Improvement of Diet after an Early Nutritional Intervention in Pediatric Oncology
Abstract
:1. Introduction
2. Materials and Methods
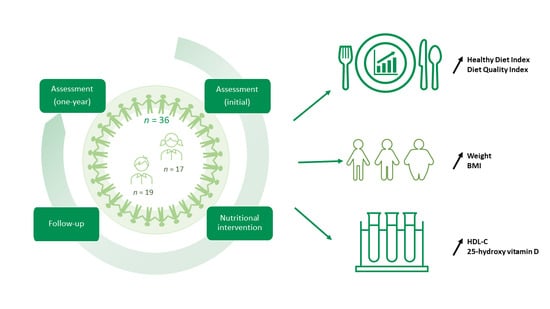
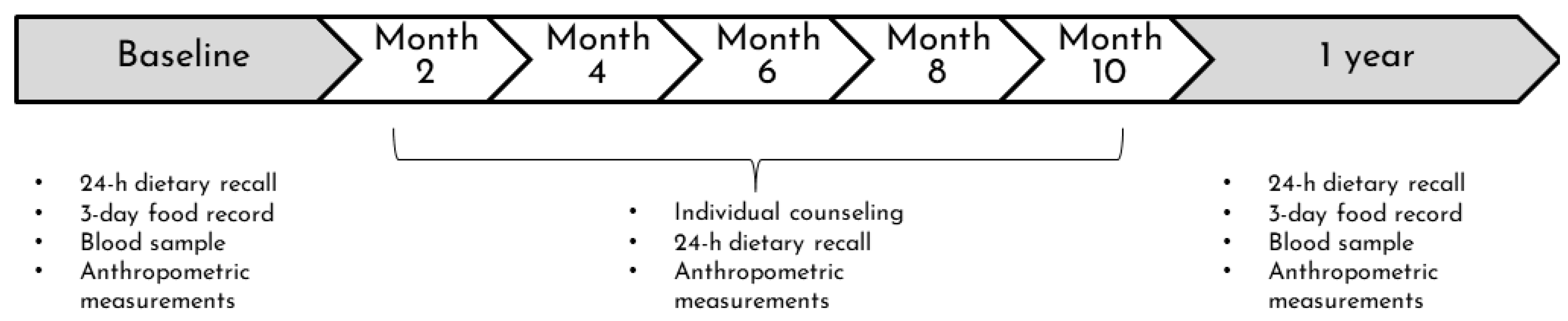
2.1. Study Design
2.2. Participants
2.3. Nutrition Intervention
2.4. Dietary Assessment Data
2.5. Anthropometric Evaluation and Blood Pressure
2.6. Biochemical Assessment
2.7. Statistical Analyses
3. Results
3.1. Description of the Cohort
3.2. Evolution of Diet Quality Scores and Dietary Intakes during the Intervention
3.3. Evolution of Clinical and Anthropometrical Characteristics during the Intervention
3.4. Biochemical Assessment at Initial Evaluation and after One Year of Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Therrien, R.; Delisle, J.-F.; Ferland, G.; Larocque, D.; McMahon, J.; Pedneault, L.; Perreault, M.; Viau, A. Le cancer chez l’enfant. Pharmactuel 2013, 46, 192–201. [Google Scholar]
- Hudson, M.M.; Link, M.P.; Simone, J.V. Milestones in the curability of pediatric cancers. J. Clin. Oncol. 2014, 32, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999-2007: Results of EUROCARE-5--a population-based study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Pui, C.H.; Pei, D.; Sandlund, J.T.; Campana, D.; Ribeiro, R.C.; Razzouk, B.I.; Rubnitz, J.E.; Howard, S.C.; Hijiya, N.; Jeha, S.; et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2005, 23, 7936–7941. [Google Scholar] [CrossRef]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Hood, A.F. Cutaneous side effects of cancer chemotherapy. Med. Clin. N. Am. 1986, 70, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.; Ludwig, K.; Pacenta, H.L.; Klesse, L.J.; Watt, T.C.; Laetsch, T.W. Recent progress in the treatment of cancer in children. CA Cancer J. Clin. 2021, 71, 315–332. [Google Scholar] [CrossRef]
- Landier, W.; Armenian, S.; Bhatia, S. Late effects of childhood cancer and its treatment. Pediatr. Clin. N. Am. 2015, 62, 275–300. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Neglia, J.P.; Leisenring, W.; Robison, L.L.; Mertens, A.C. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2328–2338. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Sklar, C. Second cancers in survivors of childhood cancer. Nat. Rev. Cancer 2002, 2, 124–132. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Berrington de Gonzalez, A.; Schulte, R.; Lee, C. A Review of Radiotherapy-Induced Late Effects Research after Advanced Technology Treatments. Front. Oncol. 2016, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, C.; Sun, L.R. Neurological complications of pediatric cancer. Cancer Metastasis Rev. 2020, 39, 3–23. [Google Scholar] [CrossRef] [PubMed]
- van Waas, M.; Neggers, S.J.; Pieters, R.; van den Heuvel-Eibrink, M.M. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann. Oncol. 2010, 21, 1121–1126. [Google Scholar] [CrossRef]
- Chow, E.J.; Leger, K.J.; Bhatt, N.S.; Mulrooney, D.A.; Ross, C.J.; Aggarwal, S.; Bansal, N.; Ehrhardt, M.J.; Armenian, S.H.; Scott, J.M.; et al. Paediatric cardio-oncology: Epidemiology, screening, prevention, and treatment. Cardiovasc. Res. 2019, 115, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.D.; Nagarajan, R.; Godown, J. Pediatric Cardio-Oncology: Development of Cancer Treatment-Related Cardiotoxicity and the Therapeutic Approach to Affected Patients. Curr. Treat. Opt. Oncol. 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Reulen, R.C.; Winter, D.L.; Frobisher, C.; Lancashire, E.R.; Stiller, C.A.; Jenney, M.E.; Skinner, R.; Stevens, M.C.; Hawkins, M.M. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010, 304, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Belle, F.N.; Wenke-Zobler, J.; Cignacco, E.; Spycher, B.D.; Ammann, R.A.; Kuehni, C.E.; Zimmermann, K. Overweight in childhood cancer patients at diagnosis and throughout therapy: A multicentre cohort study. Clin. Nutr. 2019, 38, 835–841. [Google Scholar] [CrossRef]
- Lange, B.J.; Gerbing, R.B.; Feusner, J.; Skolnik, J.; Sacks, N.; Smith, F.O.; Alonzo, T.A. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA 2005, 293, 203–211. [Google Scholar] [CrossRef]
- Chow, E.J.; Pihoker, C.; Friedman, D.L.; Lee, S.J.; McCune, J.S.; Wharton, C.; Roth, C.L.; Baker, K.S. Glucocorticoids and insulin resistance in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2013, 60, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Oeffinger, K.C.; Adams-Huet, B.; Victor, R.G.; Church, T.S.; Snell, P.G.; Dunn, A.L.; Eshelman-Kent, D.A.; Ross, R.; Janiszewski, P.M.; Turoff, A.J.; et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2009, 27, 3698–3704. [Google Scholar] [CrossRef] [Green Version]
- Beauloye, V.; Steffens, M.; Zech, F.; Vermylen, C.; Maiter, D. Characterization of insulin resistance in young adult survivors of childhood acute lymphoblastic leukaemia and non-Hodgkin lymphoma. Clin. Endocrinol. (Oxf.) 2013, 78, 790–798. [Google Scholar] [CrossRef]
- Esbenshade, A.J.; Simmons, J.H.; Koyama, T.; Lindell, R.B.; Friedman, D.L. Obesity and insulin resistance in pediatric acute lymphoblastic leukemia worsens during maintenance therapy. Pediatr. Blood Cancer 2013, 60, 1287–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, M.C.; Sharp, E.; Kalpatthi, R.; Zullo, J.; Gurtunca, N.; Zhang, J.; Krafty, R.; Raman, S. Hyperglycemia requiring insulin during acute lymphoblastic leukemia induction chemotherapy is associated with increased adverse outcomes and healthcare costs. Pediatr. Blood Cancer 2020, 67, e28475. [Google Scholar] [CrossRef]
- Morel, S.; Leahy, J.; Fournier, M.; Lamarche, B.; Garofalo, C.; Grimard, G.; Poulain, F.; Delvin, E.; Laverdière, C.; Krajinovic, M.; et al. Lipid and lipoprotein abnormalities in acute lymphoblastic leukemia survivors. J. Lipid Res. 2017, 58, 982–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, E.J.; Pihoker, C.; Hunt, K.; Wilkinson, K.; Friedman, D.L. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer 2007, 110, 2313–2320. [Google Scholar] [CrossRef]
- Levy, E.; Samoilenko, M.; Morel, S.; England, J.; Amre, D.; Bertout, L.; Drouin, S.; Laverdière, C.; Krajinovic, M.; Sinnett, D.; et al. Cardiometabolic Risk Factors in Childhood, Adolescent and Young Adult Survivors of Acute Lymphoblastic Leukemia—A Petale Cohort. Sci. Rep. 2017, 7, 17684. [Google Scholar] [CrossRef] [Green Version]
- Nottage, K.A.; Ness, K.K.; Li, C.; Srivastava, D.; Robison, L.L.; Hudson, M.M. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia—From the St. Jude Lifetime Cohort. Br. J. Haematol. 2014, 165, 364–374. [Google Scholar] [CrossRef]
- Haddy, T.B.; Mosher, R.B.; Reaman, G.H. Hypertension and prehypertension in long-term survivors of childhood and adolescent cancer. Pediatr. Blood Cancer 2007, 49, 79–83. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nutr. 2021, 61, 1651–1669. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.G.; Lim, H.; Kim, Y.; Ju, Y.S.; Lee, H.J.; Jang, H.B.; Park, S.I.; Park, K.H. The Effect of a Multidisciplinary Lifestyle Intervention on Obesity Status, Body Composition, Physical Fitness, and Cardiometabolic Risk Markers in Children and Adolescents with Obesity. Nutrients 2019, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu-Gagnon, S.; Bélanger, V.; Marcil, V. Food habits during treatment of childhood cancer: A critical review. Nutr. Res. Rev. 2019, 32, 265–281. [Google Scholar] [CrossRef]
- Cohen, J.; Goddard, E.; Brierley, M.E.; Bramley, L.; Beck, E. Poor Diet Quality in Children with Cancer During Treatment. J. Pediatr. Oncol. Nurs. 2021, 38, 313–321. [Google Scholar] [CrossRef]
- Wright, M.J.; Collins, L.; Christie, A.; Birken, K.; Dettmer, E.; Nathan, P.C. A Comprehensive Healthy Lifestyle Program for Children Receiving Treatment for Acute Lymphoblastic Leukemia: Feasibility and Preliminary Efficacy Data. Rehabil. Oncol. 2013, 31, 6–13. [Google Scholar] [CrossRef]
- Murphy, A.J.; White, M.; Davies, P.S. Body composition of children with cancer. Am. J. Clin. Nutr. 2010, 92, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, C.A.K.; Murphy-Alford, A.J.; Cohen, J.; Fleming, M.R.; Wakefield, C.E.; Naumann, F. Poor diet quality and adverse eating behaviors in young survivors of childhood cancer. Pediatr. Blood Cancer 2022, 69, e29408. [Google Scholar] [CrossRef]
- Cohen, J.E.; Wakefield, C.E.; Cohn, R.J. Nutritional interventions for survivors of childhood cancer. Cochrane Database Syst. Rev. 2016, 2016, Cd009678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, P.C. Nutritional status as a prognostic indicator for pediatric malignancies. J. Clin. Oncol. 2014, 32, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Pencharz, P.; Barr, R.D. Children, cancer, and nutrition—A dynamic triangle in review. Cancer 2004, 100, 677–687. [Google Scholar] [CrossRef]
- Hughes, W.T.; Price, R.A.; Sisko, F.; Havron, W.S.; Kafatos, A.G.; Schonland, M.; Smythe, P.M. Protein-calorie malnutrition. A host determinant for Pneumocystis carinii infection. Am. J. Dis. Child. 1974, 128, 44–52. [Google Scholar] [CrossRef]
- Taj, M.M.; Pearson, A.D.; Mumford, D.B.; Price, L. Effect of nutritional status on the incidence of infection in childhood cancer. Pediatr. Hematol. Oncol. 1993, 10, 283–287. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Stern, M.; Bachar, E.; Ronen Ackerman, E.; Rancourt, D.; Bonne, O.; Weintraub, M. Weight Trajectories of Israeli Pediatric Cancer Survivors. J. Pediatr. Psychol. 2017, 42, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.L.; Loman, D.G.; Balakas, K.; Flowers, M. Nutritional screening and early intervention in children, adolescents, and young adults with cancer. J. Pediatr. Oncol. Nurs. 2012, 29, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J. Nutritional assessment and intervention in a pediatric oncology unit. Indian J. Cancer 2015, 52, 186–190. [Google Scholar] [CrossRef]
- Walters, M.; Mowbray, C.; Jubelirer, T.; Jacobs, S.; Kelly, K.M.; Smith, K.; Yao, Y.; Jin, Z.; Ladas, E.J. A bilingual dietary intervention early in treatment is feasible and prevents weight gain in childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2021, 68, e28910. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, V.; Delorme, J.; Napartuk, M.; Bouchard, I.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; Marcil, V. Early Nutritional Intervention to Promote Healthy Eating Habits in Pediatric Oncology: A Feasibility Study. Nutrients 2022, 14, 1024. [Google Scholar] [CrossRef]
- Caru, M.; Duhamel, G.; Marcil, V.; Sultan, S.; Meloche, C.; Bouchard, I.; Drouin, S.; Bertout, L.; Laverdiere, C.; Sinnett, D.; et al. The VIE study: Feasibility of a physical activity intervention in a multidisciplinary program in children with cancer. Support Care Cancer 2020, 28, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef] [Green Version]
- Huijbregts, P.; Feskens, E.; Räsänen, L.; Fidanza, F.; Nissinen, A.; Menotti, A.; Kromhout, D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: Longitudinal cohort study. BMJ 1997, 315, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Body Measurements (Anthropometry) Manual, National Health and Nutrition Examination Survey III. Available online: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/audio.pdf (accessed on 3 March 2023).
- Sharma, A.K.; Metzger, D.L.; Daymont, C.; Hadjiyannakis, S.; Rodd, C.J. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: Association with cardio-metabolic risks. Pediatr. Res. 2015, 78, 723–729. [Google Scholar] [CrossRef]
- Addo, O.Y.; Himes, J.H. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am. J. Clin. Nutr. 2010, 91, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Addo, O.Y.; Himes, J.H.; Zemel, B.S. Reference ranges for midupper arm circumference, upper arm muscle area, and upper arm fat area in US children and adolescents aged 1-20 y. Am. J. Clin. Nutr. 2017, 105, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Provincial Health Services Authority. Tools and calculators. British Colombia, Canada. 29 October 2020. Available online: http://www.bcchildrens.ca/health-professionals/clinical-resources/endocrinology-diabetes/tools-calculators (accessed on 8 July 2022).
- Organisation Mondiale de la Santé. Calcul des Centiles et des Valeurs du z Pour la Taille-Pourl’âge, le Poids-Pour-L’âge et L’imc-Pour-L’âge. 2007. Available online: https://cdn.who.int/media/docs/default-source/child-growth/growth-reference-5-19-years/calcule.pdf?sfvrsn=78ae165c_2&fbclid=IwAR2hSl38tzM8BcysY3jQWTH5gxEAfU1V3W_SXrRUDZeOYXX-hY0eKb1Xpb0 (accessed on 8 July 2022).
- Zhang, F.F.; Kelly, M.; Du, M.; Welch, J.G.; Santacruz, N.; Rhoades, J.; Kamins, C.L.; Dreyer, Z.; Scheurer, M.E. Early Lifestyle Intervention for Obesity Prevention in Pediatric Survivors of Acute Lymphoblastic Leukemia. Nutrients 2019, 11, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.L.; McLaughlin, R.A.; Rai, S.N.; Steen, B.D.; Hudson, M.M. Adolescent survivors: A secondary analysis of a clinical trial targeting behavior change. Pediatr. Blood Cancer 2005, 45, 144–154. [Google Scholar] [CrossRef]
- Hill, R.; Hamby, T.; Bashore, L.; Rapisand, S.; Galipp, K.; Heym, K.; Bowman, W.P. Early Nutrition Intervention Attenuates Weight Gain for Pediatric Acute Lymphoblastic Leukemia Patients in Maintenance Therapy. J. Pediatr. Hematol. Oncol. 2018, 40, 104–110. [Google Scholar] [CrossRef]
- Huang, J.S.; Dillon, L.; Terrones, L.; Schubert, L.; Roberts, W.; Finklestein, J.; Swartz, M.C.; Norman, G.J.; Patrick, K. Fit4Life: A weight loss intervention for children who have survived childhood leukemia. Pediatr. Blood Cancer 2014, 61, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.R.; Raber, M.P.; Crawford, K.D.; Grasse, L.; Wartenberg, L.; Wu, J.; Dibaj, S.S.; Chandra, J. High Rates of Obesity at Presentation Persist into Survivorship across Childhood Cancer Types. Child Obes. 2020, 16, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.K.; Madan-Swain, A.; Locher, J.L.; Desmond, R.A.; de Los Santos, J.; Affuso, O.; Glover, T.; Smith, K.; Carley, J.; Lipsitz, M.; et al. Harvest for health gardening intervention feasibility study in cancer survivors. Acta Oncol. 2013, 52, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Donnella, H.; Knouse, P.; Raber, M.; Crawford, K.; Swartz, M.C.; Wu, J.; Liu, D.; Chandra, J. A randomized nutrition counseling intervention in pediatric leukemia patients receiving steroids results in reduced caloric intake. Pediatr. Blood Cancer 2017, 64, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Moyer-Mileur, L.J.; Ransdell, L.; Bruggers, C.S. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: Response to a home-based exercise and nutrition program. J. Pediatr. Hematol. Oncol. 2009, 31, 259–266. [Google Scholar] [CrossRef]
- Revuelta Iniesta, R.; Paciarotti, I.; Davidson, I.; McKenzie, J.M.; Brougham, M.F.H.; Wilson, D.C. Nutritional status of children and adolescents with cancer in Scotland: A prospective cohort study. Clin. Nutr. ESPEN 2019, 32, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Brennan, B.M. Sensitive measures of the nutritional status of children with cancer in hospital and in the field. Int. J. Cancer Suppl. 1998, 11, 10–13. [Google Scholar] [CrossRef]
- Garófolo, A.; dos Santos Maia-Lemos, P. Low Levels of High-density Lipoprotein in Patients with Pediatric Cancer at Diagnosis. Rev. Bras. Cancerol. 2020, 65, e-15528. [Google Scholar] [CrossRef]
- Moschovi, M.; Trimis, G.; Apostolakou, F.; Papassotiriou, I.; Tzortzatou-Stathopoulou, F. Serum lipid alterations in acute lymphoblastic leukemia of childhood. J. Pediatr. Hematol. Oncol. 2004, 26, 289–293. [Google Scholar] [CrossRef]
- Halton, J.M.; Nazir, D.J.; McQueen, M.J.; Barr, R.D. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer 1998, 83, 379–384. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Kawashima, T.; Leisenring, W.; Stratton, K.; Stovall, M.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; Oeffinger, K.C. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J. Clin. Oncol. 2014, 32, 1218–1227. [Google Scholar] [CrossRef]
- Poirier, P.; Eckel, R.H. Obesity and cardiovascular disease. Curr. Atheroscler. Rep. 2002, 4, 448–453. [Google Scholar] [CrossRef]
- Cohen, H.; Bielorai, B.; Harats, D.; Toren, A.; Pinhas-Hamiel, O. Conservative treatment of L-asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2010, 54, 703–706. [Google Scholar] [CrossRef]
- Morel, S.; Amre, D.; Teasdale, E.; Caru, M.; Laverdière, C.; Krajinovic, M.; Sinnett, D.; Curnier, D.; Levy, E.; Marcil, V. Dietary Intakes Are Associated with HDL-Cholesterol in Survivors of Childhood Acute Lymphoblastic Leukaemia. Nutrients 2019, 11, 2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackmann, N.; Mäkitie, O.; Harila-Saari, A.; Gustafsson, J.; Nezirevic Dernroth, D.; Frisk, P. Vitamin D status in children with leukemia, its predictors, and association with outcome. Pediatr. Blood Cancer 2020, 67, e28163. [Google Scholar] [CrossRef]
- Aristizabal, P.; Sherer, M.; Perdomo, B.P.; Castelao, E.; Thornburg, C.D.; Proudfoot, J.; Jacobs, E.; Newfield, R.S.; Zage, P.; Roberts, W.; et al. Sociodemographic and clinical characteristics associated with vitamin D status in newly diagnosed pediatric cancer patients. Pediatr. Hematol. Oncol. 2020, 37, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Modan-Moses, D.; Pinhas-Hamiel, O.; Munitz-Shenkar, D.; Temam, V.; Kanety, H.; Toren, A. Vitamin D status in pediatric patients with a history of malignancy. Pediatr. Res. 2012, 72, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Rosen, G.P.; Beebe, K.L.; Shaibi, G.Q. Vitamin D levels differ by cancer diagnosis and decline over time in survivors of childhood cancer. Pediatr. Blood Cancer 2013, 60, 949–952. [Google Scholar] [CrossRef]
- Mays, D.; Black, J.D.; Mosher, R.B.; Heinly, A.; Shad, A.T.; Tercyak, K.P. Efficacy of the Survivor Health and Resilience Education (SHARE) Program to Improve Bone Health Behaviors Among Adolescent Survivors of Childhood Cancer. Ann. Behav. Med. 2011, 42, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Resnicow, K.; Odom, E.; Wang, T.; Dudley, W.N.; Mitchell, D.; Vaughan, R.; Jackson, A.; Baranowski, T. Validation of three food frequency questionnaires and 24-h recalls with serum carotenoid levels in a sample of African-American adults. Am. J. Epidemiol. 2000, 152, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.B.; Obarzanek, E.; Morrison, J.; Sabry, Z.I. Comparative advantage of 3-day food records over 24-h recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J. Am. Diet. Assoc. 1994, 94, 626–630. [Google Scholar] [CrossRef]


| Characteristics | Participants |
|---|---|
| n = 36 | |
| Sex, n (%) | |
| Male | 19 (52.8) |
| Female | 17 (47.4) |
| Age, years | |
| Mean ± SD Min–max | 7.88 ± 5.00 1.57–17.1 |
| Diagnosis, n (%) | |
| Leukemia 1 | 18 (50.0) |
| Lymphoma 2 | 9 (25.0) |
| Sarcoma 3 | 4 (11.1) |
| Others 4 | 5 (13.9) |
| Number of visits | |
| Mean ± SD Min–max | 4.72 ± 1.06 2.00–6.00 |
| 4 visits and less, n (%) | 12 (33.3) |
| 5 visits and more, n (%) | 24 (66.7) |
| Level of engagement | |
| Low and moderate, n (%) | 13 (36.1) |
| High, n (%) | 23 (63.9) |
| Characteristics of Diet | Initial Assessment | After 6 Months of Nutritional Follow-Up | After One Year of Nutritional Follow-Up | Difference (6 Months–Initial) | p-Value | Difference (One Year–6 Months) | p-Value | Difference (One Year–Initial) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| n = 36 | n = 34 | n = 36 | n = 34 | n = 34 | n = 36 | ||||
| Mean ± SD (min–max) | Mean ± SD (min–max) | Mean ± SD (min–max) | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Diet Quality Scores | |||||||||
| Diet Quality Index | 48.53 ± 10.4 (12.0–66.0) | 49.51 ± 13.8 (4–74.5) | 53.75 ± 9.67 (38.0–74.5) | 0.57 ± 13.4 | 0.805 | 4.75 ± 12.98 | 0.093 | 5.22 ± 9.95 | 0.003 |
| Healthy Diet Index | 2.51 ± 1.02 (0–4.25) | 2.80 ± 1.36 (0.67–7) | 3.02 ± 1.44 (1–7) | 0.23 ± 1.48 | 0.613 | 0.16 ± 1.82 | 0.613 | 0.05 ± 1.58 | 0.065 |
| Low adherence, n (%) | 31 (86.1) | 22 (64.71) | 22 (61.11) | 0.027 | 0.209 | 0.012 | |||
| Moderate and strong adherence, n (%) | 5 (13.9) | 12 (35.29) | 14 (38.9) | ||||||
| NOVA (%) | 51.1 ± 22.5 (0–100) | 46.8 ± 22.96 (7.24–100) | 51.4 ± 20.6 (12.0–98.0) | −0.04 ± 0.25 | 0.372 | 0.03 ± 0.30 | 0.561 | 0.002 ± 0.28 | 0.96 |
| Nutrients | |||||||||
| Energy (kcal/d) | 1538 ± 742 (374–4285) | 1691 ± 766 (5466–3606) | 1871 ± 752 (925–4333) | 176 ± 975 | 0.300 | 234 ± 622 | 0.035 | 333 ± 1030 | 0.033 |
| Total fat (g/1000 kcal/d) | 38.3 ± 8.12 (21.7–55.3) | 38.3 ± 11.5 (13.4–59.3) | 37.5 ± 9.24 (23.7–57.9) | 0.65 ± 15.3 | 0.807 | −0.59 ± 15.4 | 0.826 | −0.80 ± 11.5 | 0.68 |
| Protein (g/1000 kcal/d) | 40.9 ± 8.55 (12.6–56.2) | 46.8 ± 13.5 (26.5–84.2) | 40.9 ± 10.0 (19.7–68.5) | 5.51 ± 14.9 | 0.072 | −5.89 ± 17.4 | 0.057 | −0.08 ± 13.2 | 0.97 |
| Carbohydrate (g/1000 kcal/d) | 125.91 ± 19.15 (82.1–164.7) | 118.9–24.8 (65.96–165.7) | 128 ± 23.03 (78.97–171.5) | −8.22 ± 32.7 | 0.152 | 8.68 ± 28.4 | 0.085 | 2.10 ± 25.7 | 0.63 |
| Omega-3 polyunsaturated fatty acids (g/1000 kcal/d) | 0.33 ± 0.39 (0–1.77) | 0.44 ± 0.47 (0–1.77) | 0.74 ± 2.40 (0–14.5) | 0.11 ± 0.58 | 0.283 | 0.34 ± 2.48 | 0.431 | 0.41 ± 2.46 | 0.33 |
| Fiber (g/1000 kcal/d) | 8.09 ± 2.76 (0–14.6) | 8.03 ± 3.33 (2.85–16.2) | 7.82 ± 2.73 (1.67–15.1) | −0.26 ± 4.33 | 0.732 | −0.17 ± 3.98 | 0.904 | −0.27 ± 4.26 | 0.71 |
| Characteristics | Initial Assessment | After One Year of Nutritional Follow-Up | Difference (One Year–Initial) | p-Value | |||
|---|---|---|---|---|---|---|---|
| n (total) | Mean z-score ± SD (min-max) | n (total) | Mean z-score ± SD (Min-max) | n (pairs) | Mean z-score (SD) | ||
| Weight | 36 | 0.14 ± 1.13 (−2.16–3.03) | 36 | 0.43 ± 1.18 (−1.94–3.37) | 36 | 0.29 (0.70) | 0.019 |
| Height | 36 | −0.21 ± 1.08 (−2.47–3.03) | 36 | −0.39 ± 1.03 (−3.13–1.59) | 36 | −0.17 (0.55) | 0.029 |
| BMI | 36 | 0.37 ± 1.11 (−1.64–2.81) | 36 | 0.87 ± 1.03 (−0.69–3.09) | 36 | 0.50 (0.88) | 0.002 |
| Waist circumference | 12 | 0.87 ± 0.63 (0.13–1.84) | 12 | 0.74 ± 1.17 (−0.84–2.13) | 5 | −0.21 (0.72) | 0.547 |
| MUAC | 23 | 0.37 ± 1.30 (−2.01–2.37) | 19 | 0.80 ± 1.00 (−0.70–2.51) | 13 | 0.23 (1.22) | 0.51 |
| TSFT | 18 | 0.20 ± 1.18 (−2.75–2.08) | 12 | 0.66 ± 0.70 (−0.50–1.96) | 10 | 0.75 (1.29) | 0.10 |
| SSFT | 15 | 0.18 ± 1.20 (−2.50–1.95) | 9 | 0.06 ± 0.91 (−1.39–1.28) | 7 | 0.32 (1.25) | 0.52 |
| Systolic blood pressure | 36 | 0.37 ± 0.99 (−1.89–2.14) | 32 | 0.38 ± 0.85 (−1.49–2.16) | 32 | −0.003 (1.18) | 0.99 |
| Diastolic blood pressure | 35 | 0.37 ± 0.91 (−1.63–2.17) | 32 | 0.31 ± 1.11 (−1.26–2.88) | 31 | −0.17 (0.83) | 0.32 |
| Biochemical Data | Initial Assessment | After One Year of Nutritional Follow-Up | Difference (One Year–Initial) | p-Value | |||
|---|---|---|---|---|---|---|---|
| n (total) | Mean ± SD (Min-max) | n (total) | Mean ± SD (Min-max) | n (pairs) | Mean (SD) | ||
| HbA1c (%) | 33 | 5.12 ± 0.56 (3.60–6.00) | 24 | 4.98 ± 0.43 (4.10–5.80) | 22 | −0.18 (0.82) | 0.32 |
| Vitamin D (mmol/L) | 31 | 54.2 ± 15.6 (25.3–85.3) | 24 | 68.99 ± 27.44 (28.3–160.5) | 21 | 14.52 (28.1) | 0.03 |
| C-reactive protein (mmol/L) | 32 | 7.44 ± 16.5 (0.20–85.6) | 24 | 4.09 ± 7.24 (0.20–29.30) | 22 | −4.41 (21.4) | 0.35 |
| Total cholesterol (mmol/L) | 35 | 4.08 ± 2.03 (1.69–11.9) | 25 | 4.01 ± 1.04 (2.98–8.10) | 25 | −0.20 (2.04) | 0.63 |
| HDL-C (mmol/L) | 34 | 0.94 ± 0.29 (0.31–1.55) | 25 | 1.22 ± 0.29 (0.66–1.90) | 24 | 0.27 (0.37) | 0.002 |
| LDL-C (mmol/L) | 34 | 2.62 ± 1.89 (0.89–9.83) | 25 | 2.28 ± 1.07 (0.75–6.47) | 24 | −0.48 (1.85) | 0.22 |
| Non HDL-C (mmol/L) | 34 | 3.18 ± 2.06 (1.27–11.16) | 25 | 2.79 ± 1.08 (1.77–6.98) | 24 | −0.51 (2.10) | 0.25 |
| Triglycerides (mmol/L) | 35 | 1.31 ± 0.84 (0.30–2.94) | 25 | 1.11 ± 0.75 (0.48–3.71) | 25 | −0.19 (0.89) | 0.30 |
| 0–9 years (mmol/L) | 23 | 1.29 ± 0.86 (0.45–2.94) | 16 | 0.82 ± 0.37 (0.48–1.90) | 16 | −0.43 (0.81) | 0.021 |
| 10–18 years (mmol/L) | 12 | 1.33 ± 0.84 (0.30–2.85) | 9 | 1.63 ± 0.98 (0.58–3.71) | 18 | 0.25 (1.00) | 0.510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napartuk, M.; Bélanger, V.; Bouchard, I.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; Marcil, V. Improvement of Diet after an Early Nutritional Intervention in Pediatric Oncology. Children 2023, 10, 667. https://doi.org/10.3390/children10040667
Napartuk M, Bélanger V, Bouchard I, Meloche C, Curnier D, Sultan S, Laverdière C, Sinnett D, Marcil V. Improvement of Diet after an Early Nutritional Intervention in Pediatric Oncology. Children. 2023; 10(4):667. https://doi.org/10.3390/children10040667
Chicago/Turabian StyleNapartuk, Mélanie, Véronique Bélanger, Isabelle Bouchard, Caroline Meloche, Daniel Curnier, Serge Sultan, Caroline Laverdière, Daniel Sinnett, and Valérie Marcil. 2023. "Improvement of Diet after an Early Nutritional Intervention in Pediatric Oncology" Children 10, no. 4: 667. https://doi.org/10.3390/children10040667
APA StyleNapartuk, M., Bélanger, V., Bouchard, I., Meloche, C., Curnier, D., Sultan, S., Laverdière, C., Sinnett, D., & Marcil, V. (2023). Improvement of Diet after an Early Nutritional Intervention in Pediatric Oncology. Children, 10(4), 667. https://doi.org/10.3390/children10040667