Thrombotic Events in MIS-C Patients: A Single Case Report and Literature Review
Abstract
1. Introduction
2. Materials and Methods
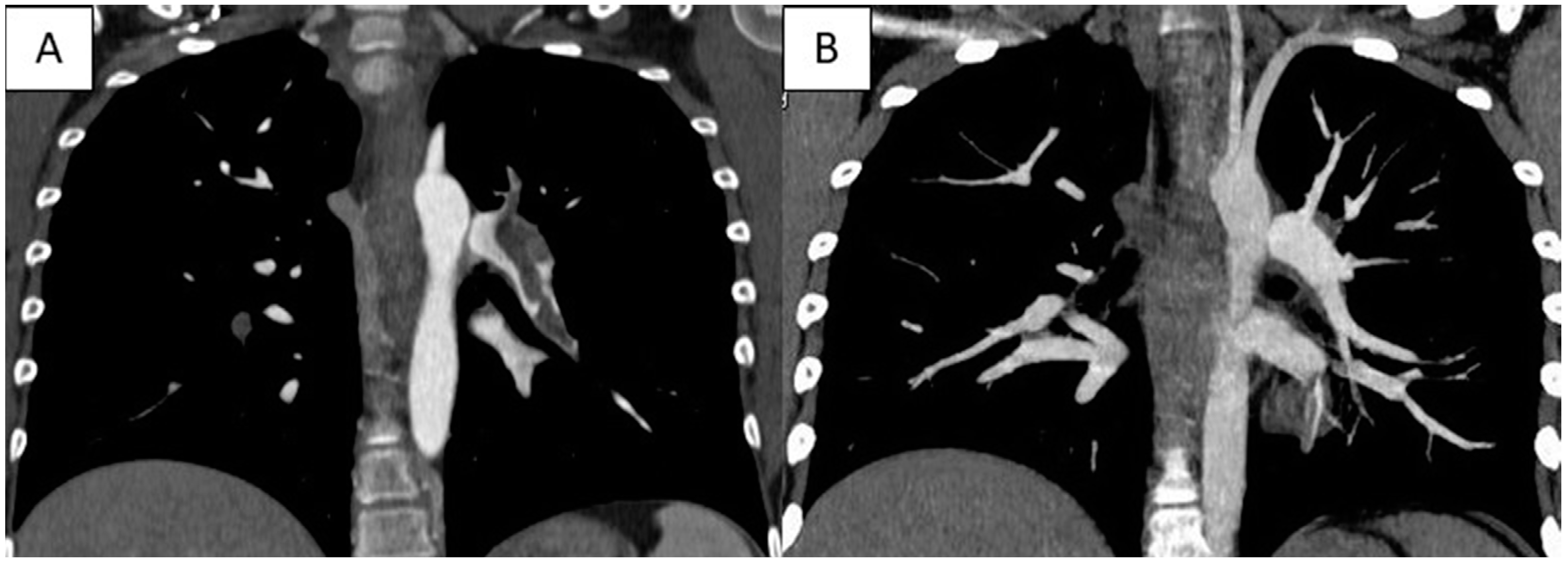
3. Case Report
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Lenge, M.; Buonsenso, D. Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group Children with Covid-19 in Pediatric Emergency Departments in Italy. N. Engl. J. Med. 2020, 383, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.M.; Hayes, D.A.; Singh-Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem Inflammatory Syndrome in Children and Kawasaki Disease: A Critical Comparison. Nat. Rev. Rheumatol. 2021, 17, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Kernan, K.F.; Schulert, G.S.; Seo, P.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022, 74, e1–e20. [Google Scholar] [CrossRef]
- Al-Ghafry, M.; Vagrecha, A.; Malik, M.; Levine, C.; Uster, E.; Aygun, B.; Appiah-Kubi, A.; Vlachos, A.; Capone, C.A.; Rajan, S.; et al. Multisystem Inflammatory Syndrome in Children (MIS-C) and the Prothrombotic State: Coagulation Profiles and Rotational Thromboelastometry in a MIS-C Cohort. J. Thromb. Haemost. 2021, 19, 1764–1770. [Google Scholar] [CrossRef]
- Morparia, K.; Spinella, P.C.; McQueen, D.; Kalyanaraman, M.; Bergel, M.; Lin, J.; Narang, S.; Saini, A. Thromboelastography Profiles in Critically Ill Children with Multisystem Inflammatory Syndrome. Pediatr. Blood Cancer 2022, 69, e29426. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. BMJ Case Rep. 2013, 2013, bcr2013201554. [Google Scholar] [CrossRef]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of Thrombosis in Children and Adolescents Hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Amonkar, P.S.; Gavhane, J.B.; Kharche, S.N.; Kadam, S.S.; Bhusare, D.B. Aortic Thrombosis in a Neonate with COVID-19-Related Fetal Inflammatory Response Syndrome Requiring Amputation of the Leg: A Case Report. Paediatr. Int. Child Health 2021, 41, 211–216. [Google Scholar] [CrossRef]
- Anastas, D.C.; Farias, A.; Runyon, J.; Laufer, M.; Sendi, P.; Totapally, B.; Sachdeva, R. Massive Pulmonary Embolism in an Adolescent With Multisystem Inflammatory Syndrome Due to COVID-19. Clin. Pediatr. 2021, 60, 341–345. [Google Scholar] [CrossRef]
- Arga, G.; Erkol, H.G.; Taskin, E.C.; Konca, H.K.; Tas, I.; Erdogan, B.O.; Ozdemir, H.; Cakmaklı, H.F.; Kahveci, F.; Demir, B.; et al. SARS-CoV-2 Infection Showing Signs of Cerebral Sinus Vein Thrombosis in the Infantile Period. Brain Disord. 2022, 7, 100051. [Google Scholar] [CrossRef] [PubMed]
- Barfuss, S.B.; Truong, D.T.; James, K.E.; Inman, C.J.; Husain, S.A.; Williams, R.V.; Minich, L.L.; Mart, C.R. Left Ventricular Thrombus in the Multisystem Inflammatory Syndrome in Children Associated with COVID-19. Ann. Pediatr. Cardiol. 2022, 15, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Beslow, L.A.; Linds, A.B.; Fox, C.K.; Kossorotoff, M.; Zuñiga Zambrano, Y.C.; Hernández-Chávez, M.; Hassanein, S.M.A.; Byrne, S.; Lim, M.; Maduaka, N.; et al. Pediatric Ischemic Stroke: An Infrequent Complication of SARS-CoV-2. Ann. Neurol. 2021, 89, 657–665. [Google Scholar] [CrossRef]
- Beslow, L.A.; Agner, S.C.; Santoro, J.D.; Ram, D.; Wilson, J.L.; Harrar, D.; Appavu, B.; Fraser, S.M.; Rossor, T.; Torres, M.D.; et al. International Prevalence and Mechanisms of SARS-CoV-2 in Childhood Arterial Ischemic Stroke during the COVID-19 Pandemic. Stroke 2022, 53, 2497–2503. [Google Scholar] [CrossRef]
- Bigdelian, H.; Sedighi, M.; Sabri, M.R.; Dehghan, B.; Mahdavi, C.; Ahmadi, A.; Ghaderian, M.; Rahimi, H.; Sadeghizadeh, A.; Emadoleslami, M.; et al. Case Report: Acute Intracardiac Thrombosis in Children with Coronavirus Disease 2019 (COVID-19). Front. Pediatr. 2021, 9, 656720. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Bulwa, Z.; Breit, H.; Cherian, L.J.; Conners, J.J.; Song, S.Y.; Dafer, R.M. Acute Large Vessel Ischemic Stroke in Patients With COVID-19-Related Multisystem Inflammatory Syndrome. Pediatr. Neurol. 2022, 126, 104–107. [Google Scholar] [CrossRef]
- Cinteză, E.; Voicu, C.; Filip, C.; Ioniță, M.; Popescu, M.; Bălgrădean, M.; Nicolescu, A.; Mahmoud, H. Myocardial Infarction in Children after COVID-19 and Risk Factors for Thrombosis. Diagnostics 2022, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Dolhnikoff, M.; Ferreira Ferranti, J.; de Almeida Monteiro, R.A.; Duarte-Neto, A.N.; Soares Gomes-Gouvêa, M.; Viu Degaspare, N.; Figueiredo Delgado, A.; Montanari Fiorita, C.; Nunes Leal, G.; Rodrigues, R.M.; et al. SARS-CoV-2 in Cardiac Tissue of a Child with COVID-19-Related Multisystem Inflammatory Syndrome. Lancet Child Adolesc. Health 2020, 4, 790–794. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Oliveira, C.R.; Guerguis, S.; Eisenberg, R.; Choi, J.; Kim, M.; Abdelhemid, A.; Agha, R.; Agarwal, S.; Aschner, J.L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. J. Pediatr. 2021, 230, 23–31.e10. [Google Scholar] [CrossRef] [PubMed]
- Ghatasheh, G.; Al Dhanhani, H.; Goyal, A.; Noureddin, M.B.; Al Awaad, D.; Peerwani, Z. COVID-19-Related Giant Coronary Aneurysms in an Infant with Multisystem Inflammatory Disorder in Children: The First Case Report from the United Arab Emirates and the Arab Region. Case Rep. Infect. Dis. 2021, 2021, 8872412. [Google Scholar] [CrossRef] [PubMed]
- Gulko, E.; Overby, P.; Ali, S.; Mehta, H.; Al-Mufti, F.; Gomes, W. Vessel Wall Enhancement and Focal Cerebral Arteriopathy in a Pediatric Patient with Acute Infarct and COVID-19 Infection. AJNR Am. J. Neuroradiol. 2020, 41, 2348–2350. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Aydin, S.I.; Derespina, K.R.; Bansal, P.B.; Kowalsky, S.; Trachtman, R.; Gillen, J.K.; Perez, M.M.; Soshnick, S.H.; Conway, E.E.; et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-Institutional Study from New York City. J. Pediatr. 2020, 224, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Ahluwalia, N.; Gangadharan, S.; Esperenza, M.; Murthy, R.; Ofori-Amanfo, G.; Aydin, S.I. ECMO Support in SARS-CoV2 Multisystem Inflammatory Syndrome in Children in a Child. Perfusion 2021, 36, 524–528. [Google Scholar] [CrossRef]
- Kavthekar, S.O.; Pawar, R.S.; Patil, R.R.; Patil, N.B.; Kurane, A.B. Intracardiac Thrombi and Pulmonary Thromboembolism in a Child with Multisystem Inflammatory Syndrome in Children (MIS-C). Indian J. Pediatr. 2022, 89, 726. [Google Scholar] [CrossRef]
- Keskin, H.; Keskin, F.; Yildirim, E.; Saritas, S.; Polat, G.; Colak, A.; Laloglu, F.; Guler, M.A.; Ozay, M.; Alp, H. Case of Venous Thromboembolia Under Enoxaparin Prophylaxis After Recovering From Acute Ischemic Stroke in Consequence of COVID-19-Related MIS-C. Pediatr. Infect. Dis. J. 2022, 41, e251–e252. [Google Scholar] [CrossRef]
- Kihira, S.; Morgenstern, P.F.; Raynes, H.; Naidich, T.P.; Belani, P. Fatal Cerebral Infarct in a Child with COVID-19. Pediatr. Radiol. 2020, 50, 1479–1480. [Google Scholar] [CrossRef]
- Kotula, J.J.; Balakumar, N.; Khan, D.; Patel, B. Bilateral Pulmonary Emboli in a Teenager with Positive SARS-CoV-2 Antibody. Pediatr. Pulmonol. 2021, 56, 271–273. [Google Scholar] [CrossRef]
- Krasic, S.; Popovic, S.; Kravljanac, R.; Prijic, S.; Vukomanovic, V. Intracardiac Thrombosis in the Three-Year-Old Boy with Normal Left Ventricle Systolic Function in MIS-C Associated with COVID-19. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022028. [Google Scholar] [CrossRef]
- Minen, F.; Hands, C.; Mustafa, M.R.; Pienaar, A.; Lillie, J. Thrombophilia in Pediatric Patients with Multisystem Inflammatory Syndrome in Children Secondary to Coronavirus Disease 2019 Supported on Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 7–11. [Google Scholar] [CrossRef]
- Manchola Narváez, K.D.; Ortíz, N.D.P.D.; Ardila Gómez, I.J.; López, P.P.; Rivera Ortíz, M.F. Giant Partially Thrombosed Coronary Aneurysm in Multisystem Inflammatory Syndrome Associated with SARS-CoV-2 in Children. Case Rep. Med. 2022, 2022, 3785103. [Google Scholar] [CrossRef]
- Pabst, L.M.; Zyck, S.A.; Youssef, P. Successful Thrombectomy in a Pediatric Patient with Large Vessel Occlusion and COVID-19 Related Multisystem Inflammatory Syndrome. Interv. Neuroradiol. 2022, 15910199221080872. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Shorofsky, M.; Leahy, R.; Friesen, R.; Khanna, A.; Cole, L.; Kim, J.S. ST-Elevation Myocardial Infarction Due to Acute Thrombosis in an Adolescent With COVID-19. Pediatrics 2021, 148, e2020049793. [Google Scholar] [CrossRef]
- Plouffe, B.; Van Hooren, T.; Barton, M.; Nashid, N.; Demirkaya, E.; Norozi, K.; Rachinsky, I.; Delport, J.; Knauer, M.; Tole, S.; et al. Renal Infarcts-A Perplexing Case in the Middle of the COVID-19 Pandemic. Front. Pediatr. 2021, 9, 669453. [Google Scholar] [CrossRef] [PubMed]
- Qasim, A.; Kaushal, S.; Bansal, M.; Lasa, J.J.; Sanchez Mejia, A.A. Echocardiographic Clues in Diagnosis of Takayasu Arteritis in a Child with Severe Acute Respiratory Syndrome Coronavirus 2-Related Multisystem Inflammatory Syndrome. CASE 2021, 5, 217–220. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.d.O.; Ribeiro, D.C.; Rocha, J.S.; Maia, S.B.S.; Moreira, A.L.E.; Silva, P.A.N.; Ito, C.R.M.; Carneiro, L.C.; Avelino, M.A.G. Severe Neurological Manifestation in a Child with Multisystem Inflammatory Syndrome. Children 2022, 9, 1653. [Google Scholar] [CrossRef]
- Schroder, J.; Lund, M.A.V.; Vejlstrup, N.; Juul, K.; Nygaard, U. Left Ventricular Thrombus in Multisystem Inflammatory Syndrome in Children Associated with COVID-19. Cardiol. Young 2022, 32, 138–141. [Google Scholar] [CrossRef]
- Schupper, A.J.; Yaeger, K.A.; Morgenstern, P.F. Neurological Manifestations of Pediatric Multi-System Inflammatory Syndrome Potentially Associated with COVID-19. Childs Nerv. Syst. 2020, 36, 1579–1580. [Google Scholar] [CrossRef]
- Shobhavat, L.; Solomon, R.; Rao, S.; Bhagat, I.; Prabhu, S.; Prabhu, S.; Chandrakar, M.; Bodhanwala, M. Multisystem Inflammatory Syndrome in Children: Clinical Features and Management-Intensive Care Experience from a Pediatric Public Hospital in Western India. Indian J. Crit. Care Med. 2020, 24, 1089–1094. [Google Scholar] [CrossRef]
- Stidham, T.; McKee, J.; Vogt, J.; Siomos, A.K. Successful Intervention for a Thrombosed Giant Coronary Artery Aneurysm in Multisystem Inflammatory Syndrome in Children. JACC Case Rep. 2022, 4, 945–949. [Google Scholar] [CrossRef]
- Tehseen, S.; Williams, S.; Robinson, J.; Morris, S.K.; Bitnun, A.; Gill, P.; Tal, T.E.; Yeh, A.; Yea, C.; Ulloa-Gutierrez, R.; et al. Thrombosis and Hemorrhage Experienced by Hospitalized Children with SARS-CoV-2 Infection or MIS-C: Results of the PICNIC Registry. Pediatr. Blood Cancer 2022, 69, e29793. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Hiremath, S.; Baju, M.M. Novel Presentation of Pediatric Inflammatory Multisystem Syndrome Temporally Associated with COVID-19. J. Pediatr. Neurol. 2022, 20, 60–62. [Google Scholar] [CrossRef]
- Tiwari, L.; Shekhar, S.; Bansal, A.; Kumar, S. COVID-19 Associated Arterial Ischaemic Stroke and Multisystem Inflammatory Syndrome in Children: A Case Report. Lancet Child Adolesc. Health 2021, 5, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Tolunay, O.; Çelik, Ü.; Arslan, İ.; Orgun, A.; Demir, H.; Demir, O.; Dağdelen, E.Ç. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19: A Case Series Experience in a Tertiary Care Hospital of Southern Turkey. J. Trop. Pediatr. 2021, 67, fmab050. [Google Scholar] [CrossRef]
- Vielleux, M.J.; Swartwood, S.; Nguyen, D.; James, K.E.; Barbeau, B.; Bonkowsky, J.L. SARS-CoV-2 Infection and Increased Risk for Pediatric Stroke. Pediatr. Neurol. 2022; in press. [Google Scholar] [CrossRef]
- Woods, G.M.; Kim, D.W.; Paden, M.L.; Viamonte, H.K. Thrombolysis in Children: A Case Report and Review of the Literature. Front. Pediatr. 2021, 9, 814033. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.A.; Hazeem, A.A.; Rashid, A. Giant Coronary Aneurysm in an Infant with Multisystem Inflammatory Syndrome. Heart Views 2022, 23, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef]
- Davies, P.; Evans, C.; Kanthimathinathan, H.K.; Lillie, J.; Brierley, J.; Waters, G.; Johnson, M.; Griffiths, B.; du Pré, P.; Mohammad, Z.; et al. Intensive Care Admissions of Children with Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS) in the UK: A Multicentre Observational Study. Lancet Child Adolesc. Health 2020, 4, 669–677. [Google Scholar] [CrossRef]
- Trapani, S.; Rubino, C.; Lasagni, D.; Pegoraro, F.; Resti, M.; Simonini, G.; Indolfi, G. Thromboembolic complications in children with COVID-19 and MIS-C: A narrative review. Front. Pediatr. 2022, 10, 944743. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, N.A.; Sochet, A.; Albisetti, M.; Biss, T.; Bonduel, M.; Jaffray, J.; MacLaren, G.; Monagle, P.; O’Brien, S.; Raffini, L.; et al. Consensus-Based Clinical Recommendations and Research Priorities for Anticoagulant Thromboprophylaxis in Children Hospitalized for COVID-19-Related Illness. J. Thromb. Haemost. 2020, 18, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Sharathkumar, A.A.; Faustino, E.V.S.; Takemoto, C.M. How We Approach Thrombosis Risk in Children with COVID-19 Infection and MIS-C. Pediatr. Blood Cancer 2021, 68, e29049. [Google Scholar] [CrossRef] [PubMed]
- Mastrolia, M.V.; Marrani, E.; Calabri, G.B.; L’Erario, M.; Maccora, I.; Favilli, S.; Duchini, P.P.; Pagnini, I.; Simonini, G. Fast recovery of cardiac function in PIMS-TS patients early using intravenous anti-IL-1 treatment. Crit. Care 2021, 25, 131. [Google Scholar] [CrossRef]
| Study | N° Patients (M/F) | Age | ≥1 Thrombosis Risk Factor | Thrombosis Site | Antiplatelet and/or Anticoagulation Prophylaxis | Thrombosis Treatment | Immunosuppressive Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| Amonkar 2021 [11] | 1 (1/0) | 1 month | No | Abdominal aorta | No | Heparin, aspirin, thrombolysis, thrombectomy | CCS | Amputation of the right leg |
| Anastas 2021 [12] | 1 (0/1) | 15 years | Yes | Bilateral pulmonary arteries, superior vena cava | No | Heparin, thrombolysis | None | Neurologic disability |
| Arga 2022 [13] | 1 (1/0) | 1 month | Yes | Cerebral sinus vein | No | Heparin, thrombectomy | IVIG, CCS | Thrombosis resolution |
| Barfuss 2022 [14] | 1 (0/1) | 3 years | Yes | Left ventricular, middle cerebral artery | No | Heparin, aspirin, thrombectomy | IVIG, CCS, anakinra | Neurologic improvement; required supplemental nasogastric feedings at the time of discharge |
| Beslow 2021 [15] | 2 (2/0) | 10–14 years | Yes | Lower limb, middle and anterior cerebral artery | No | Na | Na | Na |
| Beslow 2022 [16] | 2 (2/0) | 2–8 years | Yes | Middle cerebral artery | No | Na | Na | Na |
| Bigdelian 2021 [17] | 3 (0/3) | 7–11 years | Yes | Left atrium, left ventricular, pulmonary thrombosis | No | Heparin, thrombectomy | IVIG, CCS | Thrombosis resolution |
| Chang 2022 [18] | 2 (0/2) | 15–16 years | Yes | Internal carotid, intracardiac, stroke | No | Heparin | IVIG, CCS | Hemiparesis, aphasia |
| Cinteza 2022 [19] | 1 (1/0) | 2 years | No | Anterior descending artery, left main artery, right coronary artery | Yes | Heparin, aspirin, thrombolysis | IVIG, CCS | Thrombosis resolution |
| Dolhnikoff 2020 [20] | 1 (1/0) | 11 years | Yes | Pulmonary arterioles and renal glomerular capillaries | No | None | None | Death |
| Fernandes 2021 [21] | 1 (1/0) | 11 years | Yes | Femoral vein | No | Heparin | IVIG | Death |
| Ghatasheh 2021 [22] | 1 (1/0) | 9 months | Yes | Right coronary artery | No | Heparin, aspirin | IVIG | Na |
| Kaushik 2021 [25] | 1 (1/0) | 5 years | Yes | Cerebral infarction and subarachnoid hemorrhage | Yes | Heparin | Tocilizumab | Death |
| Kavthekar 2022 [26] | 1 (1/0) | 16 years | Yes | Right ventricle, pulmonary artery | Yes | Heparin | IVIG, CCS | Death |
| Keskin 2022 [27] | 1 (1/0) | 9 years | No | Median antebrachial vein and stroke | Yes | Heparin, aspirin | IVIG, CCS | Facial paralysis |
| Kihira 2020 [28] | 1 (1/0) | 5 years | Yes | Anterior and middle cerebral artery | Yes | Heparin | None | Death |
| Kotula 2020 [29] | 1 (0/1) | 15 years | Yes | Pulmonary arteries, superior vena cava | No | Heparin, thrombolysis | None | Spasticity |
| Krasic 2022 [30] | 1 (1/0) | 3 years | Yes | Left ventricle, middle cerebral artery | No | Heparin, aspirin | CCS | Hemiplegia and facial palsy |
| Manchola Narvaez 2022 [32] | 1 (0/1) | 4 months | Yes | Coronary artery | Yes | Heparin, aspirin | IVIG | Na |
| Minen 2021 [31] | 2 (1/1) | 13–14years | Yes | Anterior and middle cerebral artery | Yes | Heparin | CCS, infliximab | Death |
| Pabst 2022 [33] | 1 (1/0) | School-age | Yes | Middle cerebral artery | Yes | Thrombectomy, aspirin | IVIG, CCS | Mild left-sided weakness |
| Plouffe 2021 [35] | 1 (1/0) | 6 years | Yes | Renal infarct | No | Aspirin | None | Na |
| Qasim 2021 [36] | 1 (0/1) | 13 years | Yes | Thoracic and abdominal aorta, renal artery | No | Heparin | IVIG, CCS | Na |
| Riphagen 2020 [37] | 1 (1/0) | 14 years | Yes | Middle and anterior cerebral artery | No | Heparin, aspirin | IVIG, CCS | Death |
| Santos 2022 [38] | 1 (0/1) | 3 years | Yes | Stroke | No | Heparin, aspirin | IVIG, CCS | Hemiparesis, aphasia |
| Schroder 2022 [39] | 1 (1/0) | 17 years | Yes | Left ventricle | No | Heparin, aspirin | IVIG, CCS, anakinra | Thrombosis resolution |
| Schupper 2020 [40] | 2 (2/0) | 2 months–5 years | Yes | Middle and posterior cerebral artery | Yes | None | None | Death |
| Shobhavat 2020 [41] | 3 (1/2) | 12 years | Yes | Infarct of periventricular white matter and radial artery | Yes | Aspirin | CCS | Death |
| Stidham 2022 [42] | 1 (1/0) | 13 years | Yes | Left anterior descending coronary artery | No | Heparin, aspirin, cangrelor, thrombectomy | IVIG, CCS, infliximab | Thrombosis resolution |
| Tehseen 2022 [43] | 5 (4/1) | 5–10 years | Yes | Left ventricle, internal jugular vein, iliac vein, pulmonary artery, renal artery | 2/5 Yes | Heparin, aspirin, direct oral anticoagulant | IVIG, CCS, infliximab, anakinra | Na |
| Thomas 2022 [44] | 1 (1/0) | 6 years | Yes | Stroke | No | Heparin | IVIG, CCS | Resolution of neurological deficits |
| Tiwari 2021 [45] | 1 (0/1) | 9 years | Yes | Carotid artery, middle and anterior cerebral artery | No | Heparin | IVIG, CCS | Psychomotor illness |
| Tolunay 2021 [46] | 1 (0/1) | 8 years | Yes | Intracardiac | Yes | Heparin | IVIG, CCS | Na |
| Vielleux 2022 [47] | 3 (2/1) | 3–12 years | No | Middle and posterior cerebral artery | No | Heparin, apixaban, aspirin, thrombolysis, thrombectomy | CCS | Hemiparesis, hemianopia, memory impairment |
| Whitworth 2021 [10] | 9 (4/5) | 13–18 years | Yes | Stroke, deep veins of upper and lower limbs | Yes 7/9 | Na | Na | Na |
| Woods 2021 [48] | 1 (1/0) | 12 years | Yes | Pulmonary artery, ascending aorta, left and right ventricle, right atrium | No | Heparin, thrombolysis | None | Mild articulation difficulties and unsteady gait |
| Zaki 2022 [49] | 1 (1/0) | 6 months | Yes | Left coronary artery | Yes | Heparin, clopidrogrel | IVIG, CCS | Neurological insult and chronic renal failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniscalco, V.; Niccolai, R.; Marrani, E.; Maccora, I.; Bertini, F.; Pagnini, I.; Simonini, G.; Lasagni, D.; Trapani, S.; Mastrolia, M.V. Thrombotic Events in MIS-C Patients: A Single Case Report and Literature Review. Children 2023, 10, 618. https://doi.org/10.3390/children10040618
Maniscalco V, Niccolai R, Marrani E, Maccora I, Bertini F, Pagnini I, Simonini G, Lasagni D, Trapani S, Mastrolia MV. Thrombotic Events in MIS-C Patients: A Single Case Report and Literature Review. Children. 2023; 10(4):618. https://doi.org/10.3390/children10040618
Chicago/Turabian StyleManiscalco, Valerio, Rachele Niccolai, Edoardo Marrani, Ilaria Maccora, Federico Bertini, Ilaria Pagnini, Gabriele Simonini, Donatella Lasagni, Sandra Trapani, and Maria Vincenza Mastrolia. 2023. "Thrombotic Events in MIS-C Patients: A Single Case Report and Literature Review" Children 10, no. 4: 618. https://doi.org/10.3390/children10040618
APA StyleManiscalco, V., Niccolai, R., Marrani, E., Maccora, I., Bertini, F., Pagnini, I., Simonini, G., Lasagni, D., Trapani, S., & Mastrolia, M. V. (2023). Thrombotic Events in MIS-C Patients: A Single Case Report and Literature Review. Children, 10(4), 618. https://doi.org/10.3390/children10040618







