Cardiac Magnetic Resonance Derived Left Ventricular Eccentricity Index and Right Ventricular Mass Measurements Predict Outcome in Children with Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Cohort
2.2. Cardiac Magnetic Resonance
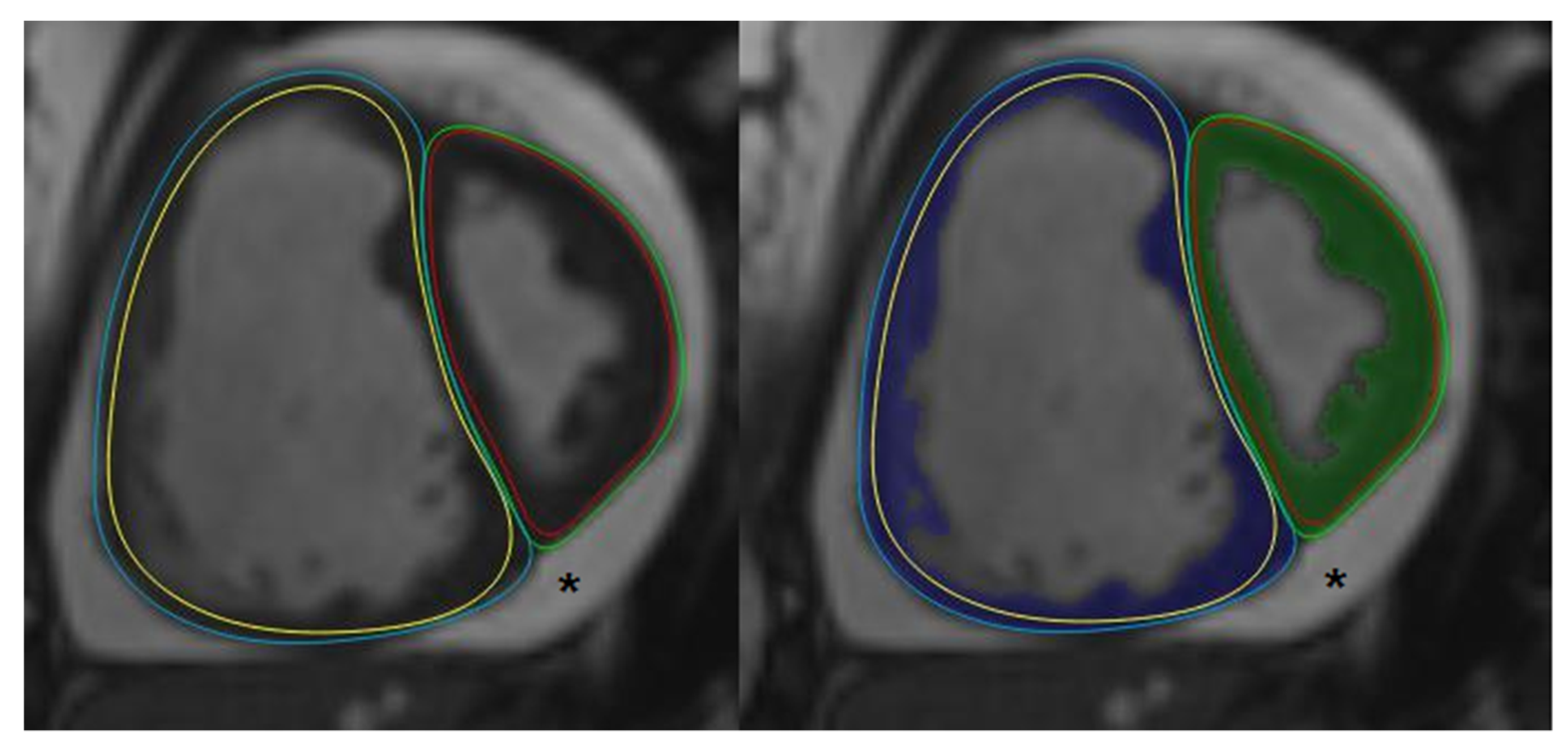
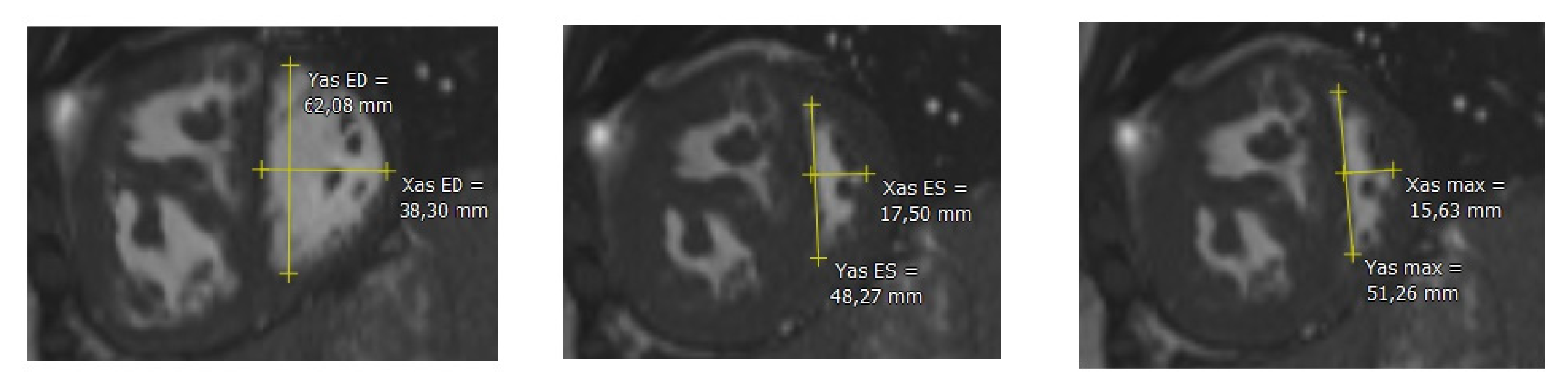
2.3. Post-Processing
2.4. Disease Severity
2.5. Statistical Analyses
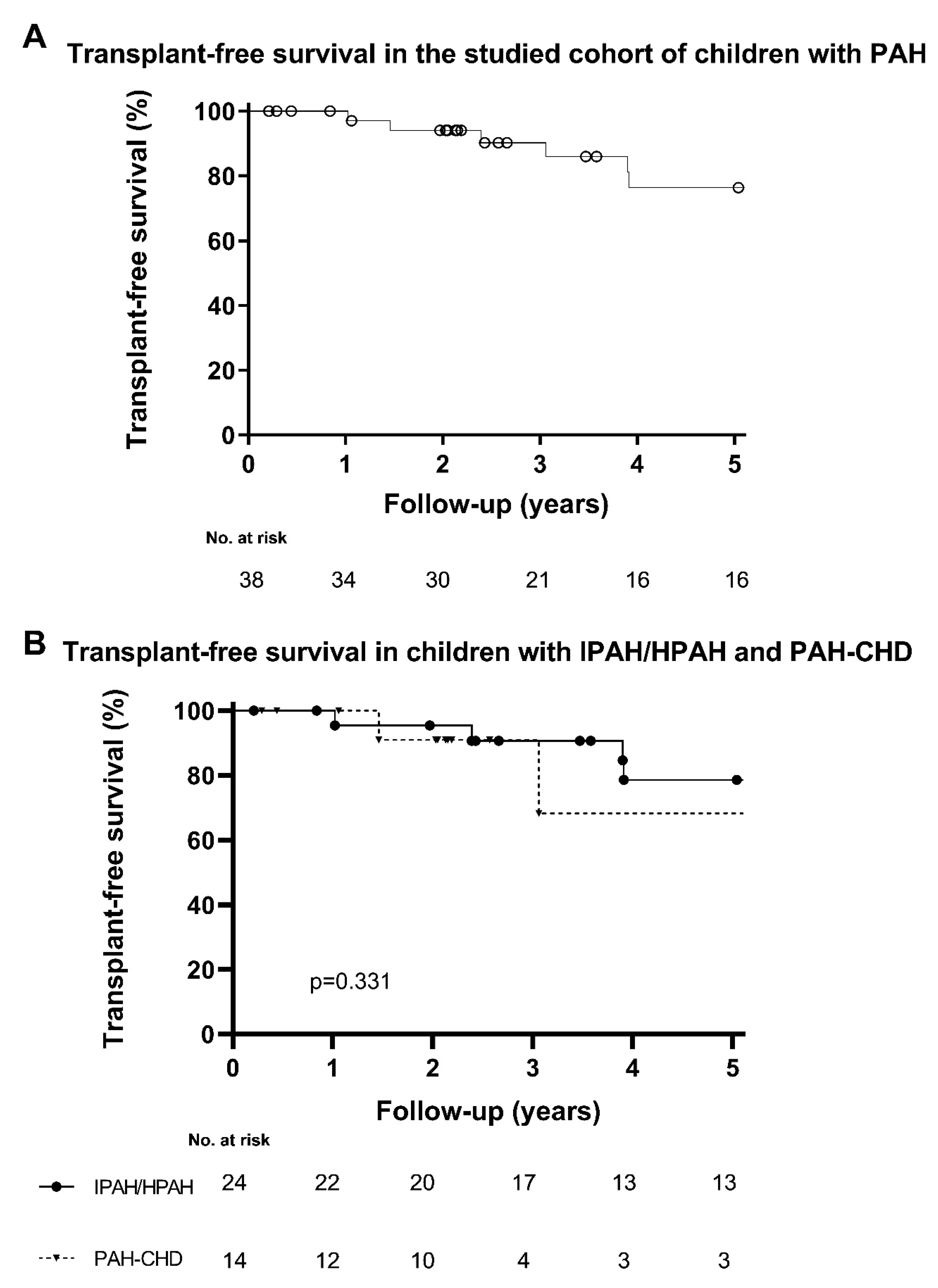
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Thilenius, O.G.; Nadas, A.S.; Jockin, H. Primary pulmonary vascular obstruction in children. Pediatrics 1965, 36, 75–87. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14313370 (accessed on 10 October 2022). [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1863023 (accessed on 10 October 2022). [CrossRef] [PubMed]
- Ploegstra, M.-J.; Douwes, J.M.; Roofthooft, M.T.R.; Zijlstra, W.M.H.; Hillege, H.L.; Berger, R.M.F. Identification of treatment goals in paediatric pulmonary arterial hypertension. Eur. Respir. J. 2014, 44, 1616–1626. [Google Scholar] [CrossRef]
- Rosenzweig, E.B.; Abman, S.H.; Adatia, I.; Beghetti, M.; Bonnet, D.; Haworth, S.; Ivy, D.; Berger, R.M. Paediatric pulmonary arterial hypertension: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019, 53, 1801916. [Google Scholar] [CrossRef] [PubMed]
- Lokhorst, C.; van der Werf, S.; Berger, R.M.F.; Douwes, J.M. Risk stratification in adult and pediatric pulmonary arterial hypertension: A systematic review. Front. Cardiovasc. Med. 2022, 9, 1035453. [Google Scholar] [CrossRef]
- Bradlow, W.M.; Gibbs, J.S.R.; Mohiaddin, R.H. Cardiovascular magnetic resonance in pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2012, 14, 1–12. [Google Scholar] [CrossRef]
- Salerno, M.; Sharif, B.; Arheden, H.; Kumar, A.; Axel, L.; Li, D.; Neubauer, S. Recent Advances in Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2017, 10, e003951. [Google Scholar] [CrossRef]
- Baggen, V.J.M.; Leiner, T.; Post, M.C.; van Dijk, A.; Roos-Hesselink, J.W.; Boersma, E.; Habets, J.; Sieswerda, G.T. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: A systematic review and meta-analysis. Eur. Radiol. 2016, 26, 3771–3780. [Google Scholar] [CrossRef]
- Simpson, C.E.; Damico, R.L.; Kolb, T.M.; Mathai, S.C.; Khair, R.M.; Sato, T.; Bourji, K.; Tedford, R.J.; Zimmerman, S.L.; Hassoun, P.M. Ventricular mass as a prognostic imaging biomarker in incident pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 30–33. [Google Scholar] [CrossRef]
- Ryan, T.; Petrovic, O.; Dillon, J.C.; Feigenbaum, H.; Conley, M.J.; Armstrong, W.F. An echocardiographic index for separation of right ventricular volume and pressure overload. J. Am. Coll. Cardiol. 1985, 5, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Burkett, D.A.; Patel, S.S.; Mertens, L.; Friedberg, M.K.; Ivy, D.D. Relationship between Left Ventricular Geometry and Invasive Hemodynamics in Pediatric Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2020, 13, e009825. [Google Scholar] [CrossRef] [PubMed]
- Ploegstra, M.-J.; Roofthooft, M.T.; Douwes, J.M.; Bartelds, B.; Elzenga, N.J.; van de Weerd, D.; Hillege, H.L.; Berger, R.M. Echocardiography in Pediatric Pulmonary Arterial Hypertension: Early Study on Assessing Disease Severity and Predicting Outcome. Circ. Cardiovasc. Imaging 2014, 8, e000878. [Google Scholar] [CrossRef] [PubMed]
- Pandya, B.; Quail, M.A.; Steeden, J.A.; McKee, A.; Odille, F.; Taylor, A.M.; Schulze-Neick, I.; Derrick, G.; Moledina, S.; Muthurangu, V. Real-time magnetic resonance assessment of septal curvature accurately tracks acute hemodynamic changes in pediatric pulmonary hypertension. Circ. Cardiovasc. Imaging 2014, 7, 706–713. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Wan, K.; Gong, C.; Li, W.; Xu, Y.; Wang, J.; He, J.; Wen, B.; Han, Y.; et al. Diagnostic and prognostic value of right ventricular eccentricity index in pulmonary artery hypertension. Pulm. Circ. 2020, 10, 2045894019899778. [Google Scholar] [CrossRef]
- Moledina, S.; Pandya, B.; Bartsota, M.; Mortensen, K.H.; McMillan, M.; Quyam, S.; Taylor, A.M.; Haworth, S.G.; Schulze-Neick, I.; Muthurangu, V. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ. Cardiovasc. Imaging 2013, 6, 407–414. [Google Scholar] [CrossRef]
- van Loon, R.L.E.; Roofthooft, M.T.; Delhaas, T.; van Osch-Gevers, M.; Harkel, A.D.T.; Strengers, J.L.; Backx, A.; Hillege, H.L.; Berger, R.M. Outcome of Pediatric Patients With Pulmonary Arterial Hypertension in the Era of New Medical Therapies. Am. J. Cardiol. 2010, 106, 117–124. [Google Scholar] [CrossRef]
- Freling, H.G.; Van Wijk, K.; Jaspers, K.; Pieper, P.G.; Vermeulen, K.M.; Van Swieten, J.M.; Willems, T.P. Impact of right ventricular endocardial trabeculae on volumes and function assessed by CMR in patients with tetralogy of Fallot. Int. J. Cardiovasc. Imaging 2013, 29, 625–631. [Google Scholar] [CrossRef]
- Freling, H.G.; Willems, T.P.; van Melle, J.P.; van Slooten, Y.J.; Bartelds, B.; Berger, R.M.; van Veldhuisen, D.J.; Pieper, P.G. Effect of right ventricular outflow tract obstruction on right ventricular volumes and exercise capacity in patients with repaired tetralogy of fallot. Am. J. Cardiol. 2014, 113, 719–723. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; A Fogel, M.; Friedrich, M.G.; Kim, R.J.; Von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J. Cardiovasc. Magn. Reson. 2013, 15, 35. [Google Scholar] [CrossRef]
- Beurskens, N.E.G.; Hagdorn, Q.A.J.; Gorter, T.M.; Berger, R.M.F.; Vermeulen, K.M.; van Melle, J.P.; Ebels, T.E.; Lui, G.K.; Ceresnak, S.R.; Chan, F.P.; et al. Risk of cardiac tachyarrhythmia in patients with repaired tetralogy of Fallot: A multicenter cardiac MRI based study. Int. J. Cardiovasc. Imaging 2019, 35, 143–151. [Google Scholar] [CrossRef]
- Jaspers, K.; Freling, H.G.; Van Wijk, K.; Romijn, E.I.; Greuter, M.J.W.; Willems, T.P. Improving the reproducibility of MR-derived left ventricular volume and function measurements with a semi-automatic threshold-based segmentation algorithm. Int. J. Cardiovasc. Imaging 2013, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; Van Melle, J.P.; Freling, H.G.; Ebels, T.; Bartelds, B.; Pieper, P.G.; Berger, R.M.F.; Van Veldhuisen, D.J.; Willems, T.P. Pulmonary regurgitant volume is superior to fraction using background-corrected phase contrast MRI in determining the severity of regurgitation in repaired tetralogy of Fallot. Int. J. Cardiovasc. Imaging 2015, 31, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Saba, T.S.; Foster, J.; Cockburn, M.; Cowan, M.; Peacock, A.J. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur. Respir. J. 2002, 20, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Ploegstra, M.J.; Zijlstra, W.M.H.; Douwes, J.M.; Hillege, H.L.; Berger, R.M.F. Prognostic factors in pediatric pulmonary arterial hypertension: A systematic review and meta-analysis. Int. J. Cardiol. 2015, 184, 198–207. [Google Scholar] [CrossRef]
- Douwes, J.M.; Hegeman, A.K.; Van Der Krieke, M.B.; Roofthooft, M.T.R.; Hillege, H.L.; Berger, R.M.F. Six-minute walking distance and decrease in oxygen saturation during the six-minute walk test in pediatric pulmonary arterial hypertension. Int. J. Cardiol. 2016, 202, 34–39. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Sanchez, M.A.G.; Kumar, R.K.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D34–D41. [Google Scholar] [CrossRef]
- Van Der Ven, J.P.G.; Sadighy, Z.; Buechel, E.R.V.; Sarikouch, S.; Robbers-Visser, D.; Kellenberger, C.J.; Kaiser, T.; Beerbaum, P.; Boersma, E.; Helbing, W.A. Multicentre reference values for cardiac magnetic resonance imaging derived ventricular size and function for children aged 0–18 years. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 102–113. [Google Scholar] [CrossRef]
- Ghio, S.; Klersy, C.; Magrini, G.; D’Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Viganò, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Brewis, M.J.; Bellofiore, A.; Vanderpool, R.R.; Chesler, N.C.; Johnson, M.K.; Naeije, R.; Peacock, A.J. Imaging right ventricular function to predict outcome in pulmonary arterial hypertension. Int. J. Cardiol. 2016, 218, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.J.; Capener, D.; Johns, C.; Hamilton, N.; Rothman, A.; Elliot, C.; Condliffe, R.; Charalampopoulos, A.; Rajaram, S.; Lawrie, A.; et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2017, 196, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.; Rajaram, S. Diagnostic accuracy of cardiovascular magnetic resonance imaging of right ventricular morphology and function in the assessment of suspected pulmonary hypertension results from the ASPIRE registry. J. Cardiovasc. Magn. Reson. 2012, 14, 40. Available online: http://www.biomedcentral.com/content/pdf/1532-429X-14-40.pdf (accessed on 10 October 2022). [CrossRef]
- Raymond, R.J.; Hinderliter, A.L.; Willis, P.W., IV; Ralph, D.; Caldwell, E.J.; Williams, W.; Ettinger, N.A.; Hill, N.S.; Summer, W.R.; de Boisblanc, B.; et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J. Am. Coll. Cardiol. 2002, 39, 1214–1219. [Google Scholar] [CrossRef]
- Said, F.; Haarman, M.G.; Roofthooft, M.T.R.; Hillege, H.L.; Ploegstra, M.J.; Berger, R.M.F. Serial Measurements of N-Terminal Pro-B-Type Natriuretic Peptide Serum Level for Monitoring Pulmonary Arterial Hypertension in Children. J. Pediatr. 2020, 220, 139–145. [Google Scholar] [CrossRef]
- Zijlstra, W.M.; Douwes, J.M.; Rosenzweig, E.B.; Schokker, S.; Krishnan, U.; Roofthooft, M.T.; Miller-Reed, K.; Hillege, H.L.; Ivy, D.D.; Berger, R.M. Survival differences in pediatric pulmonary arterial hypertension: Clues to a better understanding of outcome and optimal treatment strategies. J. Am. Coll. Cardiol. 2014, 63, 2159–2169. [Google Scholar] [CrossRef]
- Rossi, M.A.; Carillo, S.V. Cardiac hypertrophy due to pressure and volume overload: Distinctly different biological phenomena? Int. J. Cardiol. 1991, 31, 133–141. [Google Scholar] [CrossRef]
- Marcus, J.T.; Gan, C.T.-J.; Zwanenburg, J.J.; Boonstra, A.; Allaart, C.P.; Götte, M.J.; Vonk-Noordegraaf, A. Interventricular Mechanical Asynchrony in Pulmonary Arterial Hypertension. Left-to-Right Delay in Peak Shortening Is Related to Right Ventricular Overload and Left Ventricular Underfilling. J. Am. Coll. Cardiol. 2008, 51, 750–757. [Google Scholar] [CrossRef]
- Palau-Caballero, G.; Walmsley, J.; Van Empel, V.; Lumens, J.; Delhaas, T. Why septal motion is a marker of right ventricular failure in pulmonary arterial hypertension: Mechanistic analysis using a computer model. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H691–H700. [Google Scholar] [CrossRef]
- Burkett, D.A.; Slorach, C.; Patel, S.S.; Redington, A.N.; Ivy, D.D.; Mertens, L.; Younoszai, A.K.; Friedberg, M.K. Impact of Pulmonary Hemodynamics and Ventricular Interdependence on Left Ventricular Diastolic Function in Children with Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2016, 9, e004612. [Google Scholar] [CrossRef] [PubMed]
- Noordegraaf, A.V.; Chin, K.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef] [PubMed]
- Krysztofiak, H.; Młyńczak, M.; Małek, Ł.A.; Folga, A.; Braksator, W. Left ventricular mass is underestimated in overweight children because of incorrect body size variable chosen for normalization. PLoS ONE 2019, 14, e0217637. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Bossone, E.; Opotowsky, A.R.; Ghio, S.; Rudski, L.G.; Naeije, R. Strengths and weaknesses of echocardiography for the diagnosis of pulmonary hypertension. Int. J. Cardiol. 2018, 263, 177–183. [Google Scholar] [CrossRef]
- Muthurangu, V.; Lurz, P.; Critchely, J.D.; Deanfield, J.E.; Taylor, A.M.; Hansen, M.S. Real-time assessment of right and left ventricular volumes and function in patients with congenital heart disease by using high spatiotemporal resolution radial k-t SENSE. Radiology 2008, 248, 782–791. [Google Scholar] [CrossRef]
| Total Population | IPAH/HPAH | PAH-CHD | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Characteristics | N | Median (IQR), n (%) | N | Median (IQR), n (%) | N | Median (IQR), n (%) | |
| Age at diagnosis, years | 38 | 12.5 (5.4–14.4) | 24 | 13.3 (6.1–14.8) | 14 | 8.5 (4.2–14.1) | 0.263 |
| Age at CMR, years | 38 | 13.0 (10.8–15.0) | 24 | 13.5 (11.3–15.0) | 14 | 12.4 (8.7–14.7) | 0.263 |
| Sex, female | 38 | 25 (66) | 24 | 13 (54) | 14 | 12 (86) | 0.077 |
| BSA, m2 | 38 | 1.3 (1.0–1.5) | 24 | 1.4 (1.0–1.6) | 14 | 1.1 (0.9–1.4) | 0.183 |
| Follow-up from time of diagnosis, years | 38 | 6.9 (2.3–11.6) | 24 | 7.3 (2.6–14.6) | 14 | 4.9 (1.4–10.6) | 0.269 |
| Follow-up from time of first CMR, years | 38 | 3.5 (2.0–9.5) | 24 | 5.8 (2.5–9.8) | 14 | 2.1 (1.4–3.9) | 0.034 |
| Etiology | 38 | - | |||||
| IPAH/HPAH | 24 (63) | 24 (63) | - | ||||
| PAH-CHD | 14 (37) | - | 14 (37) | ||||
| Group 1 Eisenmenger syndrome | 2 | - | 2 | ||||
| Group 2 left-to-right shunt | 2 | - | 2 | ||||
| Group 3 Coincidental congenital heart disease | 8 | - | 7 | ||||
| Group 4 Post-operative PAH | 2 | - | 3 | ||||
| WHO-FC | 38 | 24 | 14 | 0.164 | |||
| I | 2 (5) | 2 (8) | - | ||||
| II | 19 (50) | 10 (42) | 9 (64) | ||||
| III | 12 (32) | 7 (29) | 5 (36) | ||||
| IV | 5 (13) | 5 (21) | - | ||||
| NT-proBNP, ng/l | 38 | 186 (103–907) | 24 | 264 (107–1074) | 14 | 156 (96–291) | 0.226 |
| 6MWD, m | 38 | 384 (339–448) | 24 | 384 (341–453) | 14 | 369 (339–400) | 0.449 |
| Hemodynamics | |||||||
| mRAP, mmHg | 26 | 5.0 (4.0–9.0) | 16 | 6.0 (4.0–13) | 10 | 5.0 (3.0–7.0) | 0.151 |
| mPAP, mmHg | 26 | 57 (36–73) | 16 | 53 (33–74) | 10 | 70 (45–74) | 0.384 |
| CI, L/min/m2 | 26 | 2.8 (2.4–3.5) | 16 | 2.6 (1.6–3.5) | 10 | 2.9 (2.6–3.9) | 0.140 |
| PVRi, WU∙m2 | 26 | 20.2 (7.2–32.1) | 16 | 22.5 (6.8–31.6) | 10 | 20.2 (10.5–32.3) | 0.916 |
| mSAP, mmHg | 26 | 67 (57–72) | 16 | 65 (53–70) | 10 | 68 (64–74) | 0.246 |
| SvO2, % | 26 | 67 (61–72) | 16 | 66 (54–71) | 10 | 69 (63–73) | 0.384 |
| Total Population | IPAH/HPAH | PAH-CHD | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Characteristics | N | Median (IQR), n (%) | N | Median (IQR), n (%) | N | Median (IQR), n (%) | |
| Right ventricle | |||||||
| RVEDVi, ml/m2 | 38 | 84 (69–104) | 24 | 85 (72–100) | 14 | 81 (68–127) | 1.000 |
| RVESVi, ml/m2 | 38 | 43 (31–66) | 24 | 44 (32–66) | 14 | 42 (30–77) | 0.928 |
| RVEF, % | 38 | 45 (33–57) | 24 | 44 (29–56) | 14 | 48 (39–57) | 0.397 |
| RVMi, g/m2 | 38 | 66 (55–85) | 24 | 66 (54–82) | 14 | 70 (61–97) | 0.304 |
| Left ventricle | |||||||
| LVEDVi, ml/m2 | 38 | 57 (49–74) | 24 | 58 (49–71) | 14 | 56 (49–83) | 0.785 |
| LVESVi, ml/m2 | 38 | 22 (17–28) | 24 | 24 (17–28) | 14 | 20 (17–29) | 0.607 |
| LVEF, % | 38 | 65 (57–71) | 24 | 65 (55–71) | 14 | 65 (61–71) | 0.506 |
| LVMi, g/m2 | 38 | 45 (41–54) | 24 | 48 (41–56) | 14 | 43 (39–52) | 0.226 |
| RVM/LVM-ratio | 38 | 1.8 (1.8–2.2) | 24 | 1.3 (1.1–1.7) | 14 | 1.6 (1.4–2.1) | 0.037 |
| RV mass/volume ratio | 38 | 1.5 (1.1–2.0) | 24 | 1.5 (1.1–1.9) | 14 | 1.6 (1.2–2.3) | 0.290 |
| Eccentricity index | |||||||
| LVEId | 38 | 1.5 (1.4–1.7) | 24 | 1.5 (1.4–1.7) | 14 | 1.5 (1.3–1.6) | 0.672 |
| LVEIs | 38 | 1.8 (1.6–2.2) | 24 | 1.8 (1.5–2.1) | 14 | 2.0 (1.6–2.4) | 0.215 |
| LVEImax | 38 | 1.9 (1.7–2.4) | 24 | 2.3 (1.9–2.8) | 14 | 2.0 (1.9–2.4) | 0.717 |
| Univariate Analysis in Total Population | Univariate Analysis in IPAH/HPAH | Univariate Analysis in PAH-CHD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N/N Events | HR (95%CI) | p-Value | N/N Events | HR (95%CI) | p-Value | N/N Events | HR (95%CI) | p-Value | |
| Right ventricle | |||||||||
| RVEDVi, ml | 38/11 | 1.016 (0.997–1.035) | 0.110 | 24/7 | 1.018 (0.992–1.046) | 0.180 | 14/4 | 1.017 (0.982–1.053) | 0.355 |
| RVESVi, ml | 38/11 | 1.018 (1.001–1.036) | 0.039 | 24/7 | 1.018 (0.997–1.039) | 0.093 | 14/4 | 1.033 (0.984–1.084) | 0.193 |
| RVEF, % | 38/11 | 0.961 (0.929–0.995) | 0.023 | 24/7 | 0.961 (0.921–1.002) | 0.060 | 14/4 | 0.907 (0.797–1.033) | 0.143 |
| RVMi, g/m2 | 38/11 | 1.046 (1.016–1.078) | 0.003 | 24/7 | 1.043 (1.001–1.086) | 0.043 | 14/4 | 1.039 (0.990–1.090) | 0.124 |
| Left ventricle | |||||||||
| LVEDVi, ml | 38/11 | 0.972 (0.930–1.016) | 0.204 | 24/7 | 0.971 (0.919–1.025) | 0.284 | 14/4 | 0.973 (0.894–1.058) | 0.520 |
| LVESVi, ml | 38/11 | 0.954 (0.866–1.051) | 0.343 | 24/7 | 0.970 (0.862–1.092) | 0.614 | 14/4 | 0.945 (0.804–1.111) | 0.492 |
| LVEF, % | 38/11 | 1.003 (0.953–1.055) | 0.923 | 24/7 | 0.992 (0.944–1.043) | 0.764 | 14/4 | 1.089 (0.901–1.315) | 0.378 |
| LVMi, g/m2 | 38/11 | 0.976 (0.911–1.046) | 0.491 | 24/7 | 0.974 (0.895–1.060) | 0.539 | 14/4 | 1.027 (0.843–1.252) | 0.791 |
| RVM/LVM-ratio, per 0.1 increase | 38/11 | 1.247 (1.085–1.433) | 0.002 | 24/7 | 1.183 (1.012–1.382) | 0.035 | 14/4 | 1.358 (0.945–1.95) | 0.098 |
| RV mass/volume ratio, per 0.1 increase | 38/11 | 0.942 (0.853–1.041) | 0.240 | 24/7 | 0.914 (0.784–1.066) | 0.255 | 14/4 | 0.926 (0.789–1.086) | 0.343 |
| Eccentricity index | |||||||||
| LVEId, per unit 10 | 38/11 | 1.429 (1.153–1.770) | 0.001 | 24/7 | 1.499 (1.095–2.051) | 0.012 | 14/4 | 3.058 (0.520–17.972 | 0.216 |
| LVEIs, per unit 10 | 38/11 | 1.343 (1.136–1.587) | 0.001 | 24/7 | 1.343 (1.091–1.653) | 0.005 | 14/4 | 1.303 (0.942–1.802) | 0.110 |
| LVEImax, per unit 10 | 38/11 | 1.110 (1.034–1.193) | 0.004 | 24/7 | 1.114 (1.020–1.218) | 0.017 | 14/4 | 1.140 (0.976–1.332) | 0.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haarman, M.G.; Coenraad, I.; Hagdorn, Q.A.J.; Hillege, H.L.; Willems, T.P.; Berger, R.M.F.; Douwes, J.M. Cardiac Magnetic Resonance Derived Left Ventricular Eccentricity Index and Right Ventricular Mass Measurements Predict Outcome in Children with Pulmonary Arterial Hypertension. Children 2023, 10, 756. https://doi.org/10.3390/children10040756
Haarman MG, Coenraad I, Hagdorn QAJ, Hillege HL, Willems TP, Berger RMF, Douwes JM. Cardiac Magnetic Resonance Derived Left Ventricular Eccentricity Index and Right Ventricular Mass Measurements Predict Outcome in Children with Pulmonary Arterial Hypertension. Children. 2023; 10(4):756. https://doi.org/10.3390/children10040756
Chicago/Turabian StyleHaarman, Meindina G., Iris Coenraad, Quint A. J. Hagdorn, Hans L. Hillege, Tineke P. Willems, Rolf M. F. Berger, and Johannes M. Douwes. 2023. "Cardiac Magnetic Resonance Derived Left Ventricular Eccentricity Index and Right Ventricular Mass Measurements Predict Outcome in Children with Pulmonary Arterial Hypertension" Children 10, no. 4: 756. https://doi.org/10.3390/children10040756
APA StyleHaarman, M. G., Coenraad, I., Hagdorn, Q. A. J., Hillege, H. L., Willems, T. P., Berger, R. M. F., & Douwes, J. M. (2023). Cardiac Magnetic Resonance Derived Left Ventricular Eccentricity Index and Right Ventricular Mass Measurements Predict Outcome in Children with Pulmonary Arterial Hypertension. Children, 10(4), 756. https://doi.org/10.3390/children10040756







