Predictor Factors for Chronicity in Immune Thrombocytopenic Purpura in Children
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. History of ITP
4.2. Pathophysiology
4.3. Risk Factors Associated with the Development of Chronic ITP
4.4. Therapeutic Regimen in Chronic ITP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M. Bleeding complications in immune thrombocytopenia. Hematol. 2014 Am. Soc. Hematol. Educ. Program Book 2015, 2015, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.; Koene, H.; Zwaginga, J.J.; Vreugdenhil, G. Chronic idiopathic thrombocytopenic purpura: Present strategy, guidelines and new insights. Neth. J. Med. 2006, 64, 356–363. [Google Scholar]
- Provan, D.; Stasi, R.; Newland, A.C.; Blanchette, V.S.; Bolton-Maggs, P.; Bussel, J.B.; Chong, B.H.; Cines, D.B.; Gernsheimer, T.B.; Godeau, B.; et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010, 115, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Lanzkowsky, P. Disorders of Platelets. In Manual of Pediatric Hematology and Oncology, 4th ed.; Elsevier: New York, NY, USA, 2005; p. 250e63. [Google Scholar]
- Donato, H.; Picón, A.; Martinez, M.; Rapetti, M.C.; Rosso, A.; Gomez, S.; Rossi, N.; Bacciedoni, V.; Schvartzman, G.; Riccheri, C.; et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: A multicentered study from Argentina. Pediatr. Blood Cancer 2009, 52, 491–496. [Google Scholar] [CrossRef]
- Stasi, R.; Newland, A.C. ITP a historical perspective. Br. J. Haematol. 2011, 153, 437–450. [Google Scholar] [CrossRef]
- Freedman, J. ITP: An over view of the conference and future directions with an abbreviated ITP history. J. Pediatr. Hematol. Oncol. 2003, 25, S77–S84. [Google Scholar] [CrossRef]
- Faki Osman, M.E. Childhood immune thrombocytopenia: Clinical presentation and management. Sudan J. Paediatr. 2012, 12, 27–39. [Google Scholar]
- Liebman, H.A.; Pullarkat, V. Diagnosis and management of immune thrombocytopenia in era of thrombopoietin mimetics. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 384–390. [Google Scholar] [CrossRef]
- McMillan, R. The Pathogenesis of Chronic Immune Thrombocytopenic Purpura. Semin. Hematol. 2007, 44, S3–S11. [Google Scholar] [CrossRef]
- Li, J.; van der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015, 6, 7737. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B. Fc receptor blockade and immune thrombocytopenic purpura. Semin. Hematol. 2000, 37, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chu, L.; Li, Y.; Wang, Q.; Zhu, J.; Wang, C.; Cui, S. miR-23a/b suppresses cGAS-mediated innate and autoimmunity. Cell. Mol. Immunol. 2021, 18, 1235–1248. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, S.; Wang, Y.; Xu, R. The Extensive Regulation of MicroRNA in Immune Thrombocytopenia. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221093595. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, X.; Tian, Y.; Gao, X.; Liu, Z.; Dong, X.; Wang, L.; He, F.; Zhou, J. Downregulation of microRNA-155–5p prevents immune thrombocytopenia by promoting macrophage M2 polarization via the SOCS1-dependent PD1/PDL1 pathway. Life Sci. 2020, 257, 118057. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Güngör, T.; Arman Bilir, Ö.; Koşan Çulha, V.; Güngör, A.; Kara, A.; Azık, F.M.; Yaralı, H.N. Retrospective evaluation of children with immune thrombocytopenic purpura and factors contributing to chronicity. Pediatr. Neonatol. 2019, 60, 411–416. [Google Scholar] [CrossRef]
- Glanz, J.; France, E.; Xu, S.; Hayes, T.; Hambidge, S. A population-based, multisite cohort study of the predictors of chronic idiopathic thrombocytopenic purpura in children. Pediatrics 2008, 121, e506–e512. [Google Scholar] [CrossRef]
- Revel-Vilk, S.; Yacobovich, J.; Frank, S.; Ben-Ami, T.; Yechieli, M.; Shkalim, V.; Lebel, A.; Semo-Oz, R.; Tamary, H. Age and duration of bleeding symptoms at diagnosis best predict resolution of childhood immune thrombocytopenia at 3, 6, and 12 months. J. Pediatr. 2013, 163, 1335–1339. [Google Scholar] [CrossRef]
- Heitink-Pollé, K.M.J.; Nijsten, J.; Boonacker, C.W.B.; de Haas, M.; Bruin, M.C.A. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: A systematic review and meta-analysis. Blood 2014, 124, 3295–3307. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.M.; Neunert, C.; Grace, R.F.; Buchanan, G.; Imbach, P.; Vesely, S.K.; Kuhne, T. Predictors of remission in children with newly diagnosed immune thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr. Blood Cancer 2017, 65, e26736–e26737. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; Wendtland Edslev, P.; Heitink-Pollé, K.M.J.; Mertens, B.; Bruin, M.C.A.; Kapur, R.; Vidarsson, G.; van der Schoot, C.E.; Porcelijn, L.; van der Bom, J.G.; et al. A clinical prediction score for transient versus per-sistent childhood immune thrombocytopenia. J. Thromb. Haemost. 2021, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Kruse, A.; Kruse, C.; Watson, S.; Morgan, M.; Provan, D.; Ghanima, W.; Arnold, D.M.; Tomiyama, Y.; Santoro, C.; et al. Immune thrombocytopenia (ITP) World Impact Survey (iWISh): Patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am. J. Hematol. 2021, 96, 188–198. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Fu, R.; Sun, T.; Lyu, C.; Yang, R. Relationship between initial absolute lymphocyte counts and the prognosis of children with primary immune thrombocytopenia. J. Appl. Clin. Pediatr. 2015, 30, 1147–1151. [Google Scholar]
- Wang, Y.X.; Huang, Y.X. Clinical value of megakaryocytes in the diagnosis and treatment of children with immune thrombocytopenic purpura. Chin. J. Prim. Med. Pharm. 2019, 26, 2830–2834. [Google Scholar]
- Sun, Y.; Long, S.; Liu, W. Risk Factors and Psychological Analysis of Chronic Immune Thrombocytopenia in Children. Int. J. Gen. Med. 2020, 13, 1675–1683. [Google Scholar] [CrossRef]
- Edslev, P.W.; Rosthøj, S.; Treutiger, I.; Rajantie, J.; Zeller, B.; Jonsson, O.G.; NOPHO ITP Working Group. A clinical score predicting a brief and uneventful course of newly diagnosed idiopathic thrombocytopenic purpura in children. Br J Haematol. 2007, 138, 513–516. [Google Scholar] [CrossRef]
- Zeng, X.L.; Sherif, M.B. Evaluating Clinical Outcomes and Potential Prognostic Factors Among 308 Children and Adolescents with Immune Thrombocytopenia (ITP): An 11-Year Retrospective Cohort Study. Blood 2021, 138, 4053. [Google Scholar] [CrossRef]
- Bussel, J.B.; Garcia, C.A. Diagnosis of immune thrombocytopenia, including secondary forms, and selection of second-line treatment. Haematologica 2022, 107, 2018–2036. [Google Scholar] [CrossRef]
- Mahévas, M.; Moulis, G.; Andres, E.; Riviere, E.; Garzaro, M.; Crickx, E.; Guillotin, V.; Malphettes, M.; Galicier, L.; Noel, N.; et al. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: A French multicentre series. Br. J. Haematol. 2020, 190, e224–e229. [Google Scholar] [CrossRef] [PubMed]
- DiMaggio, D.; Anderson, A.; Bussel, J.B. Cytomegalovirus can make immune thrombocytopenic purpura refractory. Br. J. Haematol. 2009, 146, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.M.S.; Saki, N.; Ghandali, M.V.; Ekrami, A.; Avarvand, A.Y. Effect of Helicobacter Pylori eradication on patients with ITP: A meta-analysis of studies conducted in the Middle East. Blood Res. 2021, 56, 38–43. [Google Scholar] [CrossRef]
- Osman, M.E. Chronic immune thrombocytopenia in a child responding only to thrombopoietin receptor agonist. Sudan J. Paediatr. 2012, 12, 60–64. [Google Scholar]
- Neunert, C.; Despotovic, J.; Haley, K.; Lambert, M.P.; Nottage, K.; Shimano, K.; Bennett, C.; Klaassen, R.; Stine, K.; Thompson, A.; et al. Thrombopoietin receptor agonist use in children: Data from the pediatric ITP consortium of North America ICON2 Study. Pediatr. Blood Cancer 2016, 63, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M. Thrombopoietin receptor agonists in the treatment of immune thrombocytopenia: A clinician perspective. Am. J. Hematol. 2012, 87, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Elkus, H.; Grace, R.F.; Buissereth, T.; Bennett, C.; Rifkin-Zenenberg, S.; Gunn, E.R.; Lebensburger, J.D.; Angulo, P.; Davini, M.; Rankin, A.W.; et al. Standardizing the Diagnostic and Therapeutic Approach to Newly Diagnosed Children with ITP: Prospective Data from the ITP Consortium of North America (ICON) Quality Improvement Initiative. Blood 2022, 140 (Suppl. S1), 1208–1210. [Google Scholar] [CrossRef]
- Tumaini Massaro, J.; Chen, Y.; Ke, Z. Efficacy and safety of thrombopoietin receptor agonists in children with chronic immune thrombocytopenic purpura: Meta-analysis. Platelets 2019, 30, 828–835. [Google Scholar] [CrossRef]
- Grainger, J.D.; Locatelli, F.; Chotsampancharoen, T.; Donyush, E.; Pongtanakul, B.; Komvilaisak, P.; Sosothikul, D.; Drelichman, G.; Sirachainan, N.; Holzhauer, S.; et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 386, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yagasaki, H.; Kanezawa, K.; Shimozawa, K.; Hirai, M.; Morioka, I. Incidence and outcomes of refractory immune thrombocytopenic purpura in children: A retrospective study in a single institution. Sci. Rep. 2021, 11, 14263. [Google Scholar] [CrossRef]
- Matsubara, K.; Takahashi, Y.; Hayakawa, A.; Tanaka, F.; Nakadate, H.; Sakai, M.; Maeda, N.; Oka, T.; Ishii, E.; Bessho, F.; et al. Long-term follow-up of children with refractory immune thrombocytopenia treated with rituximab. Int. J. Hematol. 2014, 99, 429–436. [Google Scholar] [CrossRef] [PubMed]
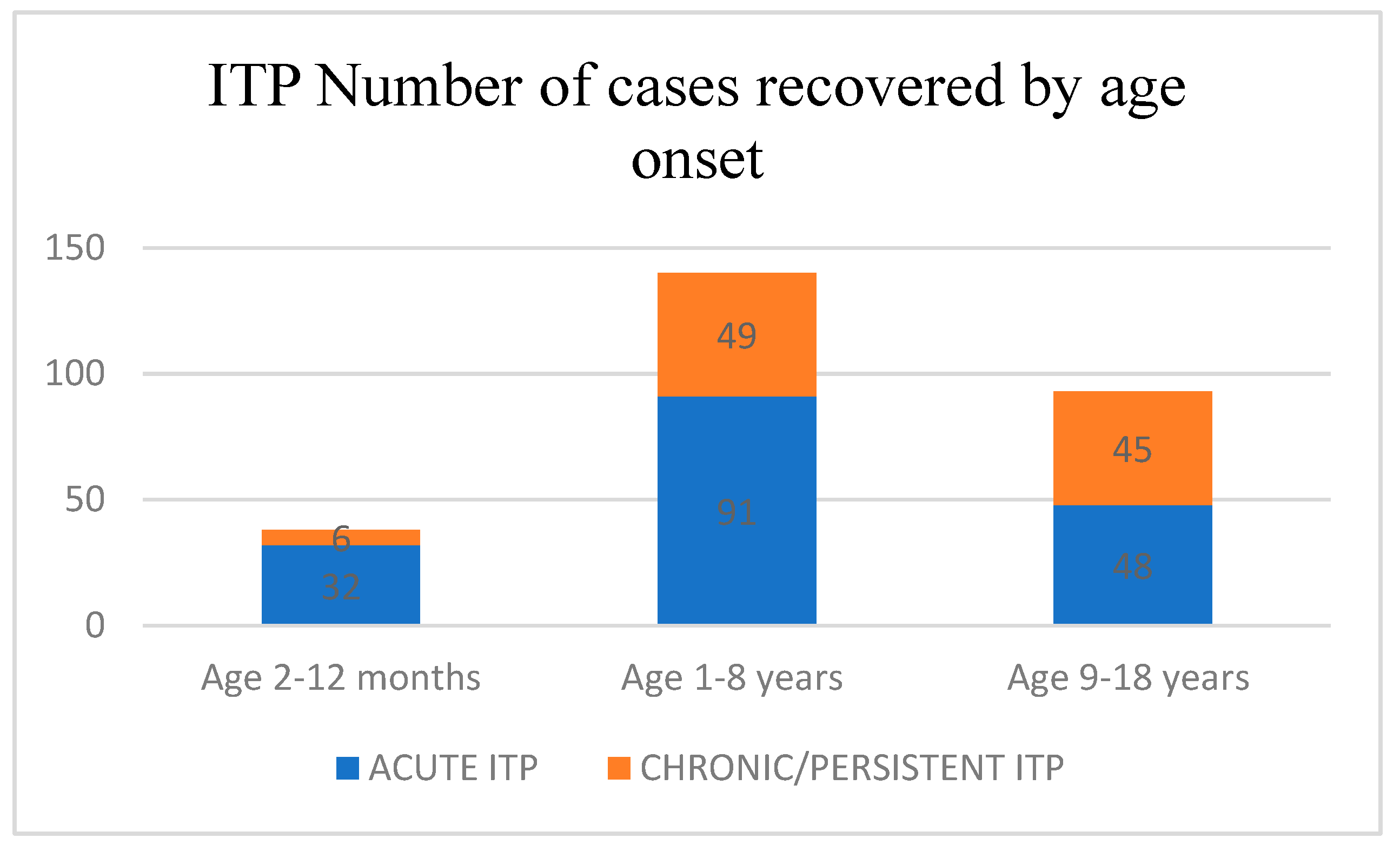


| Age Group | Platelet Count < 10 × 109/L | Platelet Count ≥ 10 × 109/L | 95% CI for Difference |
|---|---|---|---|
| 2–12 months | 10/12 (83.3%) | 22/26 (84.6%) | 14.2 to 11.6 |
| 1–8 years | 51/68 (75.0%) | 40/72 (55.6%) | 11.6 to 27.3 |
| 9–18 years | 23/41 (56.1%) | 25/52 (48.1%) | 2.4 to 18.4 |
| Total | 84/121 (69.4%) | 87/150 (58.1%) | 5.6 to 17.2 |
| Treatment | |||||
|---|---|---|---|---|---|
| Recovered | No Treatment | Corticosteroids | Corticosteroids + IGIV | Total | |
| NO | Observed | 13 | 51 | 27 | 91 |
| % within row | 14.3% | 56.0% | 29.7% | 100.0% | |
| YES | Observed | 9 | 117 | 40 | 166 |
| % within row | 5.4% | 70.5 % | 24.1% | 100.0% | |
| Total | Observed | 22 | 168 | 67 | 257 |
| % within row | 8.6% | 65.4% | 26.1% | 100.0% | |
| χ2 | df | p | |
|---|---|---|---|
| RECOVERED | 7.939 | 2 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosu, V.E.; Roșu, S.T.; Ivanov, A.V.; Starcea, I.M.; Streanga, V.; Miron, I.C.; Mocanu, A.; Lupu, A.; Lupu, V.V.; Gavrilovici, C. Predictor Factors for Chronicity in Immune Thrombocytopenic Purpura in Children. Children 2023, 10, 911. https://doi.org/10.3390/children10060911
Rosu VE, Roșu ST, Ivanov AV, Starcea IM, Streanga V, Miron IC, Mocanu A, Lupu A, Lupu VV, Gavrilovici C. Predictor Factors for Chronicity in Immune Thrombocytopenic Purpura in Children. Children. 2023; 10(6):911. https://doi.org/10.3390/children10060911
Chicago/Turabian StyleRosu, Vasile Eduard, Solange Tamara Roșu, Anca Viorica Ivanov, Iuliana Magdalena Starcea, Violeta Streanga, Ingrith Crenguta Miron, Adriana Mocanu, Ancuta Lupu, Vasile Valeriu Lupu, and Cristina Gavrilovici. 2023. "Predictor Factors for Chronicity in Immune Thrombocytopenic Purpura in Children" Children 10, no. 6: 911. https://doi.org/10.3390/children10060911





