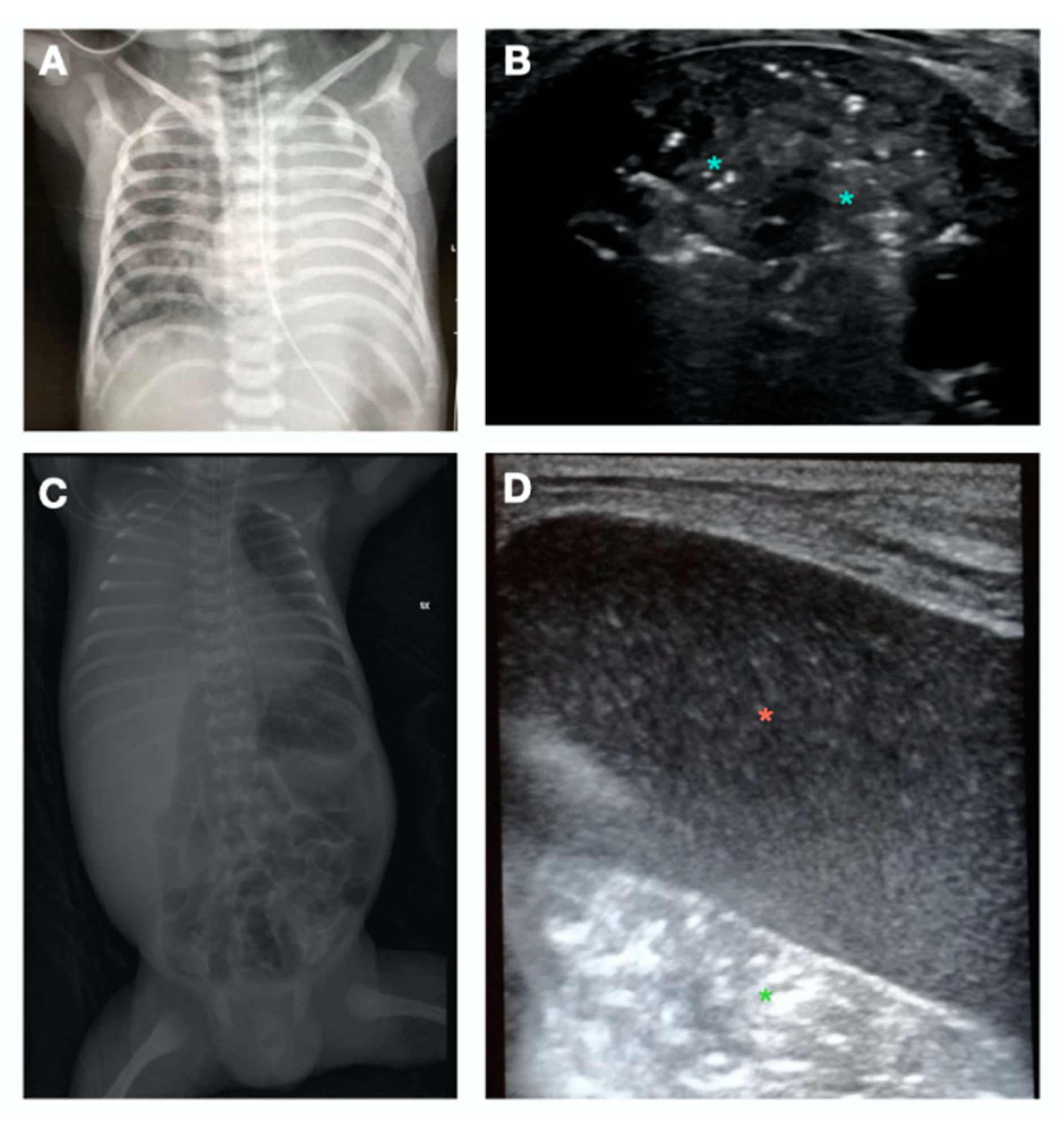
- C-Pattern (Figure 2E): complete loss of aeration (aeration score = 3) leading to lung consolidation. The C-pattern is the only LUS pattern based on the anatomical visualization of the lung. Lung collapse or consolidation remove the A-line artifacts and allow for direct visualization of the lung parenchyma. Lung consolidations (that can highly vary in size and number), generally appear as hypoechoic or “liver-like texture” images, with irregular margins, containing air bronchogram and a vascular pattern enhancing an intraparenchymal pulmonary shunt. Lung consolidation may be due to lung atelectasis, pneumonia or MAS (Figure S5).
3.4. Pleural Line and Distribution of the B-Lines
3.5. Lung Ultrasound Predictive Scores
3.6. Indication to Administer Surfactant
3.7. Detection of Lung Diseases
4. Cardiopulmonary Ultrasound
4.1. Lung Ultrasound Guided Recruitment
4.1.1. S-Pattern and D-Pattern
4.1.2. Lung Ultrasound Targeted Recruitment Protocol
4.2. Lung Ultrasound Guided Position and Postural Recruitment
4.3. Phenotyping the Acute Neonatal Respiratory Diseases near Term
4.4. Phenotyping the Chronic Respiratory Diseases
4.5. Cardiopulmonary Ultrasound Targeted Treatment
4.5.1. Cardiopulmonary Ultrasound Approach to Patent Ductus Arteriosus
4.5.2. Cardiopulmonary Ultrasound Approach to Pulmonary Hypertension
4.5.3. Cardiopulmonary Ultrasound Approach to Neonatal Shock
4.5.4. Cardiopulmonary Ultrasound Approach to Cardiac Arrest and Sudden Deterioration
4.5.5. Cardiopulmonary Ultrasound Approach to Neonatal Acute Respiratory Distress Syndrome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, S.; Chow, R.; Popovic, M.; Lam, M.; Popovic, M.; Merrick, J.; Stashefsky Margalit, R.N.; Lam, H.; Milakovic, M.; Chow, E.; et al. A Selected Review of the Mortality Rates of Neonatal Intensive Care Units. Front. Public Health 2015, 3, 225. [Google Scholar] [CrossRef] [PubMed]
- Nestaas, E. Neonatologist Performed Echocardiography for Evaluating the Newborn Infant. Front. Pediatr. 2022, 10, 853205. [Google Scholar] [CrossRef] [PubMed]
- Ben Fadel, N.; Surak, A.; Almoli, E.; Jankov, R. Implementing a successful targeted neonatal echocardiography service and a training program: The ten stages of change. J. Neonatal-Perinat. Med. 2022, 15, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Roehr, C.C.; Tissot, C.; Rogerson, S.; Gupta, S.; Bohlin, K.; Breindahl, M.; El-Khuffash, A.; de Boode, W.P. Education, training, and accreditation of Neonatologist Performed Echocardiography in Europe-framework for practice. Pediatr. Res. 2018, 84 (Suppl. S1), 13–17. [Google Scholar] [CrossRef] [PubMed]
- de Boode, W.P.; Singh, Y.; Gupta, S.; Austin, T.; Bohlin, K.; Dempsey, E.; Groves, A.; Eriksen, B.H.; van Laere, D.; Molnar, Z.; et al. Recommendations for neonatologist performed echocardiography in Europe: Consensus Statement endorsed by European Society for Paediatric Research (ESPR) and European Society for Neonatology (ESN). Pediatr. Res. 2016, 80, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.; Seri, I.; Marek, J.; Arlettaz, R.; Barker, P.; McNamara, P.; Moon-Grady, A.J.; Coon, P.D.; Noori, S.; Simpson, J.; et al. Targeted neonatal echocardiography in the neonatal intensive care unit: Practice guidelines and recommendations for training. Eur. J. Echocardiogr. 2011, 12, 715–736. [Google Scholar] [CrossRef] [PubMed]
- van Laere, D.; van Overmeire, B.; Gupta, S.; El-Khuffash, A.; Savoia, M.; McNamara, P.J.; Schwarz, C.E.; de Boode, W.P. Application of NPE in the assessment of a patent ductus arteriosus. Pediatr. Res. 2018, 84 (Suppl. S1), 46–56. [Google Scholar] [CrossRef]
- Levy, P.T.; Tissot, C.; Horsberg Eriksen, B.; Nestaas, E.; Rogerson, S.; McNamara, P.J.; El-Khuffash, A.; de Boode, W.P. Application of Neonatologist Performed Echocardiography in the Assessment and Management of Neonatal Heart Failure unrelated to Congenital Heart Disease. Pediatr. Res. 2018, 84 (Suppl. S1), 78–88. [Google Scholar] [CrossRef]
- Smith, A.; El-Khuffash, A.F. Defining “Haemodynamic Significance” of the Patent Ductus Arteriosus: Do We Have All the Answers? Neonatology 2020, 117, 225–232. [Google Scholar] [CrossRef]
- Singh, Y.; Fraisse, A.; Erdeve, O.; Atasay, B. Echocardiographic Diagnosis and Hemodynamic Evaluation of Patent Ductus Arteriosus in Extremely Low Gestational Age Newborn (ELGAN) Infants. Front. Pediatr. 2020, 8, 573627. [Google Scholar] [CrossRef]
- Arlettaz, R. Echocardiographic Evaluation of Patent Ductus Arteriosus in Preterm Infants. Front. Pediatr. 2017, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.N.; Bhombal, S.; Benitz, W.E. PDA: To treat or not to treat. Congenit. Heart Dis. 2019, 14, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; Onland, W.; Kooi, E.M.W.; Vijlbrief, D.C.; de Vries, W.B.; Dijkman, K.P.; van Kaam, A.H.; Villamor, E.; Kroon, A.A.; Visser, R.; et al. Expectant Management or Early Ibuprofen for Patent Ductus Arteriosus. N. Engl. J. Med. 2022, 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- More, K.; Soni, R.; Gupta, S. The role of bedside functional echocardiography in the assessment and management of pulmonary hypertension. Semin. Fetal Neonatal Med. 2022, 27, 101366. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Katheria, A.C.; Vora, F. Advances in Diagnosis and Management of Hemodynamic Instability in Neonatal Shock. Front. Pediatr. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- de Boode, W.P.; van der Lee, R.; Horsberg Eriksen, B.; Nestaas, E.; Dempsey, E.; Singh, Y.; Austin, T.; El-Khuffash, A. The role of Neonatologist Performed Echocardiography in the assessment and management of neonatal shock. Pediatr. Res. 2018, 84 (Suppl. S1), 57–67. [Google Scholar] [CrossRef]
- Millington, S.J.; Arntfield, R.T.; Guo, R.J.; Koenig, S.; Kory, P.; Noble, V.; Mallemat, H.; Schoenherr, J.R. The Assessment of Competency in Thoracic Sonography (ACTS) scale: Validation of a tool for point-of-care ultrasound. Crit. Ultrasound J. 2017, 9, 25. [Google Scholar] [CrossRef]
- Liu, J.; Guo, G.; Kurepa, D.; Volpicelli, G.; Sorantin, E.; Lovrenski, J.; Alonso-Ojembarrena, A.; Hsieh, K.S.; Lodha, A.; Yeh, T.F.; et al. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J. Matern.-Fetal Neonatal Med. 2022, 35, 1003–1016. [Google Scholar] [CrossRef]
- Liu, J.; Copetti, R.; Sorantin, E.; Lovrenski, J.; Rodriguez-Fanjul, J.; Kurepa, D.; Feng, X.; Cattaross, L.; Zhang, H.; Hwang, M.; et al. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J. Vis. Exp. JoVE 2019, 145, e58990. [Google Scholar] [CrossRef]
- Gomond-Le Goff, C.; Vivalda, L.; Foligno, S.; Loi, B.; Yousef, N.; De Luca, D. Effect of Different Probes and Expertise on the Interpretation Reliability of Point-of-Care Lung Ultrasound. Chest 2020, 157, 924–931. [Google Scholar] [CrossRef]
- Louis, D.; Belen, K.; Farooqui, M.; Idiong, N.; Amer, R.; Hussain, A.; ElSayed, Y. Prone versus Supine Position for Lung Ultrasound in Neonates with Respiratory Distress. Am. J. Perinatol. 2021, 38, 176–181. [Google Scholar] [CrossRef]
- Soldati, G.; Inchingolo, R.; Smargiassi, A.; Sher, S.; Nenna, R.; Inchingolo, C.D.; Valente, S. Ex vivo lung sonography: Morphologic-ultrasound relationship. Ultrasound Med. Biol. 2012, 38, 1169–1179. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Nenna, R.; Valente, S.; Inchingolo, C.D.; Corbo, G.M. Lung ultrasonography may provide an indirect estimation of lung porosity and airspace geometry. Respiration 2014, 88, 458–468. [Google Scholar] [CrossRef]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2022, 42, 309–344. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Demi, L.; Inchingolo, R. Artifactual Lung Ultrasonography: It Is a Matter of Traps, Order, and Disorder. Appl. Sci. 2020, 10, 1570. [Google Scholar] [CrossRef]
- Miller, A. Practical approach to lung ultrasound. BJA Educ. 2015, 16, 39–45. [Google Scholar] [CrossRef]
- Slavin, G.; Kreel, L.; Herbert, A.; Sandin, B. Pulmonary oedema at necropsy: A combined pathological and radiological method of study. J. Clin. Pathol. 1975, 28, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Demi, M.; Prediletto, R.; Soldati, G.; Demi, L. Physical Mechanisms Providing Clinical Information From Ultrasound Lung Images: Hypotheses and Early Confirmations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Inchingolo, R.; Smargiassi, A.; Demi, L. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J. Ultrasound Med. 2016, 35, 2075–2086. [Google Scholar] [CrossRef]
- Zong, H.; Huang, Z.; Zhao, J.; Lin, B.; Fu, Y.; Lin, Y.; Huang, P.; Sun, H.; Yang, C. The Value of Lung Ultrasound Score in Neonatology. Front. Pediatr. 2022, 10, 791664. [Google Scholar] [CrossRef]
- Brat, R.; Yousef, N.; Klifa, R.; Reynaud, S.; Shankar Aguilera, S.; De Luca, D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated With Continuous Positive Airway Pressure. JAMA Pediatr. 2015, 169, e151797. [Google Scholar] [CrossRef]
- Corsini, I.; Parri, N.; Ficial, B.; Dani, C. Lung ultrasound in the neonatal intensive care unit: Review of the literature and future perspectives. Pediatr. Pulmonol. 2020, 55, 1550–1562. [Google Scholar] [CrossRef]
- Ienghong, K.; Suzuki, T.; Celebi, I.; Bhudhisawasdi, V.; Tiamkao, S.; Gaysonsiri, D.; Apiratwarakul, K. B-Line Artifact as a Diagnostic Tool in Various Conditions at the Emergency Department. Open Access Maced. J. Med. Sci. 2021, 9, 369–372. [Google Scholar] [CrossRef]
- Gargani, L.; Bruni, C.; Romei, C.; Frumento, P.; Moreo, A.; Agoston, G.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Belloli, L.; et al. Prognostic Value of Lung Ultrasound B-Lines in Systemic Sclerosis. Chest 2020, 158, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound 2017, 20, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Vardar, G.; Karadag, N.; Karatekin, G. The Role of Lung Ultrasound as an Early Diagnostic Tool for Need of Surfactant Therapy in Preterm Infants with Respiratory Distress Syndrome. Am. J. Perinatol. 2021, 38, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Migliaro, F.; Corsini, I.; Meneghin, F.; Pierri, L.; Salomè, S.; Perri, A.; Aversa, S.; Nobile, S.; Lama, S.; et al. Neonatal Lung Ultrasound and Surfactant Administration: A Pragmatic, Multicenter Study. Chest 2021, 160, 2178–2186. [Google Scholar] [CrossRef]
- De Martino, L.; Yousef, N.; Ben-Ammar, R.; Raimondi, F.; Shankar-Aguilera, S.; De Luca, D. Lung Ultrasound Score Predicts Surfactant Need in Extremely Preterm Neonates. Pediatrics 2018, 142. [Google Scholar] [CrossRef]
- Oulego-Erroz, I.; Alonso-Quintela, P.; Terroba-Seara, S.; Jiménez-González, A.; Rodríguez-Blanco, S. Early assessment of lung aeration using an ultrasound score as a biomarker of developing bronchopulmonary dysplasia: A prospective observational study. J. Perinatol. 2021, 41, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ojembarrena, A.; Serna-Guerediaga, I.; Aldecoa-Bilbao, V.; Gregorio-Hernández, R.; Alonso-Quintela, P.; Concheiro-Guisán, A.; Ramos-Rodríguez, A.; de Las Heras-Martín, M.; Rodeño-Fernández, L.; Oulego-Erroz, I. The Predictive Value of Lung Ultrasound Scores in Developing Bronchopulmonary Dysplasia: A Prospective Multicenter Diagnostic Accuracy Study. Chest 2021, 160, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Loi, B.; Vigo, G.; Baraldi, E.; Raimondi, F.; Carnielli, V.P.; Mosca, F.; De Luca, D. Lung Ultrasound to Monitor Extremely Preterm Infants and Predict Bronchopulmonary Dysplasia. A Multicenter Longitudinal Cohort Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Mohsen, N.; Diambomba, Y.; Lashin, A.; Louis, D.; Elsayed, Y.; Shah, P.S. Lung Ultrasound for Prediction of Bronchopulmonary Dysplasia in Extreme Preterm Neonates: A Prospective Diagnostic Cohort Study. J. Pediatr. 2021, 238, 187–192.e182. [Google Scholar] [CrossRef]
- Pezza, L.; Alonso-Ojembarrena, A.; Elsayed, Y.; Yousef, N.; Vedovelli, L.; Raimondi, F.; De Luca, D. Meta-Analysis of Lung Ultrasound Scores for Early Prediction of Bronchopulmonary Dysplasia. Ann. Am. Thorac. Soc. 2022, 19, 659–667. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, J.; Jordan, I.; Balaguer, M.; Batista-Muñoz, A.; Ramon, M.; Bobillo-Perez, S. Early surfactant replacement guided by lung ultrasound in preterm newborns with RDS: The ULTRASURF randomised controlled trial. Eur. J. Pediatr. 2020, 179, 1913–1920. [Google Scholar] [CrossRef]
- Raschetti, R.; Yousef, N.; Vigo, G.; Marseglia, G.; Centorrino, R.; Ben-Ammar, R.; Shankar-Aguilera, S.; De Luca, D. Echography-Guided Surfactant Therapy to Improve Timeliness of Surfactant Replacement: A Quality Improvement Project. J. Pediatr. 2019, 212, 137–143.e131. [Google Scholar] [CrossRef]
- Guo, B.B.; Pang, L.; Yang, B.; Zhang, C.; Chen, X.Y.; OuYang, J.B.; Wu, C.J. Lung Ultrasound for the Diagnosis and Management of Neonatal Respiratory Distress Syndrome: A Minireview. Front. Pediatr. 2022, 10, 864911. [Google Scholar] [CrossRef]
- Copetti, R.; Cattarossi, L.; Macagno, F.; Violino, M.; Furlan, R. Lung ultrasound in respiratory distress syndrome: A useful tool for early diagnosis. Neonatology 2008, 94, 52–59. [Google Scholar] [CrossRef]
- Corsini, I.; Parri, N.; Gozzini, E.; Coviello, C.; Leonardi, V.; Poggi, C.; Giacalone, M.; Bianconi, T.; Tofani, L.; Raimondi, F.; et al. Lung Ultrasound for the Differential Diagnosis of Respiratory Distress in Neonates. Neonatology 2019, 115, 77–84. [Google Scholar] [CrossRef]
- Liu, J.; Cao, H.Y.; Fu, W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J. Int. Med. Res. 2016, 44, 1534–1542. [Google Scholar] [CrossRef]
- He, L.; Sun, Y.; Sheng, W.; Yao, Q. Diagnostic performance of lung ultrasound for transient tachypnea of the newborn: A meta-analysis. PLoS ONE 2021, 16, e0248827. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Stoller, J.Z.; Fraga, M.V. Point-of-care ultrasound in the neonatal ICU. Curr. Opin. Pediatr. 2020, 32, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Tissot, C.; Fraga, M.V.; Yousef, N.; Cortes, R.G.; Lopez, J.; Sanchez-de-Toledo, J.; Brierley, J.; Colunga, J.M.; Raffaj, D.; et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 2020, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J. Recruitment by sustained inflation: Time for a change. Intensive Care Med. 2011, 37, 1572–1574. [Google Scholar] [CrossRef]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.J. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.; Ji, S.H.; Jang, Y.E.; Kim, E.H.; Kim, H.S.; Kim, J.T. Effect of an ultrasound-guided lung recruitment manoeuvre on postoperative atelectasis in children: A randomised controlled trial. Eur. J. Anaesthesiol. 2020, 37, 719–727. [Google Scholar] [CrossRef]
- Arbelot, C.; Ferrari, F.; Bouhemad, B.; Rouby, J.J. Lung ultrasound in acute respiratory distress syndrome and acute lung injury. Curr. Opin. Crit. Care 2008, 14, 70–74. [Google Scholar] [CrossRef]
- Abushady, N.M.; Awad, H.A.S.; Kamel, D.R.; Fouda, E.M.; Ahmed, N.T.; Dawoud, M.O. Role of lung ultrasound in the assessment of recruitment maneuvers in ventilated preterm neonates with respiratory distress syndrome and its correlation with tracheal IL-6 levels: A randomized controlled trial. J. Neonatal-Perinat. Med. 2021, 14, 369–374. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, J.; Corsini, I.; Ortí, C.S.; Bobillo-Perez, S.; Raimondi, F. Lung ultrasound to evaluate lung recruitment in neonates with respiratory distress (RELUS study). Pediatr. Pulmonol. 2022, 57, 2502–2510. [Google Scholar] [CrossRef]
- Pierro, M.; Chioma, R.; Ciarmoli, E.; Villani, P.; Storti, E.; Copetti, R. Lung ultrasound guided pulmonary recruitment during mechanical ventilation in neonates: A case series. J. Neonatal-Perinat. Med. 2022, 15, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Chioma, R.; Amabili, L.; Ciarmoli, E.; Copetti, R.; Villani, P.; Stella, M.; Storti, E.; Pierro, M. The importance of lung recruitability: A novel ultrasound pattern to guide lung recruitment in neonates. J. Neonatal-Perinat. Med. 2022, 15, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, H.; Chen, H.; Wan, K.; Mao, R.; Xiao, P.; Chang, X. Application of ultrasonography in neonatal lung disease: An updated review. Front. Pediatr. 2022, 10, 1020437. [Google Scholar] [CrossRef]
- Chioma, R.; Amabili, L.; Ciarmoli, E.; Copetti, R.; Villani, P.G.; Natile, M.; Vento, G.; Storti, E.; Pierro, M. Lung UltraSound Targeted Recruitment (LUSTR): A Novel Protocol to Optimize Open Lung Ventilation in Critically Ill Neonates. Children 2022, 9, 1035. [Google Scholar] [CrossRef]
- Tusman, G.; Acosta, C.M.; Böhm, S.H.; Waldmann, A.D.; Ferrando, C.; Marquez, M.P.; Sipmann, F.S. Postural lung recruitment assessed by lung ultrasound in mechanically ventilated children. Crit. Ultrasound J. 2017, 9, 22. [Google Scholar] [CrossRef]
- He, P.; Wu, C.; Yang, Y.; Zheng, J.; Dong, W.; Wu, J.; Sun, Y.; Zhang, M. Effectiveness of postural lung recruitment on postoperative atelectasis assessed by lung ultrasound in children undergoing lateral thoracotomy cardiac surgery with cardiopulmonary bypass. Pediatr. Pulmonol. 2021, 56, 1724–1732. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, E.J.; Heo, B.Y.; Huh, J.; Min, J.J. Physiological benefits of lung recruitment in the semi-lateral position after laparoscopic surgery: A randomized controlled study. Sci. Rep. 2022, 12, 3909. [Google Scholar] [CrossRef]
- Rivas-Fernandez, M.; Roqué, I.F.M.; Diez-Izquierdo, A.; Escribano, J.; Balaguer, A. Infant position in neonates receiving mechanical ventilation. Cochrane Database Syst. Rev. 2016, 11, Cd003668. [Google Scholar] [CrossRef]
- Martinsson, A.; Houltz, E.; Wallinder, A.; Lindgren, S.; Thorén, A. Lung recruitment in the prone position after cardiac surgery: A randomised controlled study. Br. J. Anaesth. 2021, 126, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Rival, G.; Patry, C.; Floret, N.; Navellou, J.C.; Belle, E.; Capellier, G. Prone position and recruitment manoeuvre: The combined effect improves oxygenation. Crit. Care 2011, 15, R125. [Google Scholar] [CrossRef]
- Acosta, C.M.; Volpicelli, G.; Rudzik, N.; Venturin, N.; Gerez, S.; Ricci, L.; Natal, M.; Tusman, G. Feasibility of postural lung recruitment maneuver in children: A randomized, controlled study. Ultrasound J. 2020, 12, 34. [Google Scholar] [CrossRef]
- Alhassen, Z.; Vali, P.; Guglani, L.; Lakshminrusimha, S.; Ryan, R.M. Recent Advances in Pathophysiology and Management of Transient Tachypnea of Newborn. J. Perinatol. 2021, 41, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Omran, A.; AbdAllah, N.B.; Ibrahim, M.; El-Sharkawy, S. Lung ultrasound in early diagnosis of neonatal transient tachypnea and its differentiation from other causes of neonatal respiratory distress. J. Neonatal-Perinat. Med. 2018, 11, 281–287. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, H.M.A.; Aladawy, M.A.; Mansor, T.M.; Abo El Magd, H.A. Comparative Study between Chest X-Ray and Lung Ultrasound in Neonatal Respiratory Distress. Ann. Neonatol. J. 2021, 3, 125–143. [Google Scholar] [CrossRef]
- Chen, S.W.; Fu, W.; Liu, J.; Wang, Y. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine. 2017, 96, e5826. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Chioma, R.; Benincasa, C.; Gagliardi, G.; Amabili, L.; Lelli, F.; De Luca, G.; Storti, E. Cardiopulmonary Ultrasound Patterns of Transient Acute Respiratory Distress of the Newborn: A Retrospective Pilot Study. Children 2023, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Jensen, E.A.; White, A.M.; Wang, Y.; Biko, D.M.; Nilan, K.; Fraga, M.V.; Mercer-Rosa, L.; Zhang, H.; Kirpalani, H. Characterization of Disease Phenotype in Very Preterm Infants with Severe Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2020, 201, 1398–1406. [Google Scholar] [CrossRef]
- Pierro, M.; Villamor-Martinez, E.; van Westering-Kroon, E.; Alvarez-Fuente, M.; Abman, S.H.; Villamor, E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: A systematic review, meta-analysis and meta-regression. Thorax 2022, 77, 268–275. [Google Scholar] [CrossRef]
- Pierro, M.; Van Mechelen, K.; van Westering-Kroon, E.; Villamor-Martínez, E.; Villamor, E. Endotypes of Prematurity and Phenotypes of Bronchopulmonary Dysplasia: Toward Personalized Neonatology. J. Pers. Med. 2022, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Sallmon, H.; Roehr, C.C.; Kourembanas, S.; Austin, E.D.; Koestenberger, M. Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr. Res. 2021, 89, 446–455. [Google Scholar] [CrossRef]
- Nakos, G.; Tsangaris, I.; Kostanti, E.; Nathanail, C.; Lachana, A.; Koulouras, V.; Kastani, D. Effect of the prone position on patients with hydrostatic pulmonary edema compared with patients with acute respiratory distress syndrome and pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2000, 161, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Brion, L.P. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 2011, 2011, Cd001453. [Google Scholar] [CrossRef] [PubMed]
- Late hydrocortisone does not prevent bronchopulmonary dysplasia. J. Paediatr. Child Health 2022, 58, 1279. [CrossRef] [PubMed]
- Bruno, G.; Chioma, R.; Storti, E.; De Luca, G.; Fantinato, M.; Antonazzo, P.; Pierro, M. Targeted management of evolving and established chronic lung disease of prematurity assisted by cardiopulmonary ultrasound: A case report of four patients. Front. Pediatr. 2023, 10, 1112313. [Google Scholar] [CrossRef]
- Yu, L.F.; Xu, C.K.; Zhao, M.; Niu, L.; Huang, X.M.; Zhang, Z.Q. Bedside cardiopulmonary ultrasonography evaluates lung water content in very low-weight preterm neonates with patent ductus arteriosus. World J. Clin. Cases 2021, 9, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Vahdatpour, C.A.; Ryan, J.J.; Zimmerman, J.M.; MacCormick, S.J.; Palevsky, H.I.; Alnuaimat, H.; Ataya, A. Advanced airway management and respiratory care in decompensated pulmonary hypertension. Heart Fail. Rev. 2022, 27, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Bataille, B.; Riu, B.; Ferre, F.; Moussot, P.E.; Mari, A.; Brunel, E.; Ruiz, J.; Mora, M.; Fourcade, O.; Genestal, M.; et al. Integrated use of bedside lung ultrasound and echocardiography in acute respiratory failure: A prospective observational study in ICU. Chest 2014, 146, 1586–1593. [Google Scholar] [CrossRef]
- Li, L.; Ai, Y.; Wang, X.; Zhang, H.; Ma, X.; Huang, L.; Ai, M.; Peng, Q.; Zhang, L. Effect of focused cardiopulmonary ultrasonography on clinical outcome of septic shock: A randomized study. J. Int. Med. Res. 2021, 49, 3000605211013176. [Google Scholar] [CrossRef]
- Elsayed, Y.; Wahab, M.G.A.; Mohamed, A.; Fadel, N.B.; Bhombal, S.; Yousef, N.; Fraga, M.V.; Afifi, J.; Suryawanshi, P.; Hyderi, A.; et al. Point-of-care ultrasound (POCUS) protocol for systematic assessment of the crashing neonate-expert consensus statement of the international crashing neonate working group. Eur. J. Pediatr. 2022, 182, 53–66. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Huang, D.; Ma, H.; Xiao, Z.; Blaivas, M.; Chen, Y.; Wen, J.; Guo, W.; Liang, J.; Liao, X.; Wang, Z.; et al. Diagnostic value of cardiopulmonary ultrasound in elderly patients with acute respiratory distress syndrome. BMC Pulm. Med. 2018, 18, 136. [Google Scholar] [CrossRef]
- Lazzeri, C.; Bonizzoli, M.; Batacchi, S.; Socci, F.; Matucci-Cerinic, M.; Peris, A. Combined lung and cardiac ultrasound in COVID-related acute respiratory distress syndrome. Intern. Emerg. Med. 2021, 16, 1779–1785. [Google Scholar] [CrossRef]
- De Luca, D.; van Kaam, A.H.; Tingay, D.G.; Courtney, S.E.; Danhaive, O.; Carnielli, V.P.; Zimmermann, L.J.; Kneyber, M.C.J.; Tissieres, P.; Brierley, J.; et al. The Montreux definition of neonatal ARDS: Biological and clinical background behind the description of a new entity. Lancet. Respir. Med. 2017, 5, 657–666. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D.; Tingay, D.G.; van Kaam, A.H.; Courtney, S.E.; Kneyber, M.C.J.; Tissieres, P.; Tridente, A.; Rimensberger, P.C.; Pillow, J.J. Epidemiology of Neonatal Acute Respiratory Distress Syndrome: Prospective, Multicenter, International Cohort Study. Pediatr. Crit. Care Med. 2022, 23, 524–534. [Google Scholar] [CrossRef] [PubMed]
- El-Khuffash, A.; Herbozo, C.; Jain, A.; Lapointe, A.; McNamara, P. Targeted neonatal echocardiography (TnECHO) service in a Canadian neonatal intensive care unit: A 4-year experience. J. Perinatol. 2013, 33, 687–690. [Google Scholar] [CrossRef]
- Fathi, E.M.; Narchi, H.; Chedid, F. Noninvasive hemodynamic monitoring of septic shock in children. World J. Methodol. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Fu, W.; Yang, C.-S.; Huang, J.-J. Diagnosis of Neonatal Transient Tachypnea and Its Differentiation From Respiratory Distress Syndrome Using Lung Ultrasound. Medicine 2014, 93, e197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, W.; Qin, S.-J. Lung ultrasound to guide the administration of exogenous pulmonary surfactant in respiratory distress syndrome of newborn infants: A retrospective investigation study. Front. Pediatr. 2022, 10, 1768. [Google Scholar] [CrossRef]
- Olicker, A.; Raffay, T.; Ryan, R. Neonatal Respiratory Distress Secondary to Meconium Aspiration Syndrome. Children 2021, 8, 246. [Google Scholar] [CrossRef]
- Zani, A.; Chung, W.K.; Deprest, J.; Harting, M.T.; Jancelewicz, T.; Kunisaki, S.M.; Patel, N.; Antounians, L.; Puligandla, P.S.; Keijzer, R. Congenital diaphragmatic hernia. Nat. Rev. Dis. Prim. 2022, 8, 37. [Google Scholar] [CrossRef]
- Corsini, I.; Parri, N.; Coviello, C.; Leonardi, V.; Dani, C. Lung ultrasound findings in congenital diaphragmatic hernia. Eur. J. Pediatr. 2019, 178, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa-Bilbao, V.; Velilla, M.; Teresa-Palacio, M.; Esponera, C.B.; Barbero, A.H.; Sin-Soler, M.; Sanz, M.I.; Roigés, M.D.S. Lung Ultrasound in Bronchopulmonary Dysplasia: Patterns and Predictors in Very Preterm Infants. Neonatology 2021, 118, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Baston, C.; West, T.E. Lung ultrasound in acute respiratory distress syndrome and beyond. J. Thorac. Dis. 2016, 8, E1763–E1766. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, F.; Liu, Y.; Wang, H.-W.; Feng, Z.-C. Lung Ultrasonography for the Diagnosis of Severe Neonatal Pneumonia. Chest 2014, 146, 383–388. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarmoli, E.; Storti, E.; Cangemi, J.; Leone, A.; Pierro, M. Use of Cardio-Pulmonary Ultrasound in the Neonatal Intensive Care Unit. Children 2023, 10, 462. https://doi.org/10.3390/children10030462
Ciarmoli E, Storti E, Cangemi J, Leone A, Pierro M. Use of Cardio-Pulmonary Ultrasound in the Neonatal Intensive Care Unit. Children. 2023; 10(3):462. https://doi.org/10.3390/children10030462
Chicago/Turabian StyleCiarmoli, Elena, Enrico Storti, Jessica Cangemi, Arianna Leone, and Maria Pierro. 2023. "Use of Cardio-Pulmonary Ultrasound in the Neonatal Intensive Care Unit" Children 10, no. 3: 462. https://doi.org/10.3390/children10030462




