Investigating the Link between Early Life and Breast Anomalies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Dietary Factors
3.2. Lifestyle Factors
3.2.1. Alcohol
3.2.2. Physical Activity (PA)
3.3. Anthropometric Factors
3.3.1. Body Mass Index and Weight
3.3.2. Growth Velocity and Height
3.4. Age at Menarche
3.5. Age at Thelarche
3.6. Ionizing Radiation
3.7. Anthracyclines
3.8. Socioeconomic Status
3.9. Smoking and Drug Abuse
3.10. Other Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frazier, A.L.; Rosenberg, S.M. Preadolescent and adolescent risk factors for benign breast disease. J. Adolesc. Health 2013, 52, S36–S40. [Google Scholar] [CrossRef] [Green Version]
- Berkey, C.S.; Willett, W.C.; Tamimi, R.M.; Rosner, B.; Frazier, A.L.; Colditz, G.A. Diary intakes in older girls and risk of benign breast disease in young women. Cancer Epidemiol.Biomark. Prev. 2013, 22, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Baer, H.J.; Tworoger, S.S.; Hankinson, S.E.; Willett, W.C. Body fatness at young ages and risk of breast cancer throughout life. Am. J. Epidemiol. 2010, 171, 1183–1194. [Google Scholar] [CrossRef]
- Somdat, M. Association between diet during preadolescence and adolescence and risk for breast cancer during adulthood. J. Adollesc. Health 2013, 52, S30–S35. [Google Scholar]
- Berkey, C.S.; Willett, W.C.; Tamimi, R.M.; Rosner, B.; Frazier, A.L.; Colditz, G.A. Vegetable protein and vegetable fat intakes in pre-aolescence and adolescent girls, and risk for benign breast disease in young women. Breast Cancer Res. Treat. 2013, 141, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, H.J.; Schnitt, S.J.; Connolly, J.L.; Byrne, C.; Cho, E.; Willett, W.C.; Colditz, G.A. Adolescent diet and incidence of proliferative benign breast disease. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1159–1167. [Google Scholar]
- Linos, E.; Willett, W.C.; Cho, E.; Colditz, G.; Frazier, L. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2146–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farvid, M.S.; Chen, W.Y.; Michels, K.B.; Cho, E.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: Population based cohort study. BMJ 2016, 353, i2343. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Tamimi, R.M.; Collins, L.C.; Baer, H.J.; Cho, E.; Sampson, L.; Willett, W.C.; Schnitt, S.J.; Connolly, J.L.; Rosner, B.A.; et al. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control 2010, 21, 1033–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Colditz, G.A.; Cotterchio, M.; Boucher, B.A.; Kreiger, N. Adolescent dietary fiber, vegetable fat, vegetable protein, and nut intakes and breast cancer risk. Breast Cancer Res. Treat. 2014, 145, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Korde, L.A.; Wu, A.H.; Fears, T.; Nomura, A.M.; West, D.W.; Kolonel, L.N.; Pike, M.C.; Hoover, R.N.; Ziegler, R.G. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.A.; Shu, X.O.; Li, H.; Yang, G.; Cai, H.; Wen, W.; Ji, B.T.; Gao, J.; Gao, Y.T.; Zheng, W. Adolescent and adult soy food intake and breast cancer risk: Results from the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009, 89, 1920–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkey, C.S.; Tamimi, R.M.; Willett, W.C.; Rosner, B.; Hickey, M.; Toriola, A.T.; Lindsay Frazier, A.; Colditz, G.A. Dietary intake from birth through adolescence in relation to risk of benign breast disease in young women. Breast Cancer Res. Treat. 2019, 177, 513–525. [Google Scholar] [CrossRef]
- Liu, Y.; Colditz, G.A.; Rosner, B.; Berkey, C.S.; Collins, L.C.; Schnitt, S.J.; Connolly, J.L.; Chen, W.Y.; Willett, W.C.; Tamimi, R.M. Alcohol intake between menarche and first pregnancy: A prospective study of breast cancer risk. J. Nat. Cancer Inst. 2013, 105, 1571–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garland, M.; Hunter, D.J.; Colditz, G.A.; Spiegelman, D.L.; Manson, J.E.; Stampfer, M.J.; Willett, W.C. Alcohol consumption in relation to breast cancer risk in a cohort of United States women 25–42 years of age. Cancer Epidemiol. Biomark. Prev. 1999, 8, 1017–1021. [Google Scholar]
- Horn-Ross, P.L.; Canchola, A.J.; West, D.W.; Stewart, S.L.; Bernstein, L.; Deapen, D.; Pinder, R.; Ross, R.K.; Anton-Culver, H.; Peel, D.; et al. Patterns of alcohol consumption and breast cancer risk in the California Teachers Study cohort. Cancer Epidemiol. Biomark. Prev. 2004, 13, 405–411. [Google Scholar] [CrossRef]
- Theodoratou, E.; Timofeeva, M.; Li, X.; Meng, X.; Ioannidis, J.P.A. Nature, Nurture, and Cancer Risks: Genetic and Nutritional Contributions to Cancer. Ann. Rev. Nutr. 2017, 37, 293–320. [Google Scholar] [CrossRef]
- Berkey, C.S.; Willett, W.C.; Frazier, A.L.; Rosner, B.; Tamimi, R.M.; Rockett, H.R.; Colditz, G.A. Prospective study of adolescent alcohol consumption and risk of benign breast disease in young women. Pediatrics 2010, 125, e1081–e1087. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tamimi, R.M.; Berkey, C.S.; Willett, W.C.; Collins, L.C.; Schnitt, S.J.; Connolly, J.L.; Colditz, G.A. Intakes of alcohol and folate during adolescence and risk of proliferative benign breast disease. Pediatrics 2012, 129, e1192–e1198. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Page, D.L.; Chlebowski, R.T.; Beresford, S.A.; Hendrix, S.L.; Lane, D.S.; Rohan, T.E. Alcohol and folate consumption and risk of benign proliferative epithelial disorders of the breast. Int. J. Cancer 2007, 121, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Berkey, C.S.; Tamimi, R.M.; Rosner, B.; Frazier, A.L.; Colditz, G.A. Young women with family history of breast cancer and their risk factors for benign breast disease. Cancer 2012, 118, 2796–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, H.K.; Pelucchi, C.; Bagnardi, V.; La Vecchia, C. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol Alcohol. 2012, 47, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monninkhof, E.M.; Elias, S.G.; Vlems, F.A.; van der Tweel, I.; Schuit, A.J.; Voskuil, D.W.; van Leeuwen, F.E. Physical activity and breast cancer: A systematic review. Epidemiology 2007, 18, 137–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, T.M.; Moore, S.C.; Gierach, G.L.; Wareham, N.J.; Ekelund, U.; Hollenbeck, A.R.; Schatzkin, A.; Leitzmann, M.F. Intensity and timing of physical activity in relation to postmenopausal breast cancer risk: The prospective NIH-AARP diet and health study. BMC Cancer 2009, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Maruti, S.S.; Willett, W.C.; Feskanich, D.; Rosner, B.; Colditz, G.A. A Prospective Study of Age-Specific Physical Activity and Premenopausal Breast Cancer. J. Natl. Cancer Inst. 2008, 100, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Boeke, C.E.; Eliassen, A.H.; Oh, H.; Spiegelman, D.; Willett, W.C.; Tamimi, R.M. Adolescent physical activity in relation to breast cancer risk. Breast Cancer Res. Treat. 2014, 145, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Baer, H.J.; Schnitt, S.J.; Connolly, J.L.; Byrne, C.; Willett, W.C.; Rosner, B.; Colditz, G.A. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2889–2897. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.M.; Colditz, G.A.; Collins, L.C.; Schnitt, S.J.; Connolly, J.L.; Tamimi, R.M. Lifetime physical activity and the incidence of proliferative benign breast disease. Cancer Causes Control 2011, 22, 1297–1305. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Rosner, B.A.; Willet, W.C.; Hankinson, S.E. The combined influence of multiple sex and growth hormons on risk of postmenopausal breast cancer: A nested case-control study. Breast Cancer Res. 2011, 13, R99. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, K.; Zhou, H.J.; Shi, B.M. Influence of physical activity at a young age and lifetime physical activity on the risks of 3 obesity-related cancers: Systematic review and meta-analysis of observational studies. Nutr. Rev. 2020, 78, 1–18. [Google Scholar] [CrossRef] [PubMed]
- van den Brandt, P.A.; Spiegelman, D.; Yaun, S.-S.; Adami, H.-O.; Beeson, L.; Folsom, A.R.; Fraser, G.; Goldbohm, R.A.; Graham, S.; Kushi, L.; et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am. J. Epidemiol. 2000, 152, 514–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amadou, A.; Ferrari, P.; Muwonge, R.; Moskal, A.; Biessy, C.; Romieu, I.; Hainaut, P. Overweight, obesity and risk of premenopausal breat cancer according to ethnicity: Review and dose-response meta-analysis. Obes. Rev. 2013, 14, 665–678. [Google Scholar] [CrossRef]
- Berkey, C.S.; Willett, W.C.; Frazier, A.L.; Rosner, B.; Tamimi, R.M.; Colditz, G.A. Prospective study of growth and development in older girls and risk of benign breast disease in young women. Cancer 2011, 117, 1612–1620. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.; Rice, M.S.; Warner, E.T.; Bertrand, K.A.; Fowler, E.E.; Eliassen, A.H.; Rosner, B.A.; Heine, J.J.; Tamimi, R.M. Early-Life and Adult Anthropometrics in Relation to Mammographic Image Intensity Variation in the Nurses’ Health Studies. Cancer Epidemiol. Biomark. Prev. 2020, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Ahlgren, M.; Melbye, M.; Wohlfahrt, J.; Sorensen, T.I. Growth patterns and the risk of breast cancer in women. N. Engl. J. Med. 2004, 351, 1619–1626. [Google Scholar] [CrossRef]
- De Stavola, B.L.; Dos Santos Silva, I.; Mc Cormack, V.; Hardy, R.J.; Kuh, D.J.; Wadsworth, M.E. Childhood growth and breast cancer. Am. J. Epidemiol. 2004, 159, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.; D’Aloisio, A.A.; O’Brien, K.M.; Zhao, S.; Sandler, D.P. Pubertal timing and breast cancer risk in the Sister Study cohort. Breast Cancer Res. 2020, 22, 112. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Shi, L.; Remer, T.; Buyken, A.E.; Hartman, M.F.; Hoffmann, P.; Wudy, S.A. Prepubertal urinary estrogen excretion and its relationship with pubertal timing. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E990–E997. [Google Scholar] [CrossRef]
- Madigan, M.P.; Troisi, R.; Potischman, N.; Dorgan, J.F.; Brinton, L.A.; Hoover, R.N. Serum hormone levels in relation to reproductive and lifestyle factors in postmenopausal women (United States). Cancer Causes Control 1998, 9, 199–207. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Monroe, K.R.; Wilkens, L.R.; Kolonel, L.N.; Pike, M.C.; Henderson, B.E. Breast cancer risk factors defined by estrogen and progesterone receptor status:The multiethnic cohort study. Am. J. Epidemiol. 2009, 169, 1251–1259. [Google Scholar] [CrossRef] [Green Version]
- Coldritz, G.A.; Bohlke, K.; Berkey, C.S. Breast cancer risk accumulation starts early-prevention must also. Breast Cancer Res. Treat. 2014, 145, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Silvera, S.A.; Rohan, T.E. Benign proliferative epithelial disorders of the breast: A review of epidemiologic evidence. Breast Cancer Res. Treat. 2008, 110, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, R.M.; Rosner, B.; Colditz, G.A. Evaluation of a breast cancer risk prediction model expanded to include category of prior benign breast disease lesion. Cancer 2010, 116, 4944–4953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karapanou, O.; Papadimitriou, A. Determinants of menarche. Reproductive biology and endocrinology. Reprod. Biol. Endocrinol. 2010, 8, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, D.H.; Jones, M.E.; Schoemaker, M.J.; Ashoworth, A.; Swerdlow, A.J. Determinants of age at menarche in the UK: Analysis from the Breakthrough Generations Study. Br. J. Cancer 2010, 103, 1760–1764. [Google Scholar] [CrossRef] [Green Version]
- Berkey, C.S.; Gardner, J.D.; Frazier, A.L.; Golditz, G.A. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am. J. Epidemiol. 2000, 152, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.D.; Boughey, J.C. Management of pediatric and adolescent breast masses. Semin. Plast. Surg. 2013, 27, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.O.; Amsterdam, A.; Bhatia, S.; Hudson, M.M.; Meadows, A.T.; Neglia, J.P.; Diller, L.R.; Constine, L.S.; Smith, R.A.; Mahoney, M.C.; et al. Surveillance for breast cancer in women treated with chest radiation for a childhood, adolescent or young adult cancer: A report from the children’s oncology group. Ann. Intern. Med. 2010, 152, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Inskip, P.D.; Robison, L.L.; Stovall, M.; Smith, S.A.; Hammond, S.; Mertens, A.C.; Whitton, J.A.; Diller, L.; Kenney, L.; Donaldson, S.S.; et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J. Clin. Oncol. 2009, 27, 3901. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.S.; Chou, J.F.; Wolden, S.L.; Bernstein, J.L.; Malhotra, J.; Friedman, D.N.; Mubdi, N.Z.; Leisenring, W.M.; Stovall, M.; Hammond, S.; et al. Breast cancer after chest radiation therapy for childhood cancer. J. Clin. Oncol. 2014, 32, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Sparidans, J.; van’tVeer, M.B.; Noordijk, E.M.; Louwman, M.W.J.; Zijlstra, J.M.; van den Berg, H.; Russell, N.S.; Broeks, A.; Baaijens, M.H.A.; et al. Breast Cancer risk in Female Survivors of Hodgkin’s Lymphoma: Lower Risk After Smaller Radiation Volumes. J. Clin. Oncol. 2009, 27, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Schellong, G.; Riepenhausen, M.; Ehlert, K.; Brämswig, J.; Dörffel, W.; Schmutzler, R.K.; Rhiem, K.; Bick, U. Breast Cancer in Young Women After Treatment for Hodgkin’s Disease During Childhood or Adolescence. Dtsch. Aerzteblatt Int. 2014, 111, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Demoor-Goldschmidt, C.; Allodji, R.S.; Jackson, A.; Vu-Bezin, G.; Souchard, V.; Fresneau, B.; le Fayech, C.; Haddy, N.; Rubino, C.; Pacquement, H.; et al. Breast Cancer, Secondary Breast Cancers in Childhood Cancer Male Survivors-Characteristics and Risks. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 578–583. [Google Scholar] [CrossRef]
- Veiga, L.H.; Curtis, R.E.; Morton, L.M.; Withrow, D.R.; Howell, R.M.; Smith, S.A.; Weathers, R.E.; Oeffinger, K.C.; Moskowitz, C.S.; Henderson, T.O.; et al. Association of Breast Cancer Risk After Childhood Cancer With Radiation Dose to the Breast and Anthracycline Use: A Report From the Childhood Cancer Survivor Study. JAMA Pediatr. 2019, 173, 1171–1179. [Google Scholar] [CrossRef]
- Holmqvist, A.S.; Chen, Y.; Berano, T.J.; Sun, C.; Birch, J.M.; van den Bos, C.; Diller, L.R.; Dilley, K.; Ginsberg, J.; Martin, L.T.; et al. Risk of solid subsequent malignant neoplasms after childhood Hodgkin lymphoma-Identification of high-risk populations to guide surveillance: A report from the Late Effects Study Group. Cancer 2019, 125, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.O.; Moskowitz, C.S.; Chou, J.F.; Bradbury, A.R.; Neglia, J.P.; Dang, C.T.; Onel, K.; Novetsky Friedman, D.; Bhatia, S.; Strong, L.C.; et al. Breast Cancer Risk in Childhood Cancer Survivors Without a History of Chest Radiotherapy: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2016, 34, 910. [Google Scholar] [CrossRef]
- Ehrhardt, M.J.; Howell, C.R.; Hale, K.; Baassiri, M.J.; Rodriguez, C.; Wilson, C.L.; Joshi, S.S.; Lemond, T.C.; Shope, S.; Howell, R.M.; et al. Subsequent Breast Cancer in Female Childhood Cancer Survivors in the St. Jude Lifetime Cohort Study (SJLIFE). J Clin Oncol. 2019, 37, 1647–1656. [Google Scholar] [CrossRef]
- Hiatt, R.A.; Stewart, S.L.; Hoeft, K.S.; Kushi, L.H.; Windham, G.C.; Biro, F.M.; Pinney, S.M.; Wolff, M.S.; Teitelbaum, S.L.; Braithwaite, D. Childhood Socioeconomic Position and Pubertal Onset in a Cohort of Multiethnic Girls: Implications for Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1714–1721. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Page, D.L.; Chlebowski, R.T.; Hsia, J.; Allan Hubbell, F.; Johnson, K.C.; Rohan, T.E. Cigarette smoking and risk of benign proliferative epithelial disorders of the breast in the Women’s Health Initiative. Cancer Causes Control 2007, 18, 431–438. [Google Scholar] [CrossRef]
- Okasha, M.; McCarron, P.; Gunnell, D.; Smith, G.D. Exposures in childhood, adolescence and early adulthood and breast cancer risk: A systematic review of the literature. Breast Cancer Res. Treat. 2003, 78, 223–276. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Schoemaker, M.J.; Wright, L.B.; Ashworth, A.; Swerdlow, A.J. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 2017, 19, 118. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Gatsonis, C.A.; Baylin, A.; Buka, S.L. Prenatal exposure to cigarette smoke and benign breast disease. Epidemiology 2010, 21, 736–743. [Google Scholar] [CrossRef]
- Dahlman, D.; Magnusson, H.; Li, X.; Sundquist, J.; Sundquist, K. Drug use disorder and risk of incident and fatal breast cancer: A nationwide epidemiological study. Breast Cancer Res. Treat. 2021, 186, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Velicer, C.M.; Heckbert, S.R.; Lampe, J.W.; Potter, J.D.; Robertson, C.A.; Taplin, S.H. Antibiotic use in relation to the risk of breast cancer. JAMA 2004, 291, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Simin, J.; Tamimi, R.M.; Engstrand, L.; Callens, S.; Brusselaers, N. Antibiotic use and the risk of breast cancer: A systematic review and dose-response meta-analysis. Pharmacol. Res. 2020, 160, 105072. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.Y.; Ansell, C.; Nawaz, H.; Yang, C.H.; Wood, P.A.; Hrushesky, W.J. Global breast cancer seasonality. Breast Cancer Res. Treat. 2010, 123, 233–243. [Google Scholar] [CrossRef]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Young, J.F. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev Med. 1990, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Howell, A.; Evans, D.G. Can diet and lifestyle prevent breast cancer: What is the evidence? Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Bradbury, A. Family-based Breast Cancer Prevention Efforts in Adolescence. Pediatrics 2016, 138 (Suppl. 1), S78–S80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 : the risk increases,
: the risk increases,  : the risk decreases.
: the risk decreases.
 : the risk increases,
: the risk increases,  : the risk decreases.
: the risk decreases.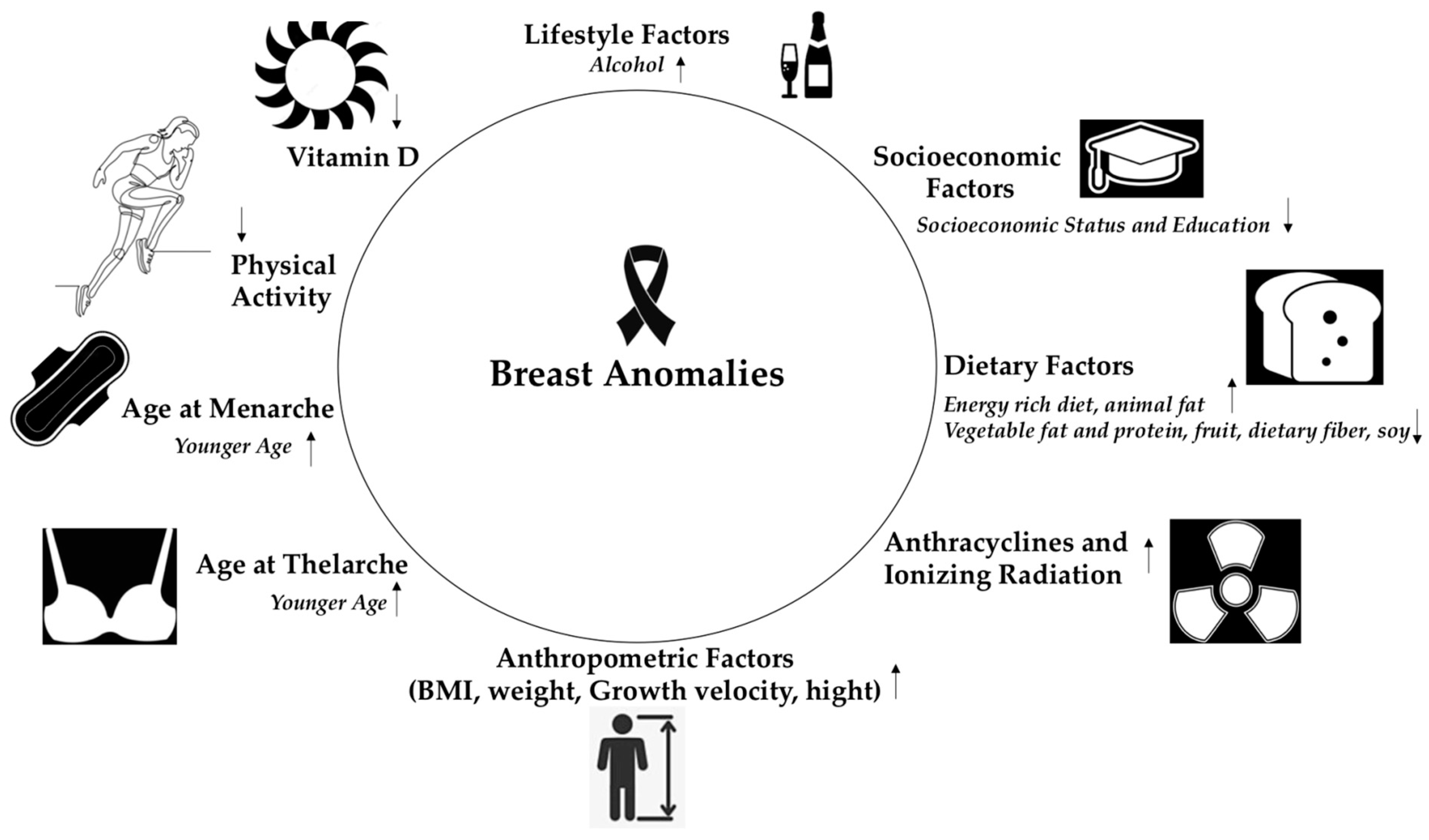
| Factors | Effect | Reference |
|---|---|---|
| Dietary factors |
| Trichopoulos [4] |
| Berkeley et al. [5], Baer et al. [6], Linos et al. [7] | |
| Fruit consumption is associated with a lower risk of breast cancer | Farvid et al. [8] | |
| Dietary fiber and vegetable protein consumption reduces the risk for breast cancer | Liu et al. [10] | |
| Soy intake reduces the risk for breast cancer | Lee et al. [13] | |
| Alcohol | Few studies available, limited data demonstrate the link between alcohol consumption and the occurrence of BBD and breast cancer | Berkey et al. [18] |
| Physical activity |
| Maruti et al. [26] |
| BMI and weight | A BMI> or equivalent to 25 at age 18 was associated with a reduction in BBD risk | Baer et al. [28] |
| Growth velocity and height | A faster height velocity was associated with a higher risk for BBD and breast cancer | Berkey et al. [34], Ahlgren et al. [37], De Stavola et al. [38] |
| Age at Menarche | Each one-year decrease in age at menarche increased the risk of breast cancer by 5% | Collaborative Group, The lancet oncology [40] |
| The relationship between age at menarche and risk of BBD is not yet well established | ||
| Age at Thelarche | Each one-year delay in age at thelarche was associated with a 3% decrease of breast cancer risk | Goldberg et al. [39] |
| The relationship between age at thelarche and risk of BBD is not yet well established | ||
| Ionizing Radiation | Chest radiation for pediatric malignancies increases the risk for breast cancer | Henderson et al. [51] |
| Anthracyclines | Exposure to anthracyclines or alkylators increases the risk for breast cancer | Henderson et al. [59] |
| Socioeconomic Status | SEP could be indirectly related to breast pathologies | Hiatt et al. [61] |
| Smoking | Few studies available, conflicting data | Cui et al. [62] |
| Vitamin D | Anticancer properties | Muñoz et al. [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulos, P.; Matsas, A.; Eleftheriades, M.; Kotsira, G.; Eleftheriades, A.; Vlahos, N.F. Investigating the Link between Early Life and Breast Anomalies. Children 2023, 10, 601. https://doi.org/10.3390/children10030601
Christopoulos P, Matsas A, Eleftheriades M, Kotsira G, Eleftheriades A, Vlahos NF. Investigating the Link between Early Life and Breast Anomalies. Children. 2023; 10(3):601. https://doi.org/10.3390/children10030601
Chicago/Turabian StyleChristopoulos, Panagiotis, Alkis Matsas, Makarios Eleftheriades, Georgia Kotsira, Anna Eleftheriades, and Nikolaos F. Vlahos. 2023. "Investigating the Link between Early Life and Breast Anomalies" Children 10, no. 3: 601. https://doi.org/10.3390/children10030601
APA StyleChristopoulos, P., Matsas, A., Eleftheriades, M., Kotsira, G., Eleftheriades, A., & Vlahos, N. F. (2023). Investigating the Link between Early Life and Breast Anomalies. Children, 10(3), 601. https://doi.org/10.3390/children10030601











