Genetic Testing in Children with Developmental and Epileptic Encephalopathies: A Review of Advances in Epilepsy Genomics
Abstract
1. Introduction
2. Background of Epilepsy Genetics
3. Advances in Disease-Related Gene Discovery
4. Major Advances in Epilepsy Genomics
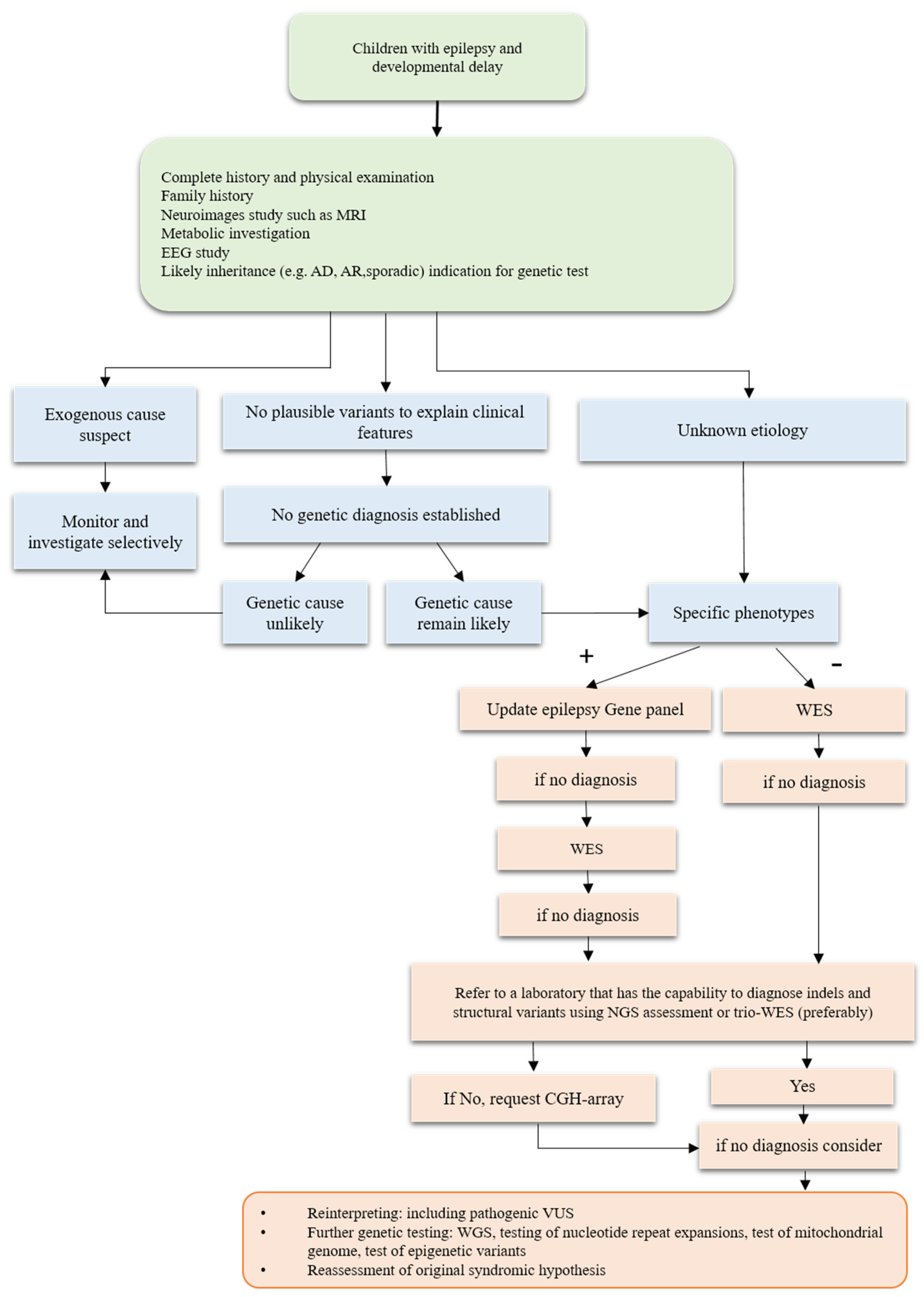
4.1. Clinical Testing including Comprehensive Gene Panels, Exomes, and Genomes
4.2. Impact of Genetic Testing on Diagnostic Rates and Disease Understanding
5. Discovery and Clinical Application of Genetic Testing for DEEs
5.1. Use of Genetic Testing for Precision Therapy Approaches
5.2. Limitations and Challenges of Genetic Testing in DEEs
- Limited availability of testing: In many regions, access to genetic testing is limited, particularly comprehensive testing, which may require specialized facilities and expertise.
- Interpreting test results: Interpreting genetic test results can be challenging, particularly when multiple genes are involved, and not all genetic variations have clear implications for the diagnosis or treatment of DEEs.
- False positive results: False positive results can occur, leading to anxiety and further testing of patients and families, which may result in the misallocation of limited healthcare resources.
- Limitations of current genetic tests: Current genetic tests are limited by their inability to detect some mutations and structural variations, and their potential for missing disease-causing mutations.
- Cost: The cost of genetic testing can be a barrier for some families, especially if insurance excludes it.
- Ethical concerns: Genetic testing raises ethical concerns, including the potential for discrimination based on genetic information, privacy, and security of genetic information and the potential for emotional distress caused by the knowledge of genetic risk.
- Difficulty extrapolating test results: Finally, it is difficult to extrapolate test results to guide therapeutic strategies. For example, even if a genetic mutation is identified, it may be unclear how it contributes to the development of DEEs or how it should inform therapeutic strategies.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perucca, P.; Bahlo, M.; Berkovic, S.F. The genetics of epilepsy. Annu. Rev. Genom. Hum. Genet. 2020, 21, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Thakran, S.; Guin, D.; Singh, P.; Singh, P.; Kukal, S.; Rawat, C.; Yadav, S.; Kushwaha, S.; Srivastava, A.; Hasija, Y.; et al. Genetic landscape of common epilepsies: Advancing towards precision in treatment. Int. J. Mol. Sci. 2020, 21, 7784. [Google Scholar] [CrossRef] [PubMed]
- Depositario-Cabacar, D.F.T.; Zelleke, T.G. Treatment of epilepsy in children with developmental disabilities. Dev. Disabil. Res. Rev. 2010, 16, 239–247. [Google Scholar] [CrossRef]
- Myers, C.T.; Mefford, H.C. Advancing epilepsy genetics in the genomic era. Genome Med. 2015, 7, 91. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Kwon, S. Early-onset epileptic encephalopathies and the diagnostic approach to underlying causes. Korean J. Pediatr. 2015, 58, 407. [Google Scholar] [CrossRef]
- Guerrini, R. Epilepsy in children. Lancet 2006, 367, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Dulac, O. Epileptic encephalopathy. Epilepsia 2001, 42, 23–26. [Google Scholar] [CrossRef]
- McTague, A.; Howell, K.B.; Cross, J.H.; Kurian, A.M.; Scheffer, E.I. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016, 15, 304–316. [Google Scholar] [CrossRef]
- Liang, J.-S.; Wang, J.-S.; Lin, L.-J.; Yang, M.-T.; Hung, K.-L.; Lu, J.-F. Genetic diagnosis in children with epilepsy and developmental delay/mental retardation using targeted gene panel analysis. Neuropsychiatry 2018, 8, 1577–1585. [Google Scholar] [CrossRef]
- Hebbar, M.; Mefford, H.C. Recent advances in epilepsy genomics and genetic testing. F1000Research 2020, 9, 185. [Google Scholar] [CrossRef]
- Souche, E.; Beltran, S.; Brosens, E.; Belmont, J.W.; Fossum, M.; Riess, O.; Gilissen, C.; Ardeshirdavani, A.; Houge, G.; van Gijn, M.; et al. Recommendations for whole genome sequencing in diagnostics for rare diseases. Eur. J. Hum. Genet. 2022, 30, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.F.; Fitzgerald, T.W.; Jones, W.D.; Clayton, S.; McRae, J.F.; van Kogelenberg, M.; King, D.A.; Ambridge, K.; Barrett, D.M.; Bayzetinova, T.; et al. Genetic diagnosis of developmental disorders in the DDD study: A scalable analysis of genome-wide research data. Lancet 2015, 385, 1305–1314. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Møller, R.S.; Larsen, L.H.; Johannesen, K.M.; Talvik, I.; Talvik, T.; Vaher, U.; Miranda, M.J.; Farooq, M.; Nielsen, J.E.; Svendsen, L.L.; et al. Gene panel testing in epileptic encephalopathies and familial epilepsies. Mol. Syndromol. 2016, 7, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Lindy, A.S.; Stosser, M.B.; Butler, E.; Downtain-Pickersgill, C.; Shanmugham, A.; Retterer, K.; Brandt, T.; Richard, G.; McKnight, D.A. Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 2018, 59, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Mercimek-Mahmutoglu, S.; Patel, J.; Cordeiro, D.; Hewson, S.; Callen, D.; Donner, E.J.; Hahn, C.; Kannu, P.; Kobayashi, J.; Minassian, B.A.; et al. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia 2015, 56, 707–716. [Google Scholar] [CrossRef]
- Ortega-Moreno, L.; Giráldez, B.G.; Soto-Insuga, V.; Pozo, R.L.-D.; Rodrigo-Moreno, M.; Alarcón-Morcillo, C.; Sánchez-Martín, G.; Díaz-Gómez, E.; Guerrero-López, R.; Serratosa, J.M.; et al. Molecular diagnosis of patients with epilepsy and developmental delay using a customized panel of epilepsy genes. PLoS ONE 2017, 12, e0188978. [Google Scholar] [CrossRef]
- Mei, D.; Parrini, E.; Marini, C.; Guerrini, R. The impact of next-generation sequencing on the diagnosis and treatment of epilepsy in paediatric patients. Mol. Diagn. Ther. 2017, 21, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Curatolo, P. Developmental and epileptic encephalopathies: What we do and do not know. Brain 2021, 144, 32–43. [Google Scholar] [CrossRef]
- Snoeijen-Schouwenaars, F.M.; van Ool, J.S.; Verhoeven, J.S.; van Mierlo, P.; Braakman, H.M.H.; Smeets, E.E.; Nicolai, J.; Schoots, J.; Teunissen, M.W.A.; Rouhl, R.P.W.; et al. Diagnostic exome sequencing in 100 consecutive patients with both epilepsy and intellectual disability. Epilepsia 2019, 60, 155–164. [Google Scholar] [CrossRef]
- Costain, G.; Cordeiro, D.; Matviychuk, D.; Mercimek-Andrews, S. Clinical application of targeted next-generation sequencing panels and whole exome sequencing in childhood epilepsy. Neuroscience 2019, 418, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.L.; Hagman, K.D.F.; Shinde, D.N.; Mroske, C.; Powis, Z.; Li, S.; Tang, S.; Helbig, I. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet. Med. 2016, 18, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Brea-Fernández, A.J.; Álvarez-Barona, M.; Amigo, J.; Tubío-Fungueiriño, M.; Caamaño, P.; Fernández-Prieto, M.; Barros, F.; De Rubeis, S.; Buxbaum, J.; Carracedo, Á. Trio-based exome sequencing reveals a high rate of the de novo variants in intellectual disability. Eur. J. Hum. Genet. 2022, 30, 938–945. [Google Scholar] [CrossRef]
- Lemke, J.R.; Riesch, E.; Scheurenbrand, T.; Schubach, M.; Wilhelm, C.; Steiner, I.; Hansen, J.; Courage, C.; Gallati, S.; Bürki, S.; et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia 2012, 53, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.C.; Kim, G.E.; Pagnamenta, A.T.; Murakami, Y.; Carvill, G.L.; Meyer, E.; Copley, R.R.; Rimmer, A.; Barcia, G.; Fleming, M.R.; et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum. Mol. Genet. 2014, 23, 3200–3211. [Google Scholar] [CrossRef] [PubMed]
- Mefford, H.C. The road to diagnosis: Shortening the diagnostic odyssey in epilepsy. Epilepsy Curr. 2019, 19, 307–309. [Google Scholar] [CrossRef]
- Fernández, I.S.; Loddenkemper, T.; Gaínza-Lein, M.; Sheidley, B.R.; Poduri, A. Diagnostic yield of genetic tests in epilepsy: A meta-analysis and cost-effectiveness study. Neurology 2019, 92, e418–e428. [Google Scholar] [CrossRef]
- Bayat, A.; Bayat, M.; Rubboli, G.; Møller, R. Epilepsy syndromes in the first year of life and usefulness of genetic testing for precision therapy. Genes 2021, 12, 1051. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, Y.; Wang, L.; Wang, Y.; Gong, Z.; Qiu, W.; Wang, J.; Zhang, H.; Ji, X.; Ye, J.; et al. Chromosomal microarray analysis in developmental delay and intellectual disability with comorbid conditions. BMC Med. Genom. 2018, 11, 49. [Google Scholar] [CrossRef]
- Ho, K.S.; Wassman, E.R.; Baxter, A.L.; Hensel, C.H.; Martin, M.M.; Prasad, A.; Twede, H.; Vanzo, R.J.; Butler, M.G. Chromosomal microarray analysis of consecutive individuals with autism spectrum disorders using an ultra-high resolution chromosomal microarray optimized for neurodevelopmental disorders. Int. J. Mol. Sci. 2016, 17, 2070. [Google Scholar] [CrossRef]
- Roberts, J.L.; Hovanes, K.; Dasouki, M.; Manzardo, A.M.; Butler, M.G. Chromosomal microarray analysis of consecutive individuals with autism spectrum disorders or learning disability presenting for genetic services. Gene 2014, 535, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Shoukier, M.; Klein, N.; Auber, B.; Wickert, J.; Schröder, J.; Zoll, B.; Burfeind, P.; Bartels, I.; Alsat, E.A.; Lingen, M.; et al. Array CGH in patients with developmental delay or intellectual disability: Are there phenotypic clues to pathogenic copy number variants? Clin. Genet. 2013, 83, 53–65. [Google Scholar] [CrossRef]
- Trujillano, D.; Bertoli-Avella, A.M.; Kandaswamy, K.K.; Weiss, M.E.; Köster, J.; Marais, A.; Paknia, O.; Schröder, R.; Garcia-Aznar, J.M.; Werber, M.; et al. Clinical exome sequencing: Results from 2819 samples reflecting 1000 families. Eur. J. Hum. Genet. 2017, 25, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Shashi, V.; McConkie-Rosell, A.; Schoch, K.; Kasturi, V.; Rehder, C.; Jiang, Y.; Goldstein, D.; McDonald, M. Practical considerations in the clinical application of whole-exome sequencing. Clin. Genet. 2016, 89, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Cohen, J.S.; Vernon, H.; Barañano, K.; McClellan, R.; Jamal, L.; Naidu, S.; Fatemi, A. Clinical whole exome sequencing in child neurology practice. Ann. Neurol. 2014, 76, 473–483. [Google Scholar] [CrossRef]
- Falco-Walter, J.J.; Scheffer, I.E.; Fisher, R.S. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018, 139, 73–79. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Liao, J. Deciphering the concepts behind “Epileptic encephalopathy” and “Developmental and epileptic encephalopathy”. Eur. J. Paediatr. Neurol. 2020, 24, 11–14. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Trivisano, M.; Specchio, N. What are the epileptic encephalopathies? Curr. Opin. Neurol. 2020, 33, 179–184. [Google Scholar] [CrossRef]
- Raga, S.; Specchio, N.; Rheims, S.; Wilmshurst, J.M. Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021, 23, 40–52. [Google Scholar] [CrossRef]
- Happ, H.C.; Carvill, G.L. A 2020 view on the genetics of developmental and epileptic encephalopathies. Epilepsy Curr. 2020, 20, 90–96. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Liao, J. When monogenic isn’t monogenic—Unravelling the oligogenic architecture of the developmental and epileptic encephalopathies. Epilepsy Curr. 2019, 19, 417–419. [Google Scholar] [CrossRef]
- Mina, E.D.; Ciccone, R.; Brustia, F.; Bayindir, B.; Limongelli, I.; Vetro, A.; Iascone, M.; Pezzoli, L.; Bellazzi, R.; Perotti, G.; et al. Improving molecular diagnosis in epilepsy by a dedicated high-throughput sequencing platform. Eur. J. Hum. Genet. 2015, 23, 354–362. [Google Scholar] [CrossRef]
- De Lange, I.M.; Gunning, B.; Sonsma, A.C.M.; van Gemert, L.; van Kempen, M.; Verbeek, N.E.; Nicolai, J.; Knoers, N.V.A.M.; Koeleman, B.P.C.; Brilstra, E.H. Influence of contraindicated medication use on cognitive outcome in Dravet syndrome and age at first afebrile seizure as a clinical predictor in SCN 1A-related seizure phenotypes. Epilepsia 2018, 59, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.I.; Isom, L.L.; Petrou, S. Role of sodium channels in epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6, a022814. [Google Scholar] [CrossRef]
- Villa, C.; Combi, R. Potassium channels and human epileptic phenotypes: An updated overview. Front. Cell. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Rusconi, R.; Scalmani, P.; Avanzini, G.; Franceschetti, S. Epileptogenic ion channel mutations: From bedside to bench and, hopefully, back again. Epilepsy Res. 2010, 92, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Heyne, H.O.; EuroEPINOMICS RES Consortium; Singh, T.; Stamberger, H.; Jamra, R.A.; Caglayan, H.; Craiu, D.; De Jonghe, P.; Guerrini, R.; Helbig, K.L.; et al. De novo variants in neurodevelopmental disorders with epilepsy. Nat. Genet. 2018, 50, 1048–1053. [Google Scholar] [CrossRef]
- Helbig, K.L.; Lauerer, R.; Bahr, J.C.; Souza, I.A.; Myers, C.T.; Uysal, B.; Schwarz, N.; Gandini, M.A.; Huang, S.; Keren, B.; et al. De novo pathogenic variants in CACNA1E cause developmental and epileptic encephalopathy with contractures, macrocephaly, and dyskinesias. Am. J. Hum. Genet. 2018, 103, 666–678. [Google Scholar] [CrossRef]
- Mulhern, M.S.; Stumpel, C.; Stong, N.; Brunner, H.G.; Bier, L.; Lippa, N.; Riviello, J.; Rouhl, R.P.W.; Kempers, M.; Pfundt, R.; et al. NBEA: Developmental disease gene with early generalized epilepsy phenotypes. Ann. Neurol. 2018, 84, 788–795. [Google Scholar] [CrossRef]
- Gregor, A.; Sadleir, L.G.; Asadollahi, R.; Azzarello-Burri, S.; Battaglia, A.; Ousager, L.B.; Boonsawat, P.; Bruel, A.-L.; Buchert, R.; Calpena, E.; et al. De novo variants in the F-box protein FBXO11 in 20 individuals with a variable neurodevelopmental disorder. Am. J. Hum. Genet. 2018, 103, 305–316. [Google Scholar] [CrossRef]
- Machol, K.; Rousseau, J.; Ehresmann, S.; Garcia, T.; Nguyen, T.T.M.; Spillmann, R.C.; Sullivan, J.A.; Shashi, V.; Jiang, Y.-H.; Stong, N.; et al. Expanding the spectrum of BAF-related disorders: De novo variants in SMARCC2 cause a syndrome with intellectual disability and developmental delay. Am. J. Hum. Genet. 2019, 104, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Castermans, D.; Wilquet, V.; Parthoens, E.; Huysmans, C.; Steyaert, J.; Swinnen, L.; Fryns, J.-P.; Van de Ven, W.; Devriendt, K. The neurobeachin gene is disrupted by a translocation in a patient with idiopathic autism. J. Med. Genet. 2003, 40, 352–356. [Google Scholar] [CrossRef]
- Calhoun, J.D.; Carvill, G.L. Unravelling the genetic architecture of autosomal recessive epilepsy in the genomic era. J. Neurogenet. 2018, 32, 295–312. [Google Scholar] [CrossRef]
- Murakami, Y.; Nguyen, T.T.M.; Baratang, N.; Raju, P.K.; Knaus, A.; Ellard, S.; Jones, G.; Lace, B.; Rousseau, J.; Ajeawung, N.F.; et al. Mutations in PIGB cause an inherited GPI biosynthesis defect with an axonal neuropathy and metabolic abnormality in severe cases. Am. J. Hum. Genet. 2019, 105, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Epilepsy Phenome/Genome Project & Epi4K Consortium. Copy number variant analysis from exome data in 349 patients with epileptic encephalopathy. Ann. Neurol. 2015, 78, 323–328. [Google Scholar] [CrossRef]
- Mefford, H.C.; Yendle, S.C.; Hsu, C.; Cook, J.; Ba, E.G.; Bsc, J.M.M.; Eeg-Olofsson, O.; Sadleir, L.G.; Gill, D.; Ben-Zeev, B.; et al. Rare copy number variants are an important cause of epileptic encephalopathies. Ann. Neurol. 2011, 70, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Shen, Y.; Avallone, J.; Sheidley, B.R.; Pinsky, R.; Bergin, A.M.; Berry, G.T.; Duffy, F.H.; Eksioglu, Y.; Harris, D.J.; et al. Copy number variation plays an important role in clinical epilepsy. Ann. Neurol. 2014, 75, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Orsini, A.; Zara, F.; Striano, P. Recent advances in epilepsy genetics. Neurosci. Lett. 2018, 667, 4–9. [Google Scholar] [CrossRef]
- Pfundt, R.; del Rosario, M.; Vissers, L.E.; Kwint, M.P.; Janssen, I.M.; de Leeuw, N.; Yntema, H.G.; Nelen, M.R.; Lugtenberg, D.; Kamsteeg, E.-J.; et al. Detection of clinically relevant copy-number variants by exome sequencing in a large cohort of genetic disorders. Genet. Med. 2017, 19, 667–675. [Google Scholar] [CrossRef]
- Schuster, J.; Laan, L.; Klar, J.; Jin, Z.; Huss, M.; Korol, S.; Noraddin, F.H.; Sobol, M.; Birnir, B.; Dahl, N. Transcriptomes of Dravet syndrome iPSC derived GABAergic cells reveal dysregulated pathways for chromatin remodeling and neurodevelopment. Neurobiol. Dis. 2019, 132, 104583. [Google Scholar] [CrossRef]
- LaCroix, A.J.; Stabley, D.; Sahraoui, R.; Adam, M.P.; Mehaffey, M.; Kernan, K.; Myers, C.T.; Fagerstrom, C.; Anadiotis, G.; Akkari, Y.M.; et al. GGC repeat expansion and exon 1 methylation of XYLT1 is a common pathogenic variant in Baratela-Scott syndrome. Am. J. Hum. Genet. 2019, 104, 35–44. [Google Scholar] [CrossRef]
- Barbosa, M.; Joshi, R.S.; Garg, P.; Martin-Trujillo, A.; Patel, N.; Jadhav, B.; Watson, C.T.; Gibson, W.; Chetnik, K.; Tessereau, C.; et al. Identification of rare de novo epigenetic variations in congenital disorders. Nat. Commun. 2018, 9, 2064. [Google Scholar] [CrossRef]
- Aref-Eshghi, E.; Bend, E.G.; Colaiacovo, S.; Caudle, M.; Chakrabarti, R.; Napier, M.; Brick, L.; Brady, L.; Carere, D.A.; Levy, M.A.; et al. Diagnostic utility of genome-wide DNA methylation testing in genetically unsolved individuals with suspected hereditary conditions. Am. J. Hum. Genet. 2019, 104, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Iriart, J.A.B. Precision medicine/personalized medicine: A critical analysis of movements in the transformation of biomedicine in the early 21st century. Cad. De Saúde Publica 2019, 35, e00153118. [Google Scholar] [CrossRef] [PubMed]
- Reif, P.S.; Tsai, M.-H.; Helbig, I.; Rosenow, F.; Klein, K.M. Precision medicine in genetic epilepsies: Break of dawn? Expert Rev. Neurother. 2017, 17, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Essajee, F.; Urban, M.; Smit, L.; Wilmshurst, J.M.; Solomons, R.; van Toorn, R.; Moosa, S. Utility of genetic testing in children with developmental and epileptic encephalopathy (DEE) at a tertiary hospital in South Africa: A prospective study. Seizure 2022, 101, 197–204. [Google Scholar] [CrossRef]
- Brunklaus, A.; Schorge, S.; Smith, A.D.; Ghanty, I.; Stewart, K.; Gardiner, S.; Du, J.; Pérez-Palma, E.; Symonds, J.D.; Collier, A.C.; et al. SCN1A variants from bench to bedside—Improved clinical prediction from functional characterization. Hum. Mutat. 2020, 41, 363–374. [Google Scholar] [CrossRef]
- Ghazala, E.; Shahin, D.A.; Wahba, Y. Polymorphisms of the sodium voltage-gated channel, alpha subunit 1 (SCN1A-A3184G) gene among children with non-lesional epilepsy: A case-control study. Ital. J. Pediatr. 2022, 48, 157. [Google Scholar] [CrossRef]
- Brunklaus, A.; Pérez-Palma, E.; Ghanty, I.; Xinge, J.; Brilstra, E.; Ceulemans, B.; Chemaly, N.; de Lange, I.; Depienne, C.; Guerrini, R.; et al. Development and validation of a prediction model for early diagnosis of SCN1A-related epilepsies. Neurology 2022, 98, e1163–e1174. [Google Scholar] [CrossRef]
- Buck, M.L.; Goodkin, H.P. Stiripentol: A novel antiseizure medication for the management of Dravet syndrome. Ann. Pharmacother. 2019, 53, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Scheffer, I.E.; Sadleir, L.G. Efficacy of cannabinoids in paediatric epilepsy. Dev. Med. Child Neurol. 2019, 61, 13–18. [Google Scholar] [CrossRef]
- Satta, V.; Alonso, C.; Díez, P.; Martín-Suárez, S.; Rubio, M.; Encinas, J.M.; Fernández-Ruiz, J.; Sagredo, O. Neuropathological characterization of a Dravet syndrome knock-in mouse model useful for investigating cannabinoid treatments. Front. Mol. Neurosci. 2021, 13, 602801. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.A.; Jurado, M.; Thaxton, T.T.; Duarte, S.E.; Barse, L.; Tatsukawa, T.; Yamakawa, K.; Nishi, T.; Kondo, S.; Miyamoto, M.; et al. Soticlestat, a novel cholesterol 24-hydroxylase inhibitor, reduces seizures and premature death in Dravet syndrome mice. Epilepsia 2021, 62, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.D.; Jiang, Y.; Villanueva, V.; Zolnowska, M.; Arkilo, D.; Hsiao, S.; Asgharnejad, M.; Dlugos, D. A phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of soticlestat as adjunctive therapy in pediatric patients with Dravet syndrome or Lennox–Gastaut syndrome (ELEKTRA). Epilepsia 2022, 63, 2671–2683. [Google Scholar] [CrossRef]
- Hahn, C.D.; Jiang, Y.; Villanueva, V.; Zolnowska, M.; Arkilo, D.; Forgacs, P.B.; Asgharnejad, M.; Yan, Y.; Dlugos, D. Efficacy, safety and tolerability of soticlestat (TAK-935/OV935) as adjunctive therapy in pediatric patients with dravet syndrome and lennox-gastaut syndrome (ELEKTRA)(4234). Neurology 2021, 96 (Suppl. S15), 4234. [Google Scholar]
- Sharawat, I.K.; Panda, P.K.; Kasinathan, A.; Panda, P.; Dawman, L.; Joshi, K. Efficacy and tolerability of fenfluramine in patients with Dravet syndrome: A systematic review and meta-analysis. Seizure 2021, 85, 119–126. [Google Scholar] [CrossRef]
- Goldberg, E.M. Gene therapy in models of severe epilepsy due to sodium channelopathy. Epilepsy Curr. 2020, 20, 214–217. [Google Scholar] [CrossRef]
- Nissenkorn, A.; Kornilov, P.; Peretz, A.; Blumkin, L.; Heimer, G.; Ben-Zeev, B.; Attali, B. Personalized treatment with retigabine for pharmacoresistant epilepsy arising from a pathogenic variant in the KCNQ2 selectivity filter. Epileptic Disord. 2021, 23, 695–705. [Google Scholar] [CrossRef]
- Kenney, C.; French, J.; Porter, R.; Perucca, E.; Brodie, M.; Rogawski, M.A.; Harden, C.; Rosenblut, C.L.; Qian, J.; Leung, J.; et al. Rapid onset of efficacy of XEN1101, a novel potassium channel opener, in adults with focal epilepsy: Results from a phase 2b study (X-TOLE). In EPILEPSIA; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Landmark, C.J.; Potschka, H.; Auvin, S.; Wilmshurst, J.M.; Johannessen, S.I.; Trenité, D.K.; Wirrell, E.C. The role of new medical treatments for the management of developmental and epileptic encephalopathies: Novel concepts and results. Epilepsia 2021, 62, 857–873. [Google Scholar] [CrossRef]
- Curatolo, P.; Nabbout, R.; Lagae, L.; Aronica, E.; Ferreira, J.C.; Feucht, M.; Hertzberg, C.; Jansen, A.C.; Jansen, F.; Kotulska, K.; et al. Management of epilepsy associated with tuberous sclerosis complex: Updated clinical recommendations. Eur. J. Paediatr. Neurol. 2018, 22, 738–748. [Google Scholar] [CrossRef]
- Zybura, A.; Hudmon, A.; Cummins, T.R. Distinctive properties and powerful neuromodulation of Nav1. 6 sodium channels regulates neuronal excitability. Cells 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Aimiuwu, O.V.; Fowler, A.M.; Sah, M.; Teoh, J.J.; Kanber, A.; Pyne, N.K.; Petri, S.; Rosenthal-Weiss, C.; Yang, M.; Harper, S.Q.; et al. RNAi-based gene therapy rescues developmental and epileptic encephalopathy in a genetic mouse model. Mol. Ther. 2020, 28, 1706–1716. [Google Scholar] [CrossRef]
- Stamberger, H.; Weckhuysen, S.; De Jonghe, P. STXBP1 as a therapeutic target for epileptic encephalopathy. Expert Opin. Ther. Targets 2017, 21, 1027–1036. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Nabbout, R. Recent advances in the drug treatment of Dravet syndrome. CNS Drugs 2019, 33, 867–881. [Google Scholar] [CrossRef]
- Zimmern, V.; Minassian, B.; Korff, C. A Review of Targeted Therapies for Monogenic Epilepsy Syndromes. Front. Neurol. 2022, 13, 829116. [Google Scholar] [CrossRef]
- Samanta, D. Changing landscape of Dravet syndrome management: An overview. Neuropediatrics 2020, 51, 135–145. [Google Scholar] [CrossRef]
- Helbig, I.; Ellis, C.A. Personalized medicine in genetic epilepsies–possibilities, challenges, and new frontiers. Neuropharmacology 2020, 172, 107970. [Google Scholar] [CrossRef] [PubMed]
- Kuersten, M.; Tacke, M.; Gerstl, L.; Hoelz, H.; Stülpnagel, C.; Borggraefe, I. Antiepileptic therapy approaches in KCNQ2 related epilepsy: A systematic review. Eur. J. Med. Genet. 2020, 63, 103628. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020, 5, 354–365. [Google Scholar] [CrossRef]
- Lim, Z.; Wong, K.; Olson, H.E.; Bergin, A.M.; Downs, J.; Leonard, H. Use of the ketogenic diet to manage refractory epilepsy in CDKL 5 disorder: Experience of >100 patients. Epilepsia 2017, 58, 1415–1422. [Google Scholar] [CrossRef]
- Lotte, J.; Bast, T.; Borusiak, P.; Coppola, A.; Cross, H.; Dimova, P.; Fogarasi, A.; Graneß, I.; Guerrini, R.; Hjalgrim, H.; et al. Effectiveness of antiepileptic therapy in patients with PCDH19 mutations. Seizure 2016, 35, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Chang, T.; Liang, J.; Li, S. KCNQ2 mutations in childhood nonlesional epilepsy: Variable phenotypes and a novel mutation in a case series. Mol. Genet. Genom. Med. 2019, 7, e00816. [Google Scholar] [CrossRef]
- Absalom, N.L.; Liao, V.W.Y.; Johannesen, K.M.H.; Gardella, E.; Jacobs, J.; Lesca, G.; Gokce-Samar, Z.; Arzimanoglou, A.; Zeidler, S.; Striano, P.; et al. Gain-of-function and loss-of-function GABRB3 variants lead to distinct clinical phenotypes in patients with developmental and epileptic encephalopathies. Nat. Commun. 2022, 13, 1822. [Google Scholar] [CrossRef]
- Jiang, X.; Raju, P.K.; D’Avanzo, N.; Lachance, M.; Pepin, J.; Dubeau, F.; Mitchell, W.G.; Bello-Espinosa, L.E.; Pierson, T.M.; Minassian, B.A.; et al. Both gain-of-function and loss-of-function de novo CACNA 1A mutations cause severe developmental epileptic encephalopathies in the spectrum of Lennox-Gastaut syndrome. Epilepsia 2019, 60, 1881–1894. [Google Scholar] [CrossRef]
- Wolff, M.; Johannesen, K.M.; Hedrich, U.B.S.; Masnada, S.; Rubboli, G.; Gardella, E.; Lesca, G.; Ville, D.; Milh, M.; Villard, L.; et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain 2017, 140, 1316–1336. [Google Scholar] [CrossRef] [PubMed]
- Berecki, G.; Howell, K.B.; Deerasooriya, Y.H.; Cilio, M.R.; Oliva, M.K.; Kaplan, D.; Scheffer, I.E.; Berkovic, S.F.; Petrou, S. Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy. Proc. Natl. Acad. Sci. USA 2018, 115, E5516–E5525. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. Innovative technologies for diagnosis and screening of genetic diseases in antenatal age. J. Lab. Precis. Med. 2020, 5, 1–9. [Google Scholar] [CrossRef]
| Gene Panel | WES | |
|---|---|---|
| Cost | Low | High |
| Diagnostic rate | Usually 15–48%, but may be 0.8% at lowest | 25–44% |
| Cost | Low | High |
| Time | Rapid | Slow |
| Advantages | Rapid Cost-effective | Covers entire coding sequence Trio exome sequencing can discover de novo variants Allows for further reanalysis Can potentially be used to detect CNV |
| Disadvantages | Only test the genes on the panel The result may quite variable depend on the genes on the panel May miss as yet unknown disease causing genes Fewer VUS than WES | Incidental findings Interpretation of multiple VUS may be necessary May identify carrier status or non-paternity Unable to detect deep intronic mutations, structural rearrangements, or large deletions/duplications |
| Disease | Pathophysiological Background | Drug | Mechanisms of Action | Reference |
|---|---|---|---|---|
| Dravet syndrome | Haploinsufficiency of the voltage-gated sodium channel α subunit NaV1.1 | Stiripentol | Allosteric modulator of benzodiazepine-sensitive/benzodiazepine-insensitive GABAA receptor; activating ATP-sensitive potassium channels | [71] |
| Cannabidiol | GPR55, TRPV1, and adenosine reuptake | [72,73] | ||
| Soticlestat | Brain specific cholesterol 24-hydroxylase inhibitor; dose-dependently reduced plasma 24S-hydroxycholesterol; decreases excitability | [74,75,76] | ||
| Fenfluramine | Serotonin (5-HT) release; increases serotonergic signaling; more specific of 5-HT 1D and 5-HT 2C receptors | [77] | ||
| dCas9-mediated Scn1a gene activation system (murine model) | Stimulates Scn1a transcription | [78] | ||
| KCNQ2 mutation | KCNQ2 mutation | Ezogabine/retigabine | Specific activator of voltage- gated potassium Kv7.2/7.3 channels; decreases excitatory neurotransmission | [79] |
| KCNQ2 loss-of-function as a more precise indication; early infantile epileptic encephalopathy type 7 (BFNS) | XEN1101 | Selective potassium channel opener; decreases excitatory neurotransmission | [80,81] | |
| TSC | Mutations in TSC1 or TSC2 | Everolimus | mTOR inhibitor; mutations lead to excessive activation of mTOR signaling pathway, abnormal cell differentiation, altered plasticity, and inflammatory signaling | [82] |
| SCN8A mutation | Gain-of-function mutations encoding the Nav1.6 channel (EIEE13) | NBI 921352 (XEN901) | Selective inhibitor of voltage-gated sodium channel subtype Nav1.6, could address the cause of this condition | [81,83] |
| DEEs | De novo variants in the gene encoding dynamin-1 (DNM1) | RNAi-based gene therapy (murine model) | Dnm1-targeted therapeutic microRNA delivered by a self-complementary adeno-associated virus vector | [84] |
| STXBP1-encephalopathy | Mutations in STXBP1 | Specific protein–protein interaction inhibition and gene therapy | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Hong, S.-Y.; Lin, W.-D.; Lin, C.-H.; Lin, S.-S.; Tsai, F.-J.; Chou, I.-C. Genetic Testing in Children with Developmental and Epileptic Encephalopathies: A Review of Advances in Epilepsy Genomics. Children 2023, 10, 556. https://doi.org/10.3390/children10030556
Chang Y-T, Hong S-Y, Lin W-D, Lin C-H, Lin S-S, Tsai F-J, Chou I-C. Genetic Testing in Children with Developmental and Epileptic Encephalopathies: A Review of Advances in Epilepsy Genomics. Children. 2023; 10(3):556. https://doi.org/10.3390/children10030556
Chicago/Turabian StyleChang, Yu-Tzu, Syuan-Yu Hong, Wei-De Lin, Chien-Heng Lin, Sheng-Shing Lin, Fuu-Jen Tsai, and I-Ching Chou. 2023. "Genetic Testing in Children with Developmental and Epileptic Encephalopathies: A Review of Advances in Epilepsy Genomics" Children 10, no. 3: 556. https://doi.org/10.3390/children10030556
APA StyleChang, Y.-T., Hong, S.-Y., Lin, W.-D., Lin, C.-H., Lin, S.-S., Tsai, F.-J., & Chou, I.-C. (2023). Genetic Testing in Children with Developmental and Epileptic Encephalopathies: A Review of Advances in Epilepsy Genomics. Children, 10(3), 556. https://doi.org/10.3390/children10030556






