Effect of Acupoint Stimulation on Controlling Pain from Heel Lance in Neonates: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of RCTs
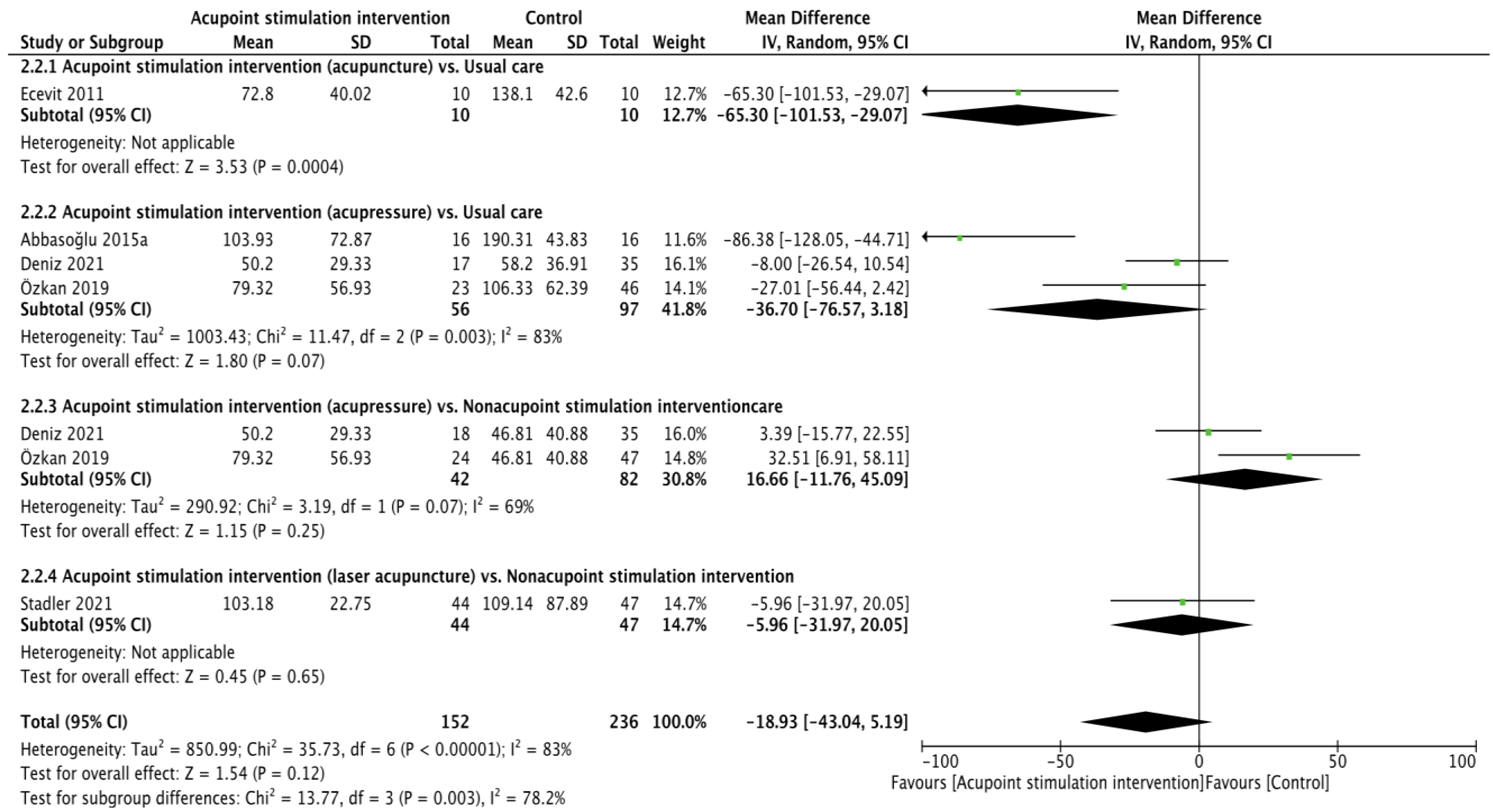
3.2. Primary Outcomes
3.2.1. Pain Score during Heel Lance
3.2.2. Pain Score after Heel Lance
3.3. Secondary Outcomes
3.3.1. Crying Time
3.3.2. Oxygenation Saturation during Heel Lance
3.3.3. Oxygenation Saturation after Heel Lance
3.3.4. Heart Rate during Heel Lance
3.3.5. Heart Rate after Heel Lance
3.3.6. Respiration Rate during Heel Lance
3.3.7. Respiration Rate after Heel Lance
3.4. Tertiary Outcomes
Duration of Procedure
3.5. Meta-Regression Analyses and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cañadas, D.C.; Perales, A.B.; Martínez, R.G.; Carreño, T.P. The impact of nonpharmacological interventions on cortisol during heel Lance procedures on preterm infants: A meta-analysis of RCTs. Pain Manag. Nurs. 2021, 22, 798–805. [Google Scholar] [CrossRef]
- Bucsea, O.; Riddell, R.P. Non-pharmacological pain management in the neonatal intensive care unit: Managing neonatal pain without drugs. Semin. Fetal Neonatal Med. 2019, 24, 10101. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Başbakkal, Z.; Yalaz, M.; Sözmen, E.Y. The effect of nesting positions on pain, stress and comfort during heel lance in premature infants. Pediatr. Neonatal 2018, 59, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.S.; Ohlsson, A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database Syst. Rev. 2011, 10, CD001452. [Google Scholar] [CrossRef]
- McPherson, C.; Miller, S.P.; El-Dib, M.; Massaro, A.N.; Inder, T.E. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 2020, 88, 168–175. [Google Scholar] [CrossRef]
- Slater, R.; Cornelissen, L.; Fabrizi, L.; Patten, D.; Yoxen, J.; Worley, A.; Boyd, S.; Meek, J.; Fitzgerald, M. Oral sucrose as an analgesic drug for procedural pain in newborn infants: A randomised controlled trial. Lancet 2010, 376, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D.; Reszel, J.; Dagg, W.; Aubertin, C.; Bueno, M.; Dunn, S.; Fuller, A.; Harrold, J.; Larocque, C.; Nicholls, S.; et al. Pain management during newborn screening—Using YouTube to disseminate effective pain management strategies. J. Perinat. Neonatal Nurs. 2017, 31, 172–177. [Google Scholar] [CrossRef]
- Akbari, N.; Mutlu, B.; Nadali, J. Effect of Non-nutritive Sucking during Heel-Stick Procedure in Pain Management of Term Infants in the Neonatal Intensive Care Unit: A Systematic Review and Meta-analysis. Curr. Pediatr. Rev. 2023, 19, 90–98. [Google Scholar]
- Gomes Neto, M.; da Silva Lopes, I.A.; Araujo, A.; Oliveira, L.S.; Saquetto, M.B. The effect of facilitated tucking position during painful procedure in pain management of preterm infants in neonatal intensive care unit: A systematic review and meta-analysis. Eur. J. Pediatr. 2020, 179, 699–709. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Ni, Z.H.; Lv, H.T. Effects of kangaroo care on pain relief in premature infants during painful procedures: A meta-analysis. J. Spec. Pediatr. Nurs. 2022, 27, e12390. [Google Scholar] [CrossRef]
- Ting, B.; Tsai, C.L.; Hsu, W.T.; Shen, M.L.; Tseng, P.T.; Chen, D.T.; Su, K.P.; Jingling, L. Music intervention for pain control in the pediatric population: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, H.; Yang, Z.; Liu, W.; Qi, L.; Dong, C.; Si, G.; Guo, Q. Efficacy and Safety of Transcutaneous Electrical Acupoint Stimulation for Postoperative Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Res. Manag. 2022, 2022, 7570533. [Google Scholar] [CrossRef]
- Mangat, A.K.; Oei, J.L.; Chen, K.; Quah-Smith, I.; Schmölzer, G.M. A review of non-pharmacological treatments for pain management in newborn infants. Children 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Guo, X.; May, B.H.; Zhang, A.L.; Liu, Y.; Lu, C.; Mao, J.J.; Xue, C.C.; Zhang, H. Clinical Evidence for Association of Acupuncture and Acupressure With Improved Cancer Pain: A Systematic Review and Meta-Analysis. JAMA Oncol. 2020, 6, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Wu, M.Y.; Huang, M.C.; Zimmerman, G.; Yang, L.Y.; Lin, C.L.; Tou, S.I.; Yen, H.R. The Association Between Acupuncture Therapies and Reduced Fracture Risk in Patients with Osteoarthritis: A Nationwide Retrospective Matched Cohort Study. J. Complement. Integr. Med. 2022, 28, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Y.; Miller, D.W.; Bolash, B.; Bauer, M.; McDonald, J.; Faggert, S.; He, H.; Li, Y.M.; Matecki, A.; Camardella, L.; et al. Acupuncture’s role in solving the opioid epidemic: Evidence, cost-effectiveness, and care availability for acupuncture as a primary, non-pharmacologic method for pain relief and management–white paper. J. Integr. Med. 2017, 15, 411–425. [Google Scholar] [CrossRef]
- Yildirim, D.; Yildiz, C.C. The effect of acupressure on vital signs, acute pain, stress and satisfaction during venipuncture: Single-blind, randomized controlled study. Eur. J. Integr. Med. 2021, 44, 101343. [Google Scholar] [CrossRef]
- Toldo, I.; Rattin, M.; Perissinotto, E.; De Carlo, D.; Bolzonella, B.; Nosadini, M.; Rossi, L.N.; Vecchio, A.; Simonati, A.; Carotenuto, M.; et al. Survey on treatments for primary headaches in 13 specialized juvenile Headache Centers: The first multicenter Italian study. Eur. J. Paediatr. Neurol. 2017, 21, 507–521. [Google Scholar] [CrossRef]
- Angelo, Z.; Polyvios, C. Alternative practices of achieving anaesthesia for dental procedures: A review. J. Dent. Anesth. Pain Med. 2018, 18, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; König, J.; König, T.; Muensterer, O. A randomized, placebo-controlled study of magnetic acupuncture for supplementary analgesia after laparoscopic appendectomy in children. J. Pediatr. Surg. 2022, 58, 64–69. [Google Scholar] [CrossRef]
- Gottschling, S.; Schmitt, R.; Meyer, S.; Graf, N.; Gortner, L. Laser acupunture for pain prevention in neonates. A randomized, placebo-controlled, double-blinded trial. Eur. J. Pediatr. 2010, 169, 383. [Google Scholar]
- Abbasoğlu, A.; Cabıoğlu, M.T.; Tuğcu, A.U.; İnce, D.A.; Tekindal, M.A.; Ecevit, A.; Tarcan, A. Acupressure at BL60 and K3 points before heel lancing in preterm infants. Explore 2015, 11, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Hall, R.W.; Golianu, B.; Yates, C.; Williams, D.K.; Chang, J.; Anand, K.J. Does noninvasive electrical stimulation of acupuncture points reduce heelstick pain in neonates? Acta Paediatr. 2016, 105, 1434–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.L.; Lindrea, K.B.; Quah-Smith, I.; Schmölzer, G.M.; Daly, M.; Schindler, T.; Oei, J.L. Magnetic noninvasive acupuncture for infant comfort (MAGNIFIC)—A single-blinded randomised controlled pilot trial. Acta Paediatr. 2017, 106, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Avian, A.; Pichler, G.; Posch, K.; Urlesberger, B.; Raith, W. Laser acupuncture versus oral glucose administration for pain prevention in term neonates: An observer-blinded non-inferiority randomized controlled clinical trial. Acupunct. Med. 2021, 39, 589–595. [Google Scholar] [CrossRef]
- Ecevit, A.; Ince, D.A.; Tarcan, A.; Cabioglu, M.T.; Kurt, A. Acupuncture in preterm babies during minor painful procedures. J. Tradit. Chin. Med. 2011, 31, 308–310. [Google Scholar] [CrossRef] [Green Version]
- Abbasoglu, A.; Cabioglu, M.T.; Tugcu, A.U.; Yapakci, E.; Tekindal, M.A.; Tarcan, A. Laser acupuncture before heel lancing for pain management in healthy term newborns: A randomised controlled trial. Acupunct. Med. 2015, 33, 445–450. [Google Scholar] [CrossRef]
- Özkan, T.K.; Küçükkelepçe, D.Ş.; Özkan, S.A. The effects of acupressure and foot massage on pain during heel lancing in neonates: A randomized controlled trial. Complement. Ther. Med. 2019, 46, 103–108. [Google Scholar] [CrossRef]
- Ibrahim Mabrouk Baraka, N.; Reda Ali El-Kest, H.; Abdl Elsalam Mohamed Elwan, S. Effect of Foot Massage and Acupressure on Pain Levels and Physiological Parameters during Heel Lancing in Full-Term Neonates. Int. Egypt. J. Nurs. Sci. Res. 2022, 3, 133–145. [Google Scholar] [CrossRef]
- Deniz, A.Ö.; Açikgöz, A. A Randomized Controlled Trial: The Effect of Acupressure and Foot Reflexology on Pain During Heel-Lancing in Neonates. Clin. Nurs. Res. 2021, 32, 306–312. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D.; Sterne, J. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; John Wiley & Sons: Chichester, UK, 2011; pp. 174–229. [Google Scholar]
- Higgins, J.P.; Deeks, J.J. Selecting studies and collecting data. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley & Sons: Chichester, UK, 2008; pp. 151–185. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis in R: A Hands-On Guide; Chapman & Hall: London, UK, 2019. [Google Scholar]
- Hudson-Barr, D.; Capper-Michel, B.; Lambert, S.; Palermo, T.M.; Morbeto, K.; Lombardo, S. Validation of the pain assessment in neonates (PAIN) scale with the neonatal infant pain scale (NIPS). Neonatal Netw. 2002, 21, 15–21. [Google Scholar] [CrossRef]
- Ballantyne, M.; Stevens, B.; McAllister, M.; Dionne, K.; Jack, A. Validation of the premature infant pain profile in the clinical setting. Clin. J. Pain 1999, 15, 297–303. [Google Scholar] [CrossRef]
- Hummel, P.; Lawlor-Klean, P.; Weiss, M.G. Validity and reliability of the N-PASS assessment tool with acute pain. Am. J. Perinatol. 2010, 30, 474–478. [Google Scholar] [CrossRef]
- Sarhangi, F.; Mollahadi, M.; Ebadi, A.; Matinzadeh, Z.K.; Tadrisi, S.D. Validity and reliability of neonatal infant pain scale in neonatal intensive care units in Iran (2010). Pak. J. Med. Sci. 2011, 27, 1087–1091. [Google Scholar]
- Stevens, B.; Johnston, C.; Petryshen, P.; Taddio, A. Premature infant pain profile: Development and initial validation. Clin. J. Pain 1996, 12, 13–22. [Google Scholar] [CrossRef]
- Schenk, K.; Stoffel, L.; Bürgin, R.; Stevens, B.; Bassler, D.; Schulzke, S.; Nelle, M.; Cignacco, E. The influence of gestational age in the psychometric testing of the Bernese Pain Scale for Neonates. BMC Pediatr. 2019, 19, 20. [Google Scholar] [CrossRef] [Green Version]
- Röver, C.; Knapp, G.; Friede, T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med. Res. Methodol. 2015, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Hartley, K.A.; Miller, C.S.; Gephart, S.M. Facilitated tucking to reduce pain in neonates: Evidence for best practice. Adv. Neonatal Care 2015, 15, 201–208. [Google Scholar] [CrossRef]
- Li, Q.; Tan, X.; Li, X.; Tang, W.; Mei, L.; Cheng, G.; Zou, Y. Efficacy and safety of combined oral sucrose and nonnutritive sucking in pain management for infants: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268033. [Google Scholar] [CrossRef]
- Vickers, A.J.; Vertosick, E.A.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Irnich, D.; Witt, C.M.; Linde, K. Acupuncture for chronic pain: Update of an individual patient data meta-analysis. J. Pain 2018, 19, 455–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Dhapte, V.; Kadam, S.; Dhapte, V. Contemporary acupressure therapy: Adroit cure for painless recovery of therapeutic ailments. J. Tradit. Complement. Med. 2017, 7, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, M.W.; Wu, X.Y.; Wu, J.C.; Wong, S.; Chung, V.C. Safety of acupuncture: Overview of systematic reviews. Sci. Rep. 2017, 7, 3369. [Google Scholar] [CrossRef]
- Lin, J.G.; Li, T.M.; Hsu, S.F. Newly Edited Color Book of Acupuncture and Moxibustion; JYIN Publishing Company: Taipei, Taiwan, 2009. [Google Scholar]
- Chen, M.C.; Yang, L.Y.; Chen, K.M.; Hsu, H.F. Systematic review and meta-analysis on using acupressure to promote the health of older adults. J. Appl. Gerontol. 2020, 39, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Duan, P.B.; Hou, Q.M.; Du, S.Z.; Sun, J.F.; Mei, S.J.; Wang, X.Q. Efficacy of auricular acupressure for chronic low back pain: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2017, 2017, 6383649. [Google Scholar] [CrossRef] [Green Version]
- Coutaux, A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Jt. Bone Spine 2017, 84, 657–661. [Google Scholar] [CrossRef]
- Liu, X.L.; Tan, J.Y.; Molassiotis, A.; Suen, L.K.; Shi, Y. Acupuncture-point stimulation for postoperative pain control: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2015, 2015, 657809. [Google Scholar] [CrossRef] [Green Version]
- Chon, T.Y.; Mallory, M.J.; Yang, J.; Bublitz, S.E.; Do, A.; Dorsher, P.T. Laser Acupuncture: A Concise Review. Med. Acupunct. 2019, 31, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Ezzo, J.M.; Richardson, M.A.; Vickers, A.; Allen, C.; Dibble, S.L.; Issell, B.F.; Lao, L.; Pearl, M.; Ramirez, G.; Roscoe, J.; et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Syst. Rev. 2006, 2, CD002285. [Google Scholar]
- Qin, Z.; Ding, Y.; Xu, C.; Kwong, J.S.W.; Ji, Y.; Wu, A.; Wu, J.; Liu, Z. Acupuncture vs noninsertive sham acupuncture in aging patients with degenerative lumbar spinal stenosis: A randomized controlled trial. Am. J. Med. 2020, 133, 500–507. [Google Scholar] [CrossRef]
| RCTs (Country) | Neonates | Numbers (Pre-/Post-) | Study Arms (Prescription) | Control | Acupoints (Position) | Outcome Measurements |
|---|---|---|---|---|---|---|
| Practitioner | ||||||
| Gottschling et al., 2010 (Germany) [21] | Term | IC: 25/25 CC: 25/25 | Laser acupuncture (dose: 0.45 J; duration: not mentioned; interval: not mentioned) | Placebo laser acupuncture | LI4 (hand), Shenmen (ear) |
|
| Not mentioned | ||||||
| Ecevit et al., 2011 (Turkey) [26] | Preterm | IC: 10/10 CC1: 30/30 | Acupuncture (duration: 30 min; interval: not mentioned) | Usual care | EX-HN3 (head) |
|
| Competent doctor | ||||||
| Abbasoğlu et al., 2015a (Turkey) [22] | Preterm | IC: 16/16 CC: 16/16 | Acupressure (duration: 3 min; interval: immediately) | Usual care | BL60 (leg), KI3 (leg) |
|
| Trained physician | ||||||
| Abbasoğlu et al., 2015b (Turkey) [27] | Term | IC: 21/21 CC: 21/21 | Laser acupuncture (dose: 0.3 J; duration: 0.5 min; interval: 2 min) | Sucrose (dose: 0.5 mL of 24% sucrose; interval: 2 min) | EX-HN3 (head) |
|
| Trained physician | ||||||
| Mitchell et al., 2016 (USA) [23] | Term | IC1: 42/37 IC2: 41/40 CC1: 39/37 CC2: 40/39 | IC1: NESAP (dose: 3.5 mA, 10 Hz; duration: 10 ± 1 min; interval: uninterrupted) IC2: NESAP + sucrose (dose: 1 ± 0.1 mL of the 24% sucrose; interval: 2 min) | CC1: sucrose (dose: 1.0 mL of 24% sucrose; interval: 2 min) CC2: usual care | ST36 (leg), SP6 (leg), BL60 (leg), KI3 (leg) |
|
| Research nurse | ||||||
| Chen et al., 2017 (Australia) [24] | Preterm+ term | IC: 21 CC: 19 | Magnetic acupuncture (dose: 100 G, 1.7 mm; duration: 3 days; interval: 2 h) | Usual care | Cingulate Gyrus, Thalamus, Omega, Point Zero, Shenmen (ear) |
|
| Experienced practitioner | ||||||
| Özkan et al., 2019 (Turkey) [28] | Term | IC: 47/47 CC1: 47/47 CC2: 46/46 | Acupressure (duration: 2 min; interval: 5 min) | CC1: massage (duration: 2 min; interval: 5 min) CC2: usual care | BL60 (leg), KI3 (leg) |
|
| Certificated researchers | ||||||
| Deniz et al., 2021 (Turkey) [30] | Term | IC: 35/35 CC1: 35/35 CC2: 35/35 | Acupressure (duration: 7 min; interval: not mentioned) | CC1: reflexology (duration: 7 min; interval: not mentioned) CC2: usual care | ST36 (leg), KI3 (leg) |
|
| Certificated researchers | ||||||
| Stadler et al., 2021 (Austria) [25] | Term | IC: 48/44 CC: 48/47 | Laser acupuncture (dose: 0.6 J; duration: 2 min; interval: 2.5 min) | Glucose (mL of the 30% glucose not mentioned) | LI4 (hand) |
|
| Not mentioned | ||||||
| Ibrahim et al., 2022 (Egypt) [29] | Term | IC: 40/40 CC1: 40/40 CC2: 40/40 | Acupressure (duration: 2 min; interval: not mentioned) | CC1: massage (duration: 2 min; interval: not mentioned) CC2: usual care | Not mentioned |
|
| Researchers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tou, S.-I.; Huang, C.-Y.; Yen, H.-R. Effect of Acupoint Stimulation on Controlling Pain from Heel Lance in Neonates: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Children 2023, 10, 1024. https://doi.org/10.3390/children10061024
Tou S-I, Huang C-Y, Yen H-R. Effect of Acupoint Stimulation on Controlling Pain from Heel Lance in Neonates: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Children. 2023; 10(6):1024. https://doi.org/10.3390/children10061024
Chicago/Turabian StyleTou, Sio-Ian, Chia-Yu Huang, and Hung-Rong Yen. 2023. "Effect of Acupoint Stimulation on Controlling Pain from Heel Lance in Neonates: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Children 10, no. 6: 1024. https://doi.org/10.3390/children10061024