Early Skin-to-Skin Contact in Preterm Infants: Is It Safe? An Italian Experience
Abstract
1. Introduction
2. Materials and Methods
Study Design
3. Statistical Analysis
4. Results
4.1. Patients and SSC Session Characteristics
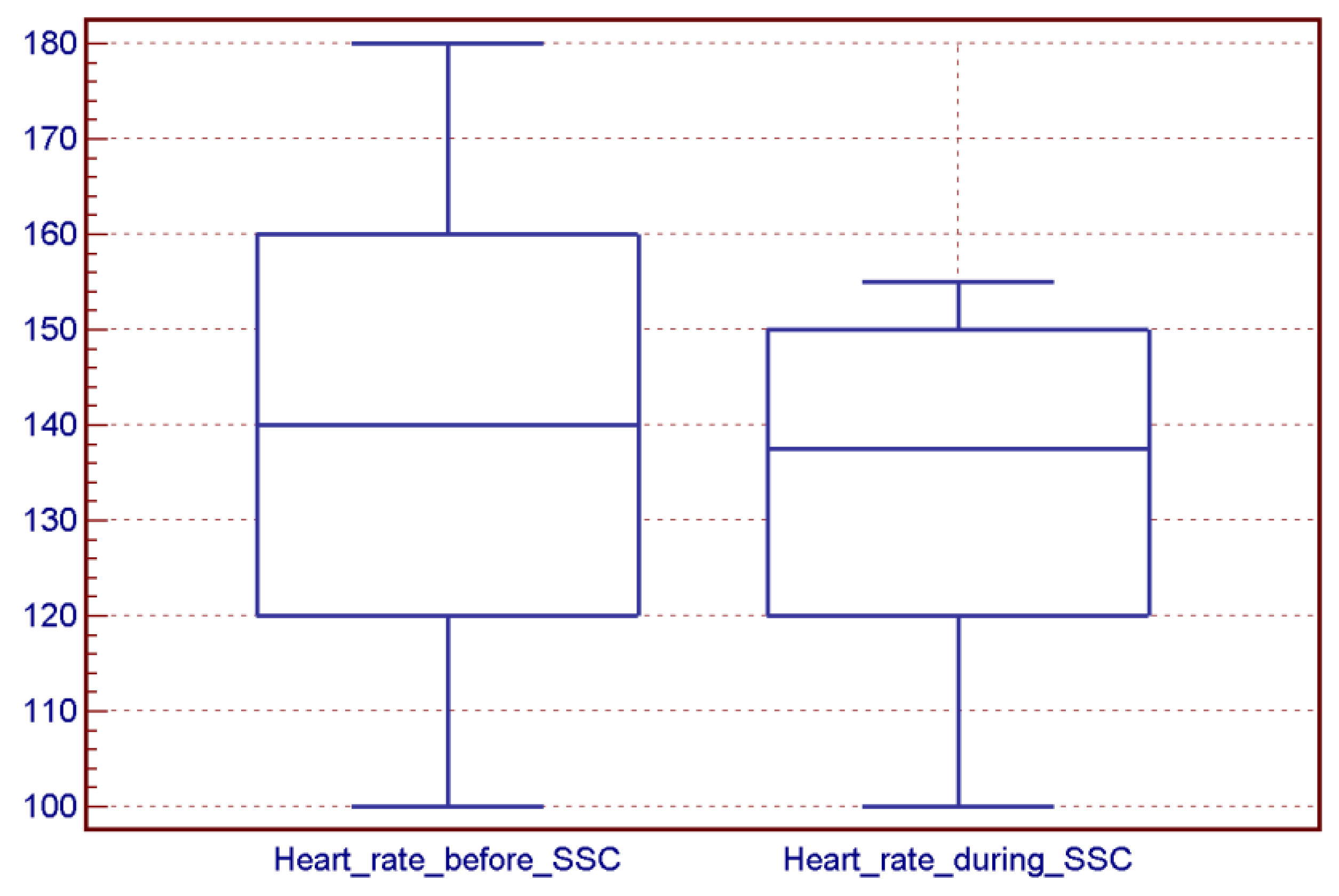
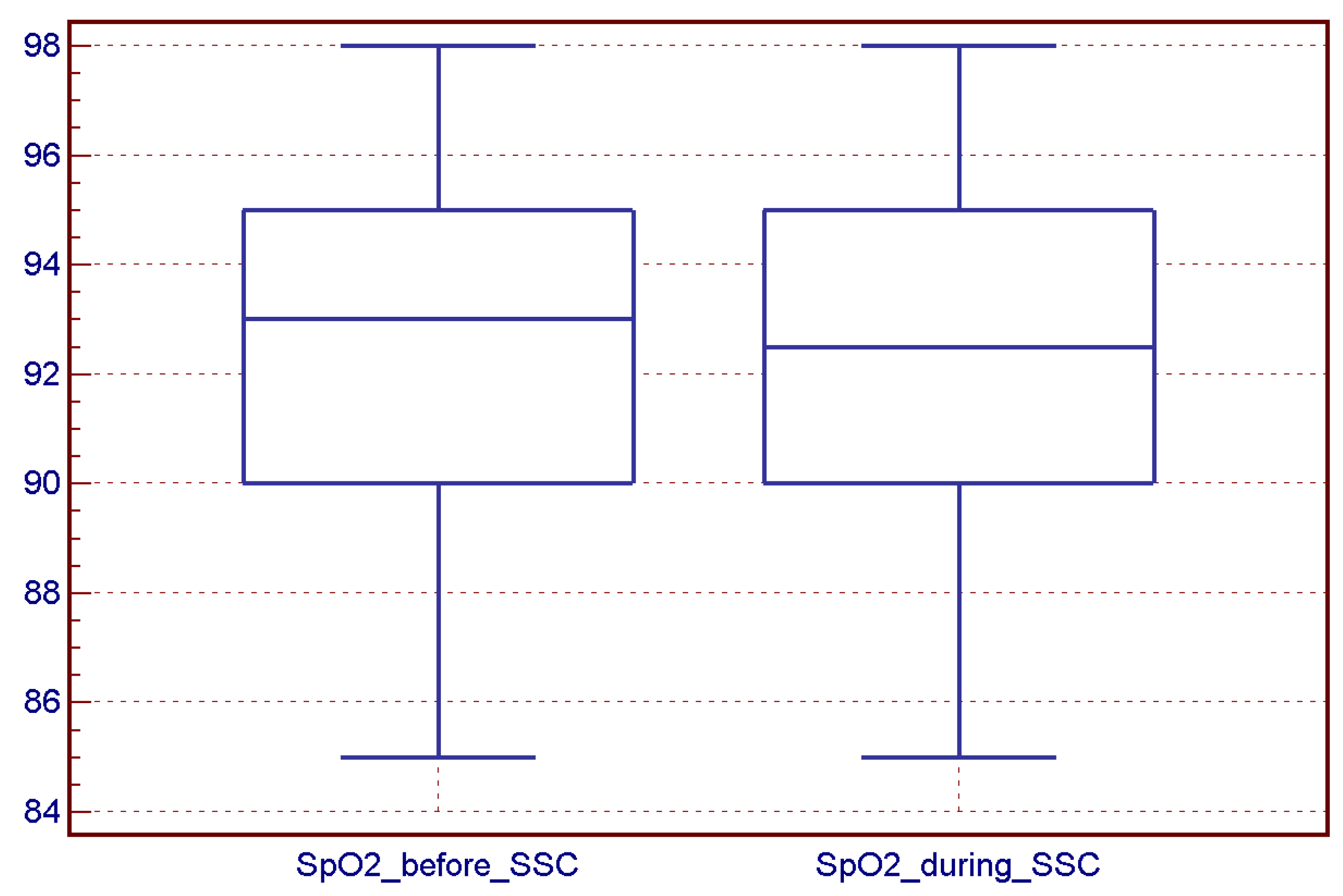
4.2. SSC Sessions and Vital Sign Stability
4.3. Further Adverse Events
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, G.J.; Valsangkar, B.; Kajeepeta, S.; Boundy, E.O.; Wall, S. What is kangaroo mother care? A systematic review of the literature. J. Glob. Health 2016, 6, 010701. [Google Scholar] [CrossRef]
- Available online: https://newborn-health-standards.org (accessed on 1 January 2020).
- Boundy, E.O.; Dastjerdi, R.; Spiegelman, D.; Fawzi, W.W.; Missmer, S.A.; Lieberman, E.; Kajeepeta, S.; Wall, S.; Chan, G.J. Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis. Pediatrics 2016, 137, e20152238. [Google Scholar] [CrossRef] [PubMed]
- Charpak, N.; Tessier, R.; Ruiz, G.J.; Hernandez, J.T.; Uriza, F.; Villegas, J.; Nadeau, L.; Mercier, C.; Maheu, F.; Marin, J.; et al. Twenty-year Follow-up of Kangaroo Mother Care Versus Traditional Care. Pediatrics 2017, 139, e20162063. [Google Scholar] [CrossRef] [PubMed]
- Nyqvist, K.; Anderson, G.; Bergman, N.; Cattaneo, A.; Charpak, N.; Davanzo, R.; Ewald, U.; Ibe, O.; Ludington-Hoe, S.; Mendoza, S.; et al. Towards universal Kangaroo Mother Care: Recommendations and report from the First European conference and Seventh International Workshop on Kangaroo Mother Care. Acta Paediatr. 2010, 99, 820–826. [Google Scholar] [CrossRef]
- Bastani, F.; Rajai, N.; Farsi, Z.; Als, H. The Effects of Kangaroo Care on the Sleep and Wake States of Preterm Infants. J. Nurs. Res. 2017, 25, 231–239. [Google Scholar] [CrossRef]
- Evereklian, M.; Posmontier, B. The Impact of Kangaroo Care on Premature Infant Weight Gain. J. Pediatr. Nurs. 2017, 34, e10–e16. [Google Scholar] [CrossRef]
- Bembich, S.; Castelpietra, E.; Cont, G.; Travan, L.; Cavasin, J.; Dolliani, M.; Traino, R.; Demarini, S. Cortical activation and oxygen perfusion in preterm newborns during kangaroo mother care: A pilot study. Acta Pediatr. 2023, 1–9. [Google Scholar] [CrossRef]
- Mehrpisheh, A.; Doorandish, Z.; Farhadi, R.; Ahmadi, M.; Moafi, M.; Elyasi, F. The Effectiveness of Kangaroo Mother Care (KMC) on attachment of mothers with premature infants. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2022, 15, 100149. [Google Scholar] [CrossRef] [PubMed]
- Vittner, D.; Casavant, S.; McGrath, J.M. A Meta-ethnography: Skin-to-Skin Holding From the Caregiver’s Perspective. Adv Neonatal Care. 2015, 15, 191–200, quiz E1-2. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Chen, D.Q.; Wang, H.; Zhang, Y.; Yang, R.; Xu, W.L.; Xu, X.F. What influences the implementation of kangaroo mother care? An umbrella review. BMC Pregnancy Childbirth 2022, 22, 851. [Google Scholar]
- Artese, C.; Paterlini, G.; Mascheroni, E.; Montirosso, R. Developmental Care Study Group (DCSG) of the Italian Neonatology Society. Barriers and facilitators to conducting Kangaroo Mother Care in Italian Neonatal Intensive Care Units. J. Pediatr. Nurs. 2021, 57, e68–e73. [Google Scholar]
- Seidman, G.; Unnikrishnan, S.; Kenny, E.; Myslinski, S.; Cairns-Smith, S.; Mulligan, B.; Engmann, C. Barriers and enablers of kangaroo mother care practice: A systematic review. PLoS ONE 2015, 10, e0125643. [Google Scholar] [CrossRef]
- Pados, B.F. Physiology of Stress and Use of Skin-to-Skin Care as a Stress-Reducing Intervention in the NICU. Nurs. Womens Health 2019, 23, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Martin-Anderson, S.; Adams Dudley, R. Clinician Perspectives on Barriers to and Opportunities for Skin-to-Skin Contact for Premature Infants in Neonatal Intensive Care Units. Breastfeed. Med. 2012, 7, 79–84. [Google Scholar] [CrossRef]
- Franck, L.S.; Bernal, H.; Gay Gale, G. Infant Holding Policies and Practices in Neonatal Units. Neonatal Netw. 2002, 21, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bergman, N.J.; Linley, L.L.; Fawcus, S.R. Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Pnediatr. 2004, 93, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, L.; Dawson, J.A.; Jones, H.; Jacobs, E.S. Skin-to-skin care in preterm infants receiving respiratory support does not lead to physiological instability. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 102, F339–F344. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Ghosh, J.; Singh, A.K.; Hazra, A.; Som, T.; Munian, D. Effect of Kangaroo mother care on vital physiological parameters of the low birth weight newborn. Indian J. Community Med. 2014, 39, 245–249. [Google Scholar] [CrossRef]
- Cañadas, C.D.; Perales, B.A.; Martínez, G.R.; Casado-Belmonte, M.D.P.; Carreño, P.T. Effects of Kangaroo Mother Care in the NICU on the Physiological Stress Parameters of Premature Infants: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 583. [Google Scholar] [CrossRef]
- Weissman, A.; Aranovitch, M.; Blazer, S.; Zimmer, E.Z. Heel lancing in newborns: Behavioral and spectral analysis assessment of pain control methods. Pediatrics 2009, 124, e921–e926. [Google Scholar] [CrossRef]
- Altimier, L.; Phillips, R.M. The Neonatal Integrative Developmental Care Model: Seven Neuroprotective Core Measures for Family-Centered Developmental Care. Newborn Infant Nurs. Rev. 2013, 13, 9–22. [Google Scholar] [CrossRef]
- NICU Brain Sensitive Care Committee. Neonatal Neuro-Protective Best Practice Guidelines; Swedish Medical Center: Seattle, WA, USA, 2015. [Google Scholar]
- Cong, X.; Ludington-Hoe, S.H.; McCain, G.; Fu, P. Kangaroo Care modifies preterm infant heart rate variability in response to heel stick pain: Pilot study. Early Hum. Dev. 2009, 85, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Bergman, N.J.; Ludwig, R.; Westrup, B.; Welch, M. Nurturescience versus neuroscience: A case for rethinking perinatal mother-infant behaviors and relationship. Birth Defects Res. 2019, 111, 1110–1127. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Tsherning, W.-W.; Robertson, N.J. Neuroscience meets nurture: Challenges of prematurity and the critical role of family-centred and developmental care as a key part of the neuroprotection care bundle. Arch. Dis. Child.-Fetal Neonatal Ed. 2022, 107, 242–249. [Google Scholar] [CrossRef]
- Artese, C.; Ferrari, F.; Perugi, S.; Cavicchioli, P.; Paterlini, G.; Mosca, F.; the Developmental Care Study Group of Italian Society and Neonatology. Surveying family access: Kangaroo mother care and breastfeeding policies across NICUs in Italy. Ital. J. Pediatr. 2021, 47, 231. [Google Scholar] [CrossRef]
- Carbasse, A.L.; Kracher, S.; Hausser, M.; Langlet, C.; Escande, B.; Donato, L.; Astruc, D.; Kuhn, P. Safety and Effectiveness of Skin-to-Skin Contact in the NICU to Support Neurodevelopment in Vulnerable Preterm Infants. J. Perinat. Neonat. Nurs. 2013, 27, 255–262. [Google Scholar] [CrossRef]
- Park, H.; Choi, B.S.; Lee, S.J.; Son, I.; Seol, I.J.; Lee, H.J. Practical application of kangaroo mother care in preterm infants: Clinical characteristics and safety of kangaroo mother care. J. Perinat. Med. 2014, 42, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Khanna, R.; Pledge, D.; Nakayama, T. Meta-analysis of physiological effects of skin-to-skin contact for newborns and mothers. Pediatr. Int. 2010, 52, 161–170. [Google Scholar] [CrossRef]
- Linner, A.; Klemming, S.; Sundbber, B.; Lilliesköld, S.; Westrup, B.; Jonas, W.; Skiöld, B. Immediate skin-to-skin contact is feasible for very preterm infants but thermal control remains a challenge. Acta Paediatr. 2020, 109, 697–704. [Google Scholar] [CrossRef]
- Maastrup, R.; Greisen, G. Extremely preterm infants tolerate skin-to-skin contact during the first weeks of life. Acta Paediatr. 2010, 99, 1145–1149. [Google Scholar] [CrossRef]
- Shattnawi, K.K.; Al-Ali, N.; Alnuami, K. Neonatal nurses’ knowledge and beliefs about kangaroo mother care in neonatal intensive care units: A descriptive, cross-sectional study. Nurs Health Sci. 2019, 21, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Nation, H.; Sanlorenzo, L.; Lebar, K.; Brandon, D. A Quality Improvement Project to Increase Frequency of Skin-to-Skin Contact for Extreme Low-Birth-Weight Infants in the Neonatal Intensive Care Unit. J. Perinatal Neonatal Nurse 2021, 35, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Díaz-Rossello, J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016, 23, CD002771. [Google Scholar] [CrossRef]
- Le Ray, I.; Kuhn, P.; Letouzey, M.; Roué, J.M.; Mitha, A.; Glorieux, I.; Foix-L’Hélias, L.; Marchand-Martin, L.; Ancel, P.-A.; Kaminski, M.; et al. Early skin-to-skin contact and risk of late-onset-sepsis in very and extremely preterm infants. Pediatr. Res. 2022. [Google Scholar] [CrossRef]
- Muñoz, K.D.; Xu, J.; Parikh, H.I.; Xu, P.; Fettweis, J.M.; Kim, Y.; Louie, M.; Buck, G.A.; Thacker, L.R.; Sheth, N.U. Skin-to-Skin Care and the Development of the Preterm Infant Oral Microbiome. Am. J. Perinatol. 2015, 32, 1205–1216. [Google Scholar]
- Lugli, L.; Bedetti, L.; Guidotti, I.; Pugliese, M.; Picciolini, O.; Roversi, M.F.; Muttini, E.D.; Lucaccioni, L.; Bertoncelli, N.; Ancora, G. Neuroprem 2: An Italian Study of Neurodevelopmental Outcomes of Very Low Birth Weight Infants. Front. Pediatr. 2021, 9, 697100. [Google Scholar] [CrossRef] [PubMed]

| Results | |
|---|---|
| Median gestational age, at birth, weeks, (range) | 29 (23–33) |
| Median gestational age at SSC, weeks, (range) | 31 (25–34) |
| Median birth weight, g, (range) | 1099 (540–1996) |
| Median body weight at SSC, g, (range) | 1130 (630–2206) |
| Median days of life at SSC (range) | 10 (1–20) |
| Respiratory Support, n (%) | |
| 19 (22.9%) |
| 6 (7.2%) |
| 57 (68.7%) |
| 1 (1.2%) |
| Catheters, n (%) | |
| 22 (26.5%) |
| 14 (16.9%) |
| 47 (56.6%) |
| Before an SSC Session (n = 83) | During an SSC Session (n = 83) | p | |
|---|---|---|---|
| Sessions with apnea, n (%) | 3 (2.7%) | 6 (4.2%) | 0.351 |
| Sessions with desaturation, n (%) | 12 (19.5%) | 20 (30.6%) | 0.314 |
| Sessions with bradycardia, n (%) | 10 (9%) | 11 (13.3%) | 0.481 |
| Duration of desaturations, seconds (mean ± ds) | 15–350 125 ± 118 | 15–560 115 ± 171 | 0.598 |
| Duration of bradycardia, seconds (mean ± ds) | 15–60 28 ± 15 | 15–135 36 ± 34 | 0.746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedetti, L.; Lugli, L.; Bertoncelli, N.; Spaggiari, E.; Garetti, E.; Lucaccioni, L.; Cipolli, F.; Berardi, A. Early Skin-to-Skin Contact in Preterm Infants: Is It Safe? An Italian Experience. Children 2023, 10, 570. https://doi.org/10.3390/children10030570
Bedetti L, Lugli L, Bertoncelli N, Spaggiari E, Garetti E, Lucaccioni L, Cipolli F, Berardi A. Early Skin-to-Skin Contact in Preterm Infants: Is It Safe? An Italian Experience. Children. 2023; 10(3):570. https://doi.org/10.3390/children10030570
Chicago/Turabian StyleBedetti, Luca, Licia Lugli, Natascia Bertoncelli, Eugenio Spaggiari, Elisabetta Garetti, Laura Lucaccioni, Federica Cipolli, and Alberto Berardi. 2023. "Early Skin-to-Skin Contact in Preterm Infants: Is It Safe? An Italian Experience" Children 10, no. 3: 570. https://doi.org/10.3390/children10030570
APA StyleBedetti, L., Lugli, L., Bertoncelli, N., Spaggiari, E., Garetti, E., Lucaccioni, L., Cipolli, F., & Berardi, A. (2023). Early Skin-to-Skin Contact in Preterm Infants: Is It Safe? An Italian Experience. Children, 10(3), 570. https://doi.org/10.3390/children10030570





