1. Introduction
The dose of any drug is determined through an understanding of pharmacokinetics (what the body does to a drug) and pharmacodynamics (what the drug does to the body). Acetaminophen pharmacodynamic effects include both beneficial (analgesia and temperature modulation) and adverse (hepatotoxicity). These two pharmacodynamic effects modulate the dose. The dose prescribed should be that determined to achieve a plasma concentration associated with beneficial effects, but without untoward effects. Both of these pharmacodynamic effects are concentration-related, rather than dose-related.
Acetaminophen dosing in the perioperative period is complicated by two further aspects. Children are often administered a loading dose before a surgical procedure to achieve that “sweet spot” concentration where beneficial effects outweigh toxic effects, known as the target concentration. That dose, invariably determined by volume of distribution, differs from the maintenance dose that maintains the target concentration in plasma. The other aspect complicating acetaminophen dosing is that there is a fear that doses above a prescribed amount (e.g., 1000 mg four times daily in an adult) contributes to toxicity. This restriction on dose means that the target concentration associated with analgesic benefit can seldom be achieved in an obese teenager [
1].
2. Current Acetaminophen Dose Estimation
Expert opinion presumes that the dose in the obese child is best determined by pharmacokinetic understanding [
2,
3,
4]. It is known that obesity has an effect on acetaminophen volume of distribution, notably through dissemination between lean body mass and fat mass, both of which increase independently in the obese individual. Drug lipophilicity is thought to be a primary determinant of the volume of distribution to the fat component of body composition [
5]. Clearance is assumed to increase, reflective of increases in lean body mass, which in turn is consistent with metabolic activity [
6,
7,
8]. Altered hepatic protein production and consequently altered protein binding in obese children can be an issue for some drugs, but acetaminophen has low protein binding and this is not a major concern for acetaminophen pharmacokinetic changes. Hepatic disease is, however, an issue. Obese adolescents have an increased prevalence of non-alcoholic fatty liver disease that may increase hepatic cytochrome P450 (CYP) 2E1 expression, an enzyme responsible for the production of metabolites that contribute to acetaminophen hepatotoxicity [
9]. Morbidly obese individuals may exhibit increased CYP2E1-mediated oxidation of acetaminophen [
10], although the clinical impact of this observation had been questioned [
11].
Hepatic dysfunction, a consequence of morbid obesity, could have a major effect on acetaminophen clearance. However, clinical scoring systems (e.g., Child–Pugh or MELD classification) are hard to quantify and relate to altered drug disposition. Each patient has an individual pattern of dysfunction and different drugs are affected differently, depending on their clearance mechanism [
12]. Altered hepatic blood flow in severe liver disease may, for example, reduce the clearance of drugs that are perfusion-limited, but this pathology has a limited impact on drugs such as acetaminophen that are capacity-limited.
Few of these obesity-related pharmacokinetic changes have been quantified in either adults or children [
13]. Acetaminophen pharmacokinetics in overweight or obese children are ill-described and there is little information to guide physicians with regard to the dose [
14]. There are some data to suggest that pharmacokinetic parameters are similar in both lean and obese adolescents [
15] or adults [
16] after single-dose therapy. The obese adolescent has been the subject of commentary [
13], but dosing recommendations for the obese adolescent remain uncertain, with suggestions for the use of ideal body weight [
17] or lean body weight [
18]. These recommendations do not consider the impact of fat mass, do not define at what degree of obesity they should be implemented, do not distinguish between loading dose and maintenance dose, and have never been validated for acetaminophen in children. They do, however, reduce the dose, when expressed per kilogram, as weight increases.
3. Size Model Foibles
Total body weight is commonly used for dosing in obese children, but that contributes to dose error because the contribution from the fat mass portion of body composition is seldom acknowledged. The fat mass may contribute to the volume of distribution (V) through drug lipid solubility altering disposition. It is also a metabolically active component of body mass and as such contributes to clearance (CL). Failure to account for fat mass contributes to dosing errors. There are few practical dose recommendations for obese children [
2,
19] even though it is known that fat mass influences the volume of distribution and clearance [
20], that effects from fat mass are drug-specific [
20], and that dosing per kilogram (weight-based linear dosing) is a contributor to dose inaccuracies. Further, obesity can be considered an inflammatory disease that both contributes to disease processes and is sometimes consequent to disease processes. An assortment of body size scalers (e.g., total body weight, body surface area, ideal body weight, lean body mass, adjusted body weight, body mass index, fat-free mass, and allometry) have been used to determine the dose in obese individuals [
21]. This assortment leaves clinicians confused about which metric to use for a particular drug for an individual child, or even the degree of obesity that commands a size metric other than total body weight. The dosing of children in the operating room may be dependent on a size metric that differs with anaesthesia phases (e.g., lean body mass for propofol induction dose and total body weight for maintenance dose rate) [
22,
23,
24].
Although recommendations for particular size scalers abound in the literature [
21], most carry the caveat that the dose in the obese child will be determined by better pharmacokinetic understanding [
2,
3,
4] and this remains lacking for many drugs used in the perioperative period. The use of pharmacokinetic (PK) and pharmacodynamic (PD) mathematical equations (known as models) is a central foundation for improving dose estimation [
25]. There are two covariates, size (reflecting body mass) and age (reflecting maturation processes in children [
26]) that are important contributors accounting for PK parameter (e.g., clearance, volume) variability [
27,
28]. Contributors to pharmacodynamics (PD: E
MAX, C
50) variability are poorly quantified, but age, particularly in neonates, is important. Size can be standardized to a 70 kg person using allometric theory [
29]. Fat is a component of body composition that certainly contributes to PK parameter variability [
30], but has been poorly investigated in children [
23].
We review the concepts behind the determination of dose for acetaminophen and endeavour to explain and quantify the impact of fat mass on dose computation.
4. Physiological Models
The pharmacokinetics of drugs used in children can be estimated using two quite different methods: physiological models (e.g., physiology-based pharmacokinetics, PBPK, top-down) or using patient data (e.g., compartment pharmacokinetic models, bottom-up). Physiological models use mathematical equations to describe an organism as a closed circulatory system consisting of compartments that represent the organs important for pharmacokinetic description, such as absorption, distribution, metabolism and elimination [
31]. They describe the anatomy, physiological processes, and chemical reactions happening in the body e.g., blood flow to organs and their metabolic activity. These models have the capacity for incorporation of so much more information, such as pharmacogenomics or environmental influences [
31,
32]. Physiological models are capable of using existing information about obesity-related physiological changes (e.g., altered organ size, composition, and function), and drug-specific properties (e.g., lipophilicity and elimination pathways) [
33]. This type of modelling has been used successfully to investigate clindamycin, trimethoprim/sulfamethoxazole, and metformin to better understand the dosing of these drugs in children with obesity [
34,
35].
This modelling technique has been used to investigate acetaminophen pharmacokinetics in humans [
36] as well as special populations such as pregnant patients [
37] and premature neonates [
38]. It is lacking for obese children, but has potential use in the investigation of pharmacokinetics in these children. Enoxyparin dose, for example, has been investigated in obese children using both PBPK [
39] and compartment models [
40]; both methods concluded that fat-free mass was a good size scaler to use for Enoxyparin dose estimation in obese children.
5. The Target Effect
The determination of dose in children cannot be made using pharmacokinetic knowledge alone. It is necessary to understand pharmacodynamics as well. A key aspect of dose determination is knowing what concentration should be targeted in order to achieve the desired effect. This target concentration strategy [
41] requires an understanding of the concentration–response relationship.
A concentration–analgesic response relationship has been described in children who have been given acetaminophen for pain after tonsillectomy. This relationship has been defined using a pharmacodynamic (PD) model, the E
MAX or Hill equation [
42,
43]:
The pharmacodynamic parameter, E
MAX, is the maximum drug effect (5.17 on a visual analogue scale 0–10), C
50 is the concentration eliciting half of E
MAX (9.97 mg/L), and the Hill exponent (Hill or N = 1) describes the steepness of the concentration–response curve [
44]. This relationship is displayed graphically in
Figure 1 and can be used to predict the target concentration known to be associated with a target effect. A target effect of 2.6 pain unit reduction (VAS 0–10) is associated with a target concentration of 10 mg/L. This acetaminophen target concentration of 10 mg/L is similar for both neonates and children [
44,
45]. Acetaminophen is a mild analgesic with a maximum effect of only 5.17 pain units. Children with an initial pain score of 10 pain units will still require remedication at this target concentration because their pain score will remain high at a VAS of 7.4 units. However, pain in those with an initial score of 6 pain units will be better managed.
6. Dose Calculation Using the Target Concentration
The target effect is the goal of drug treatment. This target effect is associated with a target concentration. The pharmacokinetic model can be used to calculate a dose [
41,
46] that achieves the target concentration [
47], a process known as the target concentration strategy. Enteral acetaminophen disposition can usually be described using a single compartment [
44,
48,
49,
50]. Time–concentration relationships for a one-compartment pharmacokinetic model, for example, are expressed in terms of the parameter clearance (CL) and volume of distribution (V):
The pharmacokinetic parameter volume of distribution (V) is used determine a loading dose that achieves a desired target concentration for a one-compartment model (Equation (3)) while clearance (CL) determines the maintenance dose rate (Equation (4)).
Acetaminophen administered enterally is often described using a one-compartment model, but that model may be inadequate to portray the time–concentration profile for acetaminophen if oral absorption is slow or if delivered intravenously, where further compartments are required to describe the time course of drug concentration in plasma [
26,
51]. This multi-compartment model is required to describe drugs administered intravenously into the central compartment (V1) that then redistributes to peripheral compartments (V2, V3, etc.;
Figure 1). The loading dose may be too small if based on V1 or too big if based on the volume of distribution at steady state (Vss). Redistribution takes place during loading dose administration. The peripheral compartment may differ between lean and obese children due to drug lipid solubility, further complicating dose calculation.
The concentration used to describe the observed response can be that in the effect compartment (Ce) rather than the plasma (Cp) [
52]. This additional compartment (the effect compartment) accounts for time delays between plasma concentration and observed response (
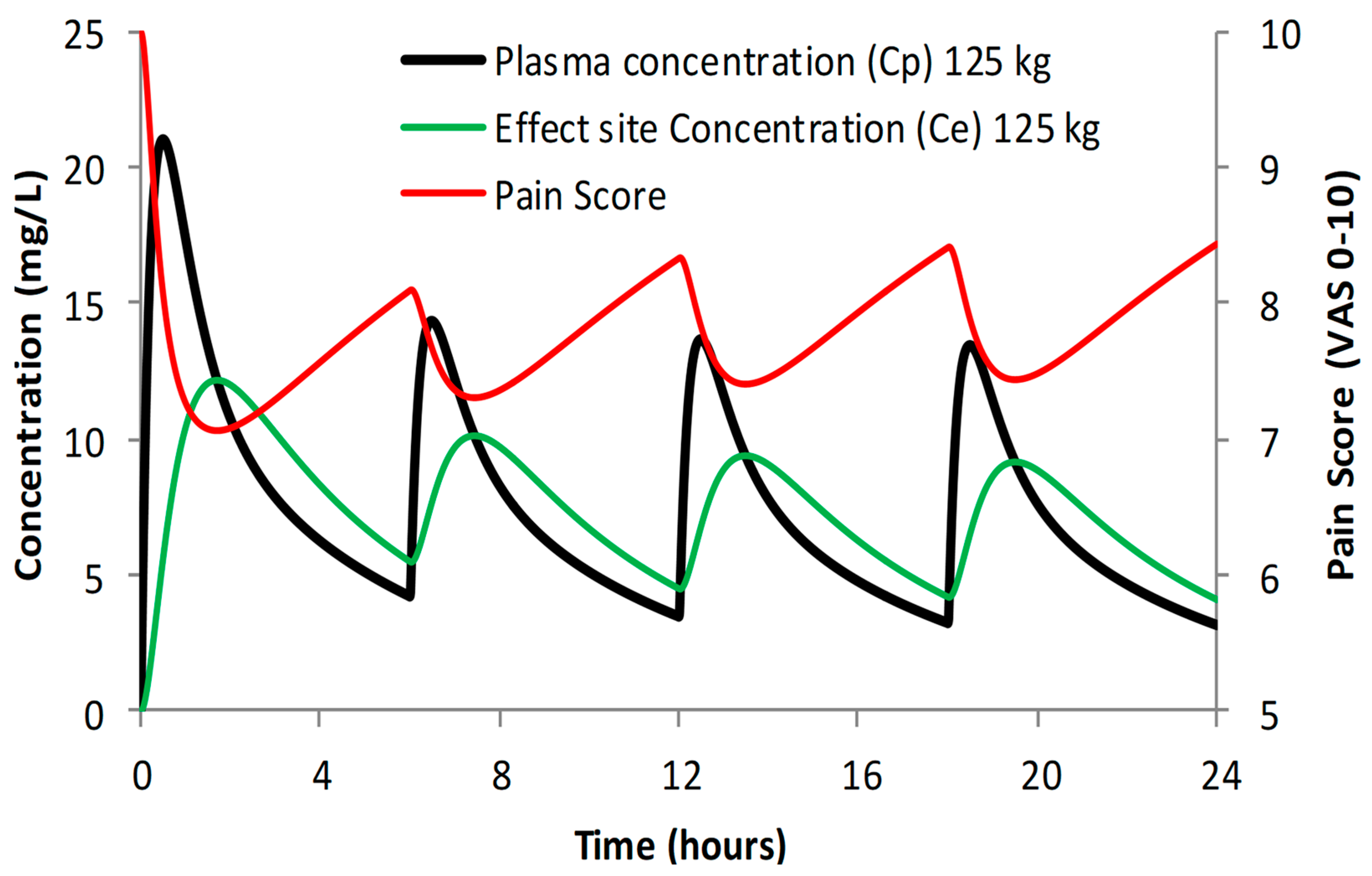
Figure 1). The delay between plasma and effect compartments is described by an equilibration half-time (T1/2keo) and this is approximately 53 min for acetaminophen (
Figure 2) [
44]. This delay has clinical implications. The drug should be given before the anticipated pain insult, or the dose should be managed so that effect compartment concentration is above the target level in the post-anaesthesia recovery room (PACU). The use of a loading dose achieves both these aims. The high plasma concentrations overshoot the target concentration and the target concentration in the effect compartment is reached earlier than if a standard maintenance dose (MD) is used (
Figure 2). In addition, the larger loading dose (LD) ensures that effect compartment concentrations are above the target level for a longer duration than the if a standard maintenance dose is used (e.g., LD 30 mg/kg vs. MD 15 mg/kg)
7. Dosing Concepts in the Child
The principles behind dose estimation involve an understanding of pharmacokinetic parameters, clearance and volume. Weight (reflecting size) and age (contributing maturation of physiological processes) are the major covariates contributing to parameter variability in children, [
28] but fat mass is also important and contributes to both these parameters, even in lean individuals. Maturation of physiological and anatomical processes has a greater impact in neonates and infants.
7.1. The Association between Size and Dose
Drugs are commonly dosed per kilogram of total body weight in children. That dose often changes with age so that the dose (per kilogram) is higher for a 2-year-old child than for a 10-year-old child. This is because the pharmacokinetic parameters (e.g., CL, V) that determine dose are based on size. It is important to separate out the impact of size so that other covariate influences (e.g., age-related changes, organ dysfunction, or obesity-associated changes) can be assessed.
Drug dose calculations are commonly made assuming a linear relationship between TBW and dose (Equation (5)):
where a standard dose (Dose
STD) is that for a person of standard weight (WT
STD e.g., 70 kg). This equation demonstrates dosing commonly known as dosing per kilogram. However, it is widely known that maintenance doses expressed as mg/kg, as in Equation (4), are too small in children when compared to adults; this linear approach is not a suitable general method for drug dosing in children [
53]. The maintenance dose should be based on clearance (Equation (3)), but clearance has a nonlinear, not linear relationship with size.
7.2. Allometry
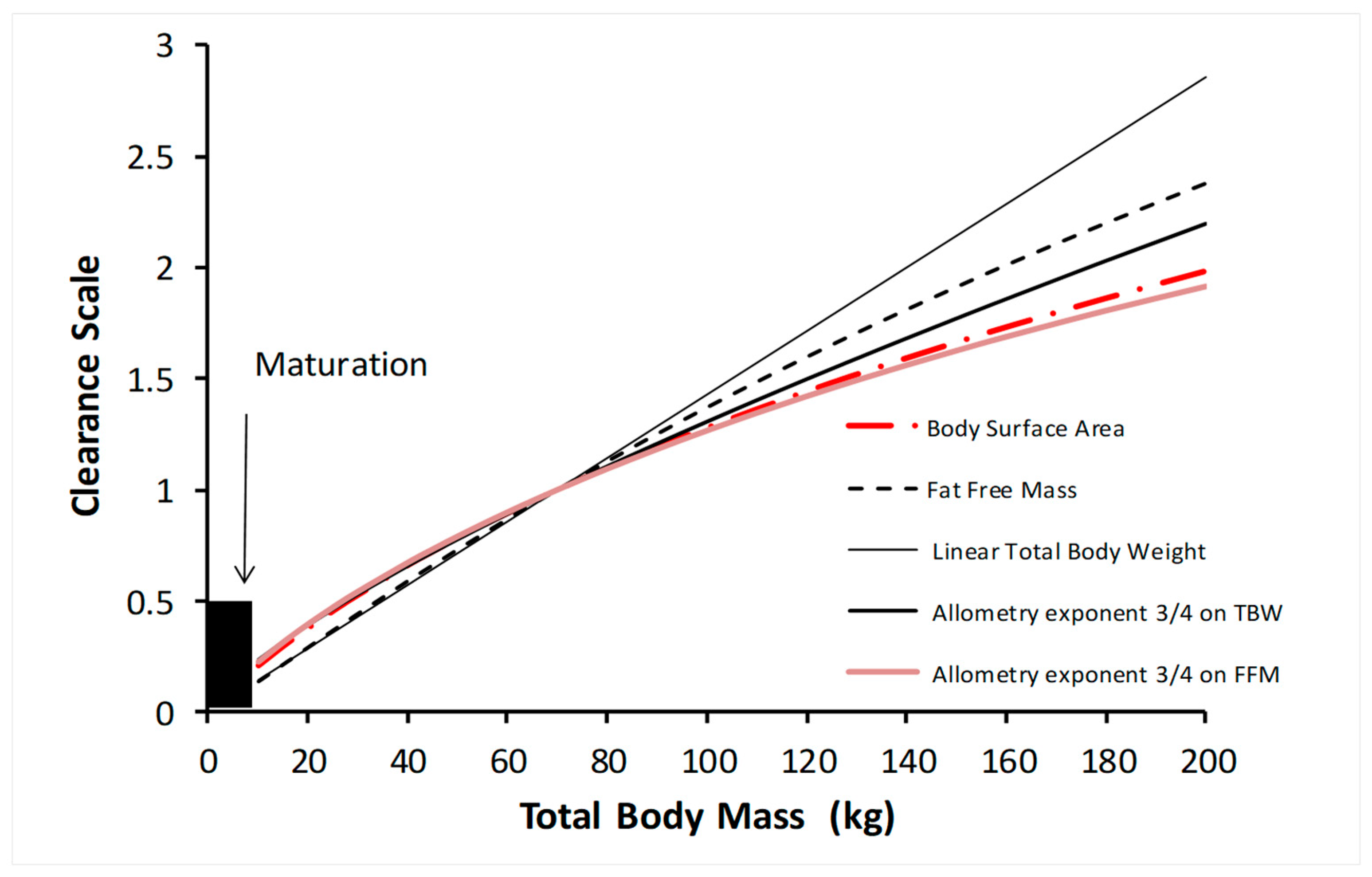
The use of allometry introduces the nonlinear relationship between size and clearance (
Figure 3). Allometry is the relationship between the size of an organism and its physiology (functional aspects), morphology (structural aspects), and life history (temporal aspects). The relationship between physiological traits (e.g., metabolic processes such as clearance) and structural components (e.g., blood volume or volume of distribution), and time-related processes (heart rate, respiratory rate, and drug half-life) and size has been used to scale pharmacokinetic parameters.
The log of basal metabolic rate plotted against the log of body weight produces a straight line with a slope of ¾ across species, with size changes that are 18 orders of magnitude. Similar relationships for volumes (e.g., blood volume) have a slope of 4/4, while time-related functions (e.g., heart rate) have a slope of ¼. Fractal geometry is used to mathematically explain this allometric scaling law [
54,
55]. Total drug clearance may be expected to scale to weight with an exponent of ¾ (Equation (6)) [
56], so that clearance in a child can be predicted from that in an adult person of standard weight, which is 70 kg (WT
STD):
Clearance maturation occurs in the first year of life and a function describing this maturation is required (
Figure 3) during that period. Maturation is usually described using another function (F
MATURATION) that uses age as an independent variable.
Figure 3 shows how clearance is less than might be expected from total body weight with the linear per kilogram model. The difference between the linear total body weight prediction and the allometric weight prediction increases with total body weight. Other bodyweight scalers shown in
Figure 3 (body surface area, fat-free mass) are also curvilinear in nature, a relationship that exists in both obese and lean individuals [
20]. There is no change at any one weight where a size scaler should be changed from one to another. The rate of clearance increase slows as size increases; consequently, the dose, when expressed per kilogram of total body weight without allometry, is invariably excessive.
Total body weight, without allometry, is a poor size scaler. Ideal body weight (IBW), which also has a non-linear relationship to clearance (i.e., rate of clearance increase slows as size increases), is currently the only alternative body size scaler to total body weight [
57] mentioned in the British National Formulary for Children [
58]. However, the calculation of IBW is not facile and there are five published methods available for its calculation [
59]. There is a poor understanding of when and how to calculate IBW among paediatricians [
60]. If IBW should be the preferred size scaler for the obese child, then uncertainty exists about an obese size measure at which it should be implemented. Even the definition of obesity changes with age and body mass index [
61]. Ideal body weight does not account for fat mass and is not the best scaler for all drugs in which fat mass has a varying impact on PK parameters [
4]. A better scaler would consider the impact of fat mass on pharmacokinetic parameters and have applicability to all children, lean or obese.
7.3. Fat Mass
It is suggested that 75% of excess weight in obese children is fat mass, and the remainder is lean mass [
23], but the very definition of obesity in children relies on variability above a mean weight for age, and excess weight is poorly quantified [
61]. It is assumed that increases in fat mass alter the distribution of lipophilic drugs and increases in lean mass alter drug clearance, but there is a paucity of evidence supporting these assumptions for most drugs [
23]. The contribution of fat mass to pathology is not acknowledged. Investigators have used an assortment of size scalers to empirically explain the contribution of fat mass for individual drugs [
24] with scant evidence for the superiority of one metric over another.
7.3.1. Lean Body Mass
It has been asserted that lean body mass (LBM) (commonly used interchangeably with lean body weight (LBW) and fat-free mass (FFM)) is the optimal size scalar for many drugs used in anaesthesia during the perioperative period [
62,
63,
64,
65,
66]. The merits of using LBM have been reviewed, with the conclusion that LBM is a good predictor of drug dose for all drugs [
67]. This extension to all drugs remains unproven [
4,
21]. Direct comparison with other size scalers has rarely been undertaken. Lean body mass does not consider fat mass, which is known to have an effect on pharmacokinetic parameters.
7.3.2. Normal Fat Mass
Any size scaler must account for fat mass and must be applicable to children of all weights. There seems little value in using total body weight for children who are lean and then switching to an alternative size scaler in those children classified as obese. The idea of adding a fraction of fat mass to FFM has been used to estimate the mass that best describes structure and function based on allometric scaling theory [
47]. This mass has been called normal fat mass (NFM) [
68]. NFM is calculated from FFM and FAT mass (Equation (7)):
The fraction of FAT (Ffat) that contributes to the structural (V) or functional (CL) size is specific to each drug (Equation (8)).
If Ffat is estimated to be zero then NFM is FFM, while if Ffat is 1 then NFM is TBW. Normal fat mass, used in conjunction with allometry, lies between FFM and TBW when used with allometry (
Figure 3) and is specific for each drug. NFM may also differ when used for clearance and when used for volume of distribution. NFM requires the determination of Ffat. While the impact of this parameter (Ffat) has been established for the renal elimination pathway over a broad range of ages from premature neonates to adults [
69], it has only been determined for a handful of drugs [
68,
70,
71], one of which is acetaminophen [
72]. The parameter Ffat was estimated as 1 for volume (i.e., TBW) and 0.82 for clearance. Total body weight can then be used as the size metric for an acetaminophen loading dose.
A negative value for Ffat for clearance (Ffat
CL) might suggest organ dysfunction. Obesity is associated with organ dysfunction in the morbidly obese. Dexmedetomidine was noted to have a negative value for Ffat
CL in morbidly obese adults [
73]. Although we might anticipate that Ffat increases with lipid solubility when used for volume of distribution, this has not yet been demonstrated.
8. Application of NFM Principles for Acetaminophen Dosing in Children
Once the impact of fat mass on pharmacokinetic parameters (CL, V) has been evaluated, then those pharmacokinetic parameters can be used in all children, lean or obese. It is not necessary to change to a different size scaler simply because the patient fulfils criteria that determine a specific grade of obesity.
8.1. Loading Dose
An acetaminophen loading dose is commonly given by mouth (per os, po) preoperatively. The use of rectal formulations was favoured in the past because of fears related to the aspiration of gastric contents during anaesthesia consequent to having an increased volume of fluid in the stomach. However, fasting guidelines for children presenting for routine procedures have been relaxed [
74,
75,
76] and acetaminophen elixir is cleared from the stomach quickly [
77]. Rectal formulations, although effective, require a larger dose because of reduced bioavailability [
78] and are associated with high plasma concentration variability and slow absorption [
48,
79].
The appropriate size matrix for a loading dose of acetaminophen is total body weight because Ffat
VOL = 1. Simulated plasma concentrations attained after a loading dose of acetaminophen of 30 mg/kg in a 6-year-old, 20 kg child (FFM 16.4 kg, BMI 15.12 kg/m
2) are shown in
Figure 2. Effect compartment concentrations of 10 mg/L are achieved at 25 min and decrease below this concentration at 3.5 h. The loading dose (30 mg/kg) is the same for obese and lean children. Clearance, however, determines the duration of time that concentrations remain above 10 mg/L. Clearance increases with weight when expressed per kilogram, and so the duration of concentration above 10 mg/L in a 6-year-old, 40 kg child (FFM 24.7 kg, BMI 30.25 kg/m
2) is longer (
Figure 2). However, while concentrations might be estimated to be below 10 mg/L at 4 h 25 min in the lean child, the impact of adding Ffat = 0.82 to the simulation is minimal. Simulation using TBW (Ffat = 1) with allometry reveals a time below 10 mg/L at 4 h 15 min, a small analgesic difference because the concentration–response curve is shallow at that concentration (
Figure 1). The impact of separating out fat mass for loading dose estimation is minimal. Total body weight is the better scaler for an acetaminophen loading dose.
8.2. Maintenance/Infusion Dose
The difference in drug clearance between an adult and a child is predictable from NFM used in conjunction with allometry (Equation (9)) [
56]:
The maintenance dose can then be calculated based on the steady-state target concentration (Equation (10)):
Simulation has been used to demonstrate the impact of size on predicted concentration in 10-year-old children (weight 30 kg, FFM 24 kg, BMI 15.3 kg/m
2; weight 50 kg, FFM 33 kg, BMI 25.5 kg/m
2; and weight 70 kg, FFM 39 kg, BMI 35.7 kg/m
2 (
Figure 4)) given a loading dose of 30 mg/kg and a maintenance dose of 15 mg/kg 6-hourly. While clearance increase is nonlinear and predicted concentrations increase with size, these higher concentrations are unlikely to contribute a meaningful improvement to analgesia. Similarly, the use of NFM (Ffat
CL = 0.82) instead of TBW (Ffat
CL = 1.0) will have minimal impact on pain scores. This clinical impact is minimal because predicted concentrations are on the flat part of the concentration–response curve, because a clinically important pain score change is more than 1 pain unit (VAS 0–10) [
80,
81], and because both PK and PD parameter estimates are associated with considerable variability [
28].
If it is assumed that the target concentration in children and adults is the same, then the relationship between doses in children and adults can be predicted (Equation (11)):
This dosing extrapolation becomes problematic in teenagers because the adult maintenance dose is commonly capped at 1000 mg. An obese teenager (e.g., weight 125 kg) administered a loading dose of acetaminophen 2 g with a maintenance dosing of 1000 mg 6-hourly will not reach the target concentration of 10 mg/L at steady-state conditions (
Figure 5). There will be a mean pain score decrease of 2 (VAS 0–10) and while this is a meaningful pain decrease, it is a small decrease and will require supplementation from other analgesic drugs.
9. Consideration of Adverse Effects
9.1. Hepatotoxicity
Acetaminophen (APAP) dosing in children is tempered by concerns of hepatotoxicity. The toxic metabolite of acetaminophen, N-acetyl-p-benzoquinone imine (NAPQI), is formed by the P450 hepatic cytochrome, CYP2E1. Hepatotoxicity is dependent on the balance between the rate of NAPQI formation, the capacity of the acetaminophen elimination clearance pathways involving hepatic glucuronide and sulfate conjugation, and the initial content and maximal rate of synthesis of hepatic glutathione that mops up NAPQI. NAPQI binds to intracellular hepatic macromolecules to produce cell necrosis and damage.
9.1.1. Loading Dose
Hepatotoxicity in children is relatable to concentration, not dose. Dose, a measure determined using pharmacokinetic parameters, is commonly used as a surrogate to assess the risk of hepatotoxicity. There remains a distinction between hepatotoxicity due to a single dose of acetaminophen (e.g., a loading dose) and that administered over a duration longer than 2–3 days at doses greater than 90 mg/kg/min. The Rumack and Matthew [
82] acetaminophen toxicity nomogram is widely used to guide the management of acetaminophen overdose in adults and children. This nomogram interprets acetaminophen clearance and relates clearance to a concentration at time points after 4 h. Acetaminophen concentrations of more than 300 mg/L at 4 h were always associated with severe hepatic lesions, but none were observed in adults with concentrations lower than 150 mg/L. The half-life was less than 4 h in all patients without liver damage.
Clearance, expressed as L/h/kg, is greater in children than in adults. The 4 h concentration determined by clearance in preschool children following accidental ingestion of acetaminophen elixir is less than that in adults. This is because the absorption of the elixir is more rapid than that noted in adults following tablet ingestion and because clearance is faster in toddlers than in adults. As a consequence, younger children (1–5 years) require larger doses than older children and adults to achieve similar concentrations at 4 h. Children (1–5 years) with reported accidental ingestion of greater than 250 mg/kg (compared to 150 mg/kg in adults) can have the serum concentration measured at 2 h after ingestion rather than the 4 h time point recommended in adults [
83]. Preoperative loading doses administered before anaesthesia induction (e.g., 30 mg/kg) are well below those associated with toxicity.
9.1.2. Maintenance Dose
Maintenance dosing in excess of 90 mg/kg/day administered over 2–3 days is of greater concern. This dose will cause higher concentrations in teenagers than in children 2–3 years of age because clearance, expressed per kilogram, is faster in the younger cohort. Hepatic and renal disease, malnutrition, and dehydration may increase the propensity for toxicity. Medications that induce NAPQI formation (e.g., phenobarbitone, phenytoin, and rifampicin) may also increase the risk of hepatotoxicity. The influence of obesity on acetaminophen toxicity is unknown and although there are occasional reports [
84], the influence of dose administered per kilogram and the underlying pathology of the child remains uncertain. Hepatotoxicity causing death or requiring liver transplantation has been reported with doses above 75 mg/kg/day in children and 90 mg/kg/day in infants. It has been suggested that even these traditional regimens may cause hepatotoxicity if used for longer than 2 to 3 days [
85]. The dose that might lead to hepatotoxicity remains speculative and ‘safe’ doses range from 60 mg/kg/day through to 90 mg/kg/day [
86,
87].
9.2. Concentration or Dose
Dosing restrictions for acetaminophen have eventuated because of toxicity fears associated with doses greater than 75 mg/kg/day in children. This lower dose became standard clinical practice following the introduction of the intravenous formulation of acetaminophen, where the dose was dictated by the pharmaceutical industry operating in a litigious environment. It is not the dose that causes toxicity, but rather a plasma concentration (or perhaps exposure, measured using the area under the curve). The dose is determined using pharmacokinetic knowledge to enable a target concentration to be reached. Fear of litigation has resulted in underdosing of obese teenagers.
9.3. The Acetaminophen–NSAID Interaction
Practitioner choices for acetaminophen maintenance dosing are limited. If the use of the target concentration is chosen, then a lower target concentration than 10 mg/L must be used, resulting in less effective analgesia. Capping a dose at 1000 mg in obese teenagers is particularly irksome for practitioners when it is known that a target concentration of 10 mg/L cannot be achieved with the 1000 mg dose. This concentration of 10 mg/L is not associated with toxicity but has a reasonable analgesic effect.
One solution is to use acetaminophen–nonsteroidal anti-inflammatory drug (NSAID) combination therapy. Acetaminophen and NSAIDs are often given together for the management of pain [
88] or fever [
89]. They can be safely combined using lower doses of each drug without increases in their associated adverse effect profiles and combination therapy is both popular [
90] and recommended for analgesia after procedures such as tonsillectomy [
91]. The maximum analgesic effect (e.g., E
MAX 5 to 6, VAS 0–10) remained the same as that for either agent alone, but that analgesic effect was sustained at 4 to 8 h after combination dosing [
92,
93,
94]. The dose for this combination therapy is often also dictated by regulatory authorities, e.g., 4.5 mg/kg ibuprofen (maximum dose 300 mg) and 15 mg/kg paracetamol (max dose 1000 mg) [
95]. The dose of ibuprofen is lower than that commonly prescribed alone [
96]. Ibuprofen has a similar FAT fraction for clearance (fFat
CL = 0.86) to acetaminophen, but with a lower FAT fraction for volume (fFat
VOL = 0.72) [
72]. However, because the ibuprofen dose is small, the dose for the mixture could be calculated based on calculations for acetaminophen alone.
There are few data concerning ibuprofen pharmacokinetics or dosing in obese children [
97]. Ibuprofen dose could be based on normal fat mass with allometry. Should ibuprofen be given separately from acetaminophen, then the dose of ibuprofen can be readily calculated using NFM (Equation (9)). Fat-free mass (FFM) can be predicted from sex, height, and total body weight (Equation (12)).
where WHS
MAX is the maximum FFM for any given height (HT, m) and WHS
50 is the TBW value when FFM is half of WHS
MAX. For men, WHS
MAX is 42.92 kg·m
−2 and WHS
50 is 30.93 kg·m
−2, and for women, WHS
MAX is 37.99 kg·m
−2 and WHS
50 is 35.98 kg·m
−2 [
98]. Computation of FFM in children has been simplified by the availability of online calculators (e.g., [
99]).
10. Conclusions
The loading dose is determined by total body weight (mg/kg) and is the same in both lean and obese children. The maintenance dose (mg/kg) in children with obesity is less than that presumed using linear scaling. There is a curvilinear relationship between clearance and weight. The dose should reflect that relationship. Ideal body weight (IBW) has been proposed as an appropriate size scaler for use in obese children. However, IBW calculation is not easy, and although it may describe a curvilinear relationship with clearance, it is neither drug-specific nor does it distinguish between clearance or volume. The fat mass has an influence on both clearance and volume and fat mass is present in children, even those considered lean.
The use of NFM as a size scaler for acetaminophen has merit, but the computations required to calculate the dose are also not facile, although online calculators are available. Acetaminophen has the advantage that the loading dose can be calculated using TBW. The maintenance dose can be better calculated using NFM; however, because the FAT fraction is large (Ffat
CL = 0.82) and because some consider the estimation of this parameter to have low precision [
100], then TBW is a reasonable proxy if used with allometric scaling.
If we assume a typical adult (70 kg) will be given a maintenance dose of 1000 mg (15 mg/kg) four times a day, then the dose for a 10-year-old weighing 30 kg can be readily calculated (Equation (14)):
A 10-year-old child 120 kg in weight will require a maintenance dose (Equation (13))
It can be noted that while weight has increased 4-fold from 30 kg to 120 kg, the dose has only increased 3-fold from 500 mg to 1500 mg, exemplifying the non-linear relationship between clearance and weight. The dose can be scaled directly from the adult dose using allometry in children, but not for infants and neonates where physiological processes are maturing.
Author Contributions
B.J.A. and L.I.C. contributed to conceptualization, methodology, formal analysis, and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Ethical approval was not required.
Informed Consent Statement
Study did not contain humans. This is a computer simulation study. No informed consent was needed or sought or used.
Data Availability Statement
No new data were generated or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hakim, M.; Anderson, B.J.; Walia, H.; Tumin, D.; Michalsky, M.P.; Syed, A.; Tobias, J.D. Acetaminophen pharmacokinetics in severely obese adolescents and young adults. Paediatr. Anaesth. 2019, 29, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendrick, J.G.; Carr, R.R.; Ensom, M.H. Pharmacokinetics and drug dosing in obese children. J. Pediatr. Pharmacol. Ther. 2010, 15, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, F.; Conti, V.; Pepe, A.; Vajro, P.; Filippelli, A.; Mandato, C. Drug dosing in children with obesity: A narrative updated review. Ital. J. Pediatr. 2022, 48, 168. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Getting the dose right for obese children. Arch. Dis. Child. 2017, 102, 54–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 2010, 49, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, M. Body size and metabolism. Hilgardia 1932, 6, 315–333. [Google Scholar] [CrossRef] [Green Version]
- Kleiber, M. The Fire of Life: An Introduction to Animal Energetics; Wiley: New York, NY, USA, 1961. [Google Scholar]
- Kleiber, M. Metabolic turnover rate: A physiological meaning of the metabolic rate per unit body weight. J. Theor. Biol. 1975, 53, 199–204. [Google Scholar] [CrossRef]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- van Rongen, A.; Välitalo, P.A.J.; Peeters, M.Y.M.; Boerma, D.; Huisman, F.W.; van Ramshorst, B.; van Dongen, E.P.A.; van den Anker, J.N.; Knibbe, C.A.J. Morbidly Obese Patients Exhibit Increased CYP2E1-Mediated Oxidation of Acetaminophen. Clin. Pharmacokinet. 2016, 55, 833–847. [Google Scholar] [CrossRef] [Green Version]
- Reith, M.E.A.; Benuck, M.; Lajtha, A. Cocaine Dispositon in the Brain after Continuous or Intermittent Treatment and Locomotor Stimulation in Mice. J. Pharmacol. Exp. Ther. 1987, 243, 281–287. [Google Scholar]
- Blaschke, T.F. Protein binding and kinetics of drugs in liver diseases. Clin. Pharmacokinet. 1977, 2, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Vaughns, J.D.; Ziesenitz, V.C.; van den Anker, J.N. Clinical pharmacology of frequently used intravenous drugs during bariatric surgery in adolescents. Curr. Pharm. Des. 2015, 21, 5650–5659. [Google Scholar] [CrossRef] [PubMed]
- Zempsky, W.T.; Bhagat, P.K.; Siddiqui, K. Practical Challenges-Use of Paracetamol in Children and Youth Who Are Overweight or Obese: A Narrative Review. Paediatr. Drugs 2020, 22, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Barshop, N.J.; Capparelli, E.V.; Sirlin, C.B.; Schwimmer, J.B.; Lavine, J.E. Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 198–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abernethy, D.R.; Divoll, M.; Greenblatt, D.J.; Ameer, B. Obesity, sex, and acetaminophen disposition. Clin. Pharmacol. Ther. 1982, 31, 783–790. [Google Scholar] [CrossRef]
- Lee, W.H.; Kramer, W.G.; Granville, G.E. The effect of obesity on acetaminophen pharmacokinetics in man. J. Clin. Pharmacol. 1981, 21, 284–287. [Google Scholar] [CrossRef]
- Green, B.; Duffull, S.B. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br. J. Clin. Pharmacol. 2004, 58, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Matson, K.L.; Horton, E.R.; Capino, A.C. Medication Dosage in Overweight and Obese Children. J. Pediatr. Pharmacol. Ther. 2017, 22, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Greenblatt, D.J.; Bruno, C.D.; Harmatz, J.S.; Zhang, Q.; Chow, C.R. Drug Disposition in Subjects with Obesity: The Research Work of Darrell R. Abernethy. J. Clin. Pharmacol. 2022, 62, 1350–1363. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. What is the best size predictor for dose in the obese child? Paediatr. Anaesth. 2017, 27, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Casati, A.; Putzu, M. Anesthesia in the obese patient: Pharmacokinetic considerations. J. Clin. Anesth. 2005, 17, 134–145. [Google Scholar] [CrossRef]
- Mulla, H.; Johnson, T.N. Dosing dilemmas in obese children. Arch. Dis. Child.-Educ. Pract. 2010, 95, 112–117. [Google Scholar] [CrossRef]
- Mortensen, A.; Lenz, K.; Abildstrom, H.; Lauritsen, T.L. Anesthetizing the obese child. Paediatr. Anaesth. 2011, 21, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.D.; Cortinez, L.I.; Anderson, B.J. Pharmacokinetic pharmacodynamic modelling contributions to improve paediatric anaesthesia practice. J. Clin. Med. 2022, 11, 3009. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Holford, N.H. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 2009, 24, 25–36. [Google Scholar] [CrossRef]
- Holford, N.; Heo, Y.A.; Anderson, B. A pharmacokinetic standard for babies and adults. J. Pharm. Sci. 2013, 102, 2941–2952. [Google Scholar] [CrossRef]
- Anderson, B.J. My child is unique; the pharmacokinetics are universal. Paediatr. Anaesth. 2012, 22, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Meakin, G.H. Scaling for size: Some implications for paediatric anaesthesia dosing. Paediatr. Anaesth. 2002, 12, 205–219. [Google Scholar] [CrossRef]
- Al-Sallami, H.S.; Goulding, A.; Grant, A.; Taylor, R.; Holford, N.; Duffull, S.B. Prediction of Fat-Free Mass in Children. Clin. Pharmacokinet. 2015, 54, 1169–1178. [Google Scholar] [CrossRef]
- Edginton, A.N.; Theil, F.P.; Schmitt, W.; Willmann, S. Whole body physiologically-based pharmacokinetic models: Their use in clinical drug development. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1143–1152. [Google Scholar] [CrossRef]
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharmacokinet. 2006, 45, 1013–1034. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, J.G.; Balevic, S.; Sinha, J.; Perrin, E.M.; Wang, J.; Edginton, A.N.; Gonzalez, D. Characterizing Pharmacokinetics in Children with Obesity-Physiological, Drug, Patient, and Methodological Considerations. Front. Pharmacol. 2022, 13, 818726. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, J.G.; Carreño, F.O.; Edginton, A.N.; Sinha, J.; Perrin, E.M.; Kumar, K.R.; Rikhi, A.; Hornik, C.P.; Harris, V.; Ganguly, S.; et al. Development and evaluation of a virtual population of children with obesity for physiologically based pharmacokinetic modeling. Clin. Pharmacokinet. 2022, 61, 307–320. [Google Scholar] [CrossRef]
- Ford, J.L.; Gerhart, J.G.; Edginton, A.N.; Yanovski, J.A.; Hon, Y.Y.; Gonzalez, D. Physiologically Based Pharmacokinetic Modeling of Metformin in Children and Adolescents With Obesity. J. Clin. Pharmacol. 2022, 62, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Zurlinden, T.J.; Reisfeld, B. Physiologically based modeling of the pharmacokinetics of acetaminophen and its major metabolites in humans using a Bayesian population approach. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Brookhuis, S.A.M.; Allegaert, K.; Hanff, L.M.; Lub-de Hooge, M.N.; Dallmann, A.; Mian, P. Modelling Tools to Characterize Acetaminophen Pharmacokinetics in the Pregnant Population. Pharmaceutics 2021, 13, 1302. [Google Scholar] [CrossRef]
- Claassen, K.; Thelen, K.; Coboeken, K.; Gaub, T.; Lippert, J.; Allegaert, K.; Willmann, S. Development of a Physiologically-Based Pharmacokinetic Model for Preterm Neonates: Evaluation with In Vivo Data. Curr. Pharm. Des. 2015, 21, 5688–5698. [Google Scholar] [CrossRef]
- Gerhart, J.G.; Carreño, F.O.; Loop, M.S.; Lee, C.R.; Edginton, A.N.; Sinha, J.; Kumar, K.R.; Kirkpatrick, C.M.; Hornik, C.P.; Gonzalez, D. Use of Real-World Data and Physiologically-Based Pharmacokinetic Modeling to Characterize Enoxaparin Disposition in Children With Obesity. Clin. Pharmacol. Ther. 2022, 112, 391–403. [Google Scholar] [CrossRef]
- Derbalah, A.; Duffull, S.; Sherwin, C.M.; Job, K.; Al-Sallami, H. Optimal dosing of enoxaparin in overweight and obese children. Br. J. Clin. Pharmacol. 2022, 88, 5348–5358. [Google Scholar] [CrossRef]
- Holford, N.; Ma, G.; Metz, D. TDM is dead. Long live TCI! Br. J. Clin. Pharmacol. 2022, 88, 1406–1413. [Google Scholar] [CrossRef]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 14, iv–vii. [Google Scholar]
- Holford, N.H.G.; Sheiner, L.B. Understanding the dose-effect relationship: Clinical application of pharmacokinetic-pharmacodynamic models. Clin. Pharmacokinet. 1981, 6, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Woollard, G.A.; Holford, N.H. Acetaminophen analgesia in children: Placebo effect and pain resolution after tonsillectomy. Eur. J. Clin. Pharmacol. 2001, 57, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Naulaers, G.; Vanhaesebrouck, S.; Anderson, B.J. The paracetamol concentration-effect relation in neonates. Paediatr. Anaesth. 2013, 23, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.H.G. The target concentration approach to clinical drug development. Clin. Pharmacokinet. 1995, 29, 287–291. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Understanding dosing: Children are small adults, neonates are immature children. Arch. Dis. Child. 2013, 98, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Woolard, G.A.; Holford, N.H. Pharmacokinetics of rectal paracetamol after major surgery in children. Paediatr. Anaesth. 1995, 5, 237–242. [Google Scholar] [PubMed]
- Anderson, B.J.; Woollard, G.A.; Holford, N.H. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Brit. J. Clin. Pharmacol. 2000, 50, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.J.; van Lingen, R.A.; Hansen, T.G.; Lin, Y.C.; Holford, N.H. Acetaminophen developmental pharmacokinetics in premature neonates and infants: A pooled population analysis. Anesthesiology 2002, 96, 1336–1345. [Google Scholar] [CrossRef]
- Anderson, B.J.; Pons, G.; Autret-Leca, E.; Allegaert, K.; Boccard, E. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: A population analysis. Paediatr. Anaesth. 2005, 15, 282–292. [Google Scholar] [CrossRef]
- Gibb, I.A.; Anderson, B.J. Paracetamol (acetaminophen) pharmacodynamics; interpreting the plasma concentration. Arch. Dis. Child. 2008, 93, 241–247. [Google Scholar] [CrossRef]
- Holford, N.H. A size standard for pharmacokinetics. Clin. Pharmacokinet. 1996, 30, 329–332. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sales, M.; Holford, N.; Bonnefois, G.; Desrochers, J. Wide size dispersion and use of body composition and maturation improves the reliability of allometric exponent estimates. J. Pharmacokinet. Pharmacodyn. 2022, 49, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, N.; Rodriguez-Gonzalvez, C.; Clarke, J. Ideal body weight in the precision era: Recommendations for prescribing in obesity require thought for computer-assisted methods. Arch. Dis. Child. 2020, 105, 516. [Google Scholar] [CrossRef] [Green Version]
- BMJ Group and Royal Pharmaceutical Society. British National Formulary for Children; Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Moylan, A.; Appelbaum, N.; Clarke, J.; Feather, C.; Tairraz, A.F.; Maconochie, I.; Darzi, A. Assessing the Agreement of 5 Ideal Body Weight Calculations for Selecting Medication Dosages for Children with Obesity. JAMA Pediatr. 2019, 173, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Collier, H.; Nasim, M.; Gandhi, A. Prescribing in obese children: How good are paediatricians? Arch. Dis. Child. 2017, 102, 61–62. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ingrande, J.; Brodsky, J.B.; Lemmens, H.J. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth. Analg. 2011, 113, 57–62. [Google Scholar] [CrossRef]
- Lemmens, H.J. Perioperative pharmacology in morbid obesity. Curr. Opin. Anaesthesiol. 2010, 23, 485–491. [Google Scholar] [CrossRef]
- Scherrer, P.D.; Mallory, M.M.; Cravero, J.P.; Lowrie, L.; Hertzog, J.H.; Berkenbosch, J.W. The impact of obesity on pediatric procedural sedation-related outcomes: Results from the Pediatric Sedation Research Consortium. Paediatr. Anaesth. 2015, 25, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.D. Remifentanil pharmacokinetics and pharmacodynamics. A preliminary appraisal. Clin. Pharmacokinet. 1995, 29, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Eleveld, D.J.; Proost, J.H.; Vereecke, H.; Absalom, A.R.; Olofsen, E.; Vuyk, J.; Struys, M. An allometric model of remifentanil pharmacokinetics and pharmacodynamics. Anesthesiology 2017, 126, 1005–1018. [Google Scholar] [CrossRef]
- Morgan, D.J.; Bray, K.M. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin. Pharmacokinet. 1994, 26, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.H.G.; Anderson, B.J. Allometric size: The scientific theory and extension to normal fat mass. Eur. J. Pharm. Sci. 2017, 109, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, C.J.; Holford, N.; Sumpter, A.; Al-Sallami, H.S. Consistent methods for fat-free mass, creatinine clearance, and glomerular filtration rate to describe renal function from neonates to adults. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 401–412. [Google Scholar] [CrossRef]
- Morse, J.D.; Cortinez, L.I.; Anderson, B.J. Considerations for intravenous anesthesia dose in obese children: Understanding PKPD. J. Clin. Med. 2023, 12, 1642. [Google Scholar] [CrossRef]
- Takahashi, S.; Tsuji, Y.; Holford, N.; Ogami, C.; Kasai, H.; Kawasuji, H.; To, H.; Yamamoto, Y. Population Pharmacokinetic Model for Unbound Concentrations of Daptomycin in Patients with MRSA Including Patients Undergoing Hemodialysis. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 201–211. [Google Scholar] [CrossRef]
- Morse, J.D.; Stanescu, I.; Atkinson, H.C.; Anderson, B.J. Population Pharmacokinetic Modelling of Acetaminophen and Ibuprofen: The Influence of Body Composition, Formulation and Feeding in Healthy Adult Volunteers. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 497–507. [Google Scholar] [CrossRef]
- Cortinez, L.I.; Anderson, B.J.; Holford, N.H.; Puga, V.; de la Fuente, N.; Auad, H.; Solari, S.; Allende, F.A.; Ibacache, M. Dexmedetomidine pharmacokinetics in the obese. Eur. J. Clin. Pharmacol. 2015, 71, 1501–1508. [Google Scholar] [CrossRef]
- Andersson, H.; Hellstrom, P.M.; Frykholm, P. Introducing the 6-4-0 fasting regimen and the incidence of prolonged preoperative fasting in children. Paediatr. Anaesth. 2018, 28, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Schmitz, A.; Frykholm, P. Preoperative fasting guidelines in pediatric anesthesia: Are we ready for a change? Curr. Opin. Anaesthesiol. 2018, 31, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frykholm, P.; Disma, N.; Andersson, H.; Beck, C.; Bouvet, L.; Cercueil, E.; Elliott, E.; Hofmann, J.; Isserman, R.; Klaucane, A.; et al. Pre-operative fasting in children: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2022, 39, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Rees, S.G.; Liley, A.; Stewart, A.W.; Wardill, M.J. Effect of preoperative paracetamol on gastric volumes and pH in children. Paediatr. Anaesth. 1999, 9, 203–207. [Google Scholar] [PubMed]
- Anderson, B.J.; Holford, N.H.; Woollard, G.A.; Kanagasundaram, S.; Mahadevan, M. Perioperative pharmacodynamics of acetaminophen analgesia in children. Anesthesiology 1999, 90, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Monteleone, J.; Holford, N.H. Variability of concentrations after rectal paracetamol. Paediatr. Anaesth. 1998, 8, 274. [Google Scholar] [PubMed]
- Bahreini, M.; Safaie, A.; Mirfazaelian, H.; Jalili, M. How much change in pain score does really matter to patients? Am. J. Emerg. Med. 2020, 38, 1641–1646. [Google Scholar] [CrossRef]
- Voepel-Lewis, T. Do 10 million ANOVAs satisfy the quest for pain score meaning? Pain 2013, 154, 2581–2582. [Google Scholar] [CrossRef]
- Rumack, B.H.; Matthew, H. Acetaminophen poisoning and toxicity. Pediatrics 1975, 55, 871–876. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H.; Armishaw, J.C.; Aicken, R. Predicting concentrations in children presenting with acetaminophen overdose. J. Pediatr. 1999, 135, 290–295. [Google Scholar] [CrossRef]
- Miles, F.K.; Kamath, R.; Dorney, S.F.A.; Gaskin, K.J.; O’Loughlin, E.V. Accidental paracetamol overdosing and fulminant hepatic failure in children. Med. J. Aust. 1999, 171, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Leeder, J.S.; Wasserman, G.S. Acetaminophen overdose with therapeutic intent. J. Pediatr. 1998, 132, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Penna, A.; Buchanan, N. Paracetamol poisoning in children and hepatotoxicity. Br. J. Clin. Pharmacol. 1991, 32, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, L.F. Therapeutic misadventure with paracetamol: Fact or fiction? Am. J. Ther. 2000, 7, 99–114. [Google Scholar] [CrossRef]
- Liu, C.; Ulualp, S.O. Outcomes of an alternating ibuprofen and acetaminophen regimen for pain relief after tonsillectomy in children. Ann. Otol. Rhinol. Laryngol. 2015, 124, 777–781. [Google Scholar] [CrossRef]
- Luo, S.; Ran, M.; Luo, Q.; Shu, M.; Guo, Q.; Zhu, Y.; Xie, X.; Zhang, C.; Wan, C. Alternating Acetaminophen and Ibuprofen versus Monotherapies in Improvements of Distress and Reducing Refractory Fever in Febrile Children: A Randomized Controlled Trial. Paediatr. Drugs 2017, 19, 479–486. [Google Scholar] [CrossRef]
- Ong, C.K.; Seymour, R.A.; Lirk, P.; Merry, A.F. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: A qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth. Analg. 2010, 110, 1170–1179. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol.-Head Neck Surg. 2019, 160, S1–S42. [Google Scholar] [CrossRef] [Green Version]
- Hannam, J.; Anderson, B.J. Explaining the acetaminophen-ibuprofen analgesic interaction using a response surface model. Paediatr. Anaesth. 2011, 21, 1234–1240. [Google Scholar] [CrossRef]
- Hannam, J.A.; Anderson, B.J.; Mahadevan, M.; Holford, N.H. Postoperative analgesia using diclofenac and acetaminophen in children. Paediatr. Anaesth. 2014, 24, 953–961. [Google Scholar] [CrossRef]
- Hannam, J.A.; Anderson, B.J.; Potts, A. Acetaminophen, ibuprofen, and tramadol analgesic interactions after adenotonsillectomy. Paediatr. Anaesth. 2018, 28, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Playne, R.; Anderson, B.J.; Frampton, C.; Stanescu, I.; Atkinson, H.C. Analgesic effectiveness, pharmacokinetics, and safety of a paracetamol/ibuprofen fixed-dose combination in children undergoing adenotonsillectomy: A randomized, single-blind, parallel group trial. Paediatr. Anaesth. 2018, 28, 1086–1095. [Google Scholar] [CrossRef]
- Anderson, B.J.; Hannam, J.A. A target concentration strategy to determine ibuprofen dosing in children. Paediatr. Anaesth. 2019, 29, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Shamsaee, E.; Huws, A.; Gill, A.; McWilliam, S.J.; Hawcutt, D.B. Ibuprofen efficacy, tolerability and safety in obese children: A systematic review. Arch. Dis. Child. 2023, 108, 67–71. [Google Scholar] [CrossRef]
- Janmahasatian, S.; Duffull, S.B.; Ash, S.; Ward, L.C.; Byrne, N.M.; Green, B. Quantification of lean bodyweight. Clin. Pharmacokinet. 2005, 44, 1051–1065. [Google Scholar] [CrossRef]
- Al-Sallami, H.; Erikson, L. Fat-Free Mass Calculator. Available online: https://www.otago.ac.nz/pharmacometrics/downloads/ffm-calculator.html (accessed on 15 March 2023).
- Wasmann, R.E.; Svensson, E.M.; Schalkwijk, S.J.; Brüggemann, R.J.; Ter Heine, R. Normal fat mass cannot be reliably estimated in typical pharmacokinetic studies. Eur. J. Clin. Pharmacol. 2021, 77, 727–733. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).