The Applicability of a 2-Transcript Signature to Identify Bacterial Infections in Children with Febrile Neutropenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Case Definition and Sampling Procedures
2.3. Sample Collection and Storage
2.4. RNA Sequencing and Analysis
2.5. Definition of Test Groups
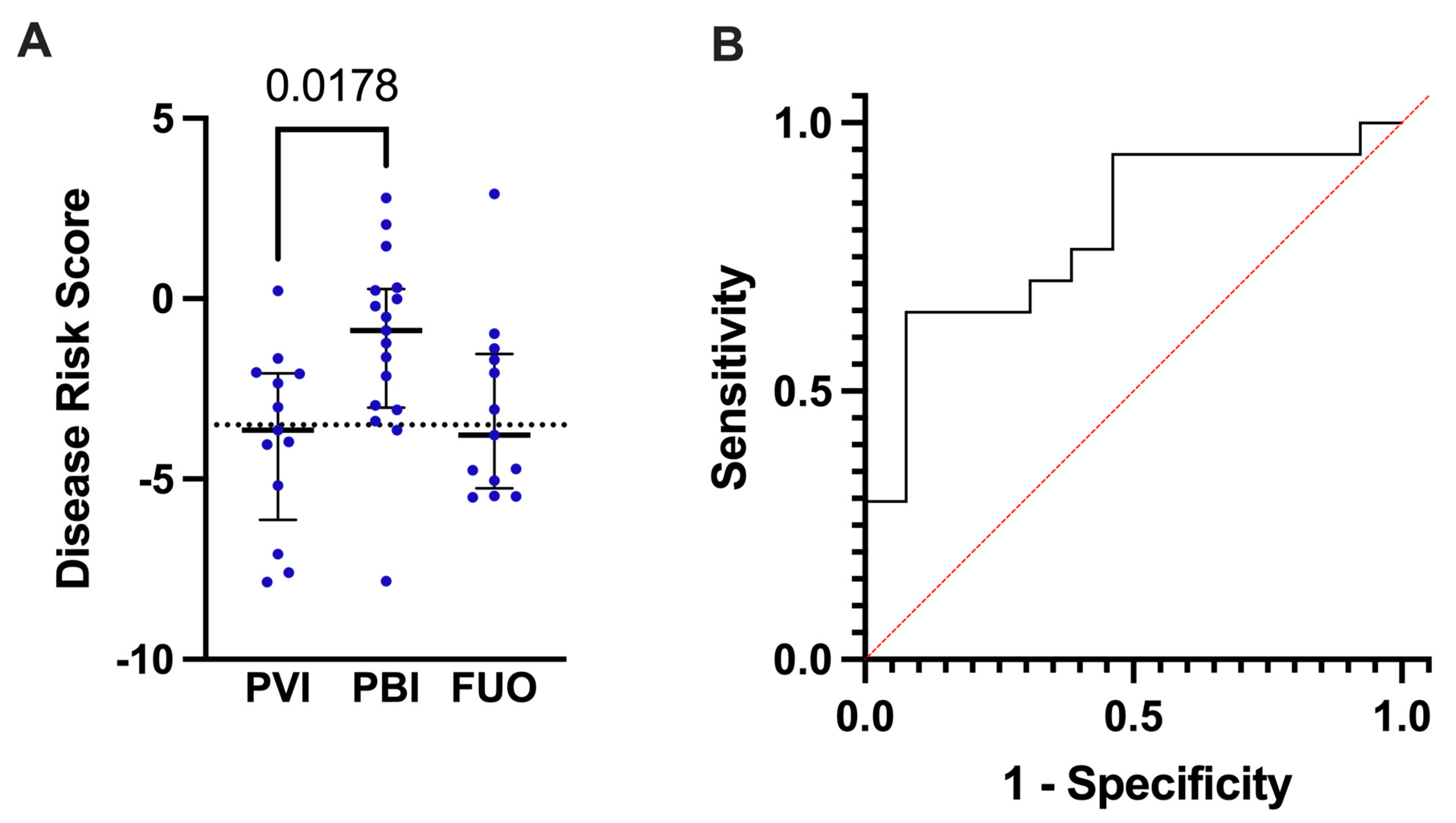
2.5.1. Test Groups Based on Microbiologically Defined Infections
2.5.2. Test Groups Based on Microbial Detection and CRP Cut-Off Values
2.5.3. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Analysis of Other Significantly Expressed Genes and Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Conflicts of Interest
References
- Rhedin, S.; Elfving, K.; Berggren, A. Novel Biomarkers Differentiating Viral from Bacterial Infection in Febrile Children: Future Perspectives for Management in Clinical Praxis. Children 2021, 8, 1070. [Google Scholar] [CrossRef]
- Karakioulaki, M.; Stolz, D. Biomarkers in Pneumonia—Beyond Procalcitonin. Int. J. Mol. Sci. 2019, 20, 2004. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sindhu, K.N.; Ramanujam, K.; Ramasamy, R.K.; Subramaniam, S.; Ganesan, S.K.; Vajja, S.; David, A.S.; Lankala, P.; Rose, W.; et al. Factors Predicting Blood Culture Positivity in Children with Enteric Fever. J. Infect. Dis. 2021, 224 (Suppl. S5), S484–S493. [Google Scholar] [CrossRef] [PubMed]
- Rampini, S.K.; Bloemberg, G.V.; Keller, P.M.; Büchler, A.C.; Dollenmaier, G.; Speck, R.F.; Böttger, E.C. Broad-range 16S rRNA gene polymerase chain reaction for diagnosis of culture-negative bacterial infections. Clin. Infect. Dis. 2011, 53, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Wilson, S. Febrile neutropenia in paediatric oncology. Paediatr. Child Health 2020, 30, 93–97. [Google Scholar] [CrossRef]
- Alali, M.; David, M.Z.; Danziger-Isakov, L.A.; Elmuti, L.; Bhagat, P.H.; Bartlett, A.H. Pediatric Febrile Neutropenia: Change in Etiology of Bacteremia, Empiric Choice of Therapy and Clinical Outcomes. J. Pediatr. Hematol. 2020, 42, e445–e451. [Google Scholar] [CrossRef]
- Castagnola, E.; Fontana, V.; Caviglia, I.; Caruso, S.; Faraci, M.; Fioredda, F.; Garrè, M.L.; Moroni, C.; Conte, M.; Losurdo, G.; et al. A Prospective Study on the Epidemiology of Febrile Episodes during Chemotherapy-Induced Neutropenia in Children with Cancer or after Hemopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2007, 45, 1296–1304. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.L.; Rolston, K.A.; Young, G.A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, A.; Bhadri, V.; Söderhäll, S.; Öhrmalm, L.; Wong, M.; Norbeck, O.; Lindau, C.; Rotzén-Östlund, M.; Allander, T.; Catchpoole, D.; et al. Respiratory viruses, a common microbiological finding in neutropenic children with fever. J. Clin. Virol. 2010, 47, 234–237. [Google Scholar] [CrossRef]
- Söderman, M.; Rhedin, S.; Tolfvenstam, T.; Rotzén-Östlund, M.; Albert, J.; Broliden, K.; Lindblom, A. Frequent Respiratory Viral Infections in Children with Febrile Neutropenia—A Prospective Follow-Up Study. PLoS ONE 2016, 11, e0157398. [Google Scholar] [CrossRef]
- Aldemir-Kocabas, B.; Karbuz, A.; Pekpak, E.; Karahan, Z.; Dolapci, G.Ü.L.L.Ü.; İnce, E.; Uysal, Z.; Yavuz, G.; Çiftçi, E.; İnce, E. Effects of respiratory viruses on febrile neutropenia attacks in children. Turk. J. Pediatr. 2017, 59, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Grecka, P.; Poulakou, G.; Anargyrou, K.; Katsilambros, N.; Giamarellou, H. Assessment of Procalcitonin as a Diagnostic Marker of Underlying Infection in Patients with Febrile Neutropenia. Clin. Infect. Dis. 2001, 32, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-W.; Wu, J.-Y.; Chen, C.-K.; Huang, S.-L.; Hsu, S.-C.; Lee, M.-T.G.; Chang, S.-S.; Lee, C.-C. Does procalcitonin, C-reactive protein, or interleukin-6 test have a role in the diagnosis of severe infection in patients with febrile neutropenia? A systematic review and meta-analysis. Support. Care Cancer 2015, 23, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Boeriu, E.; Borda, A.; Vulcanescu, D.D.; Sarbu, V.; Arghirescu, S.T.; Ciorica, O.; Bratosin, F.; Marincu, I.; Horhat, F.G. Diagnosis and Management of Febrile Neutropenia in Pediatric Oncology Patients—A Systematic Review. Diagnostics 2022, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, J.; Crosby, S.D.; Storch, G.A. Gene expression profiles in febrile children with defined viral and bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, 12792–12797. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Kuppermann, N.; Mejias, A.; Suarez, N.; Chaussabel, D.; Casper, T.C.; Smith, B.; Alpern, E.R.; Anders, J.; Atabaki, S.M.; et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA 2016, 316, 846–857. [Google Scholar] [CrossRef]
- Ramilo, O.; Allman, W.; Chung, W.; Mejias, A.; Ardura, M.; Glaser, C.; Wittkowski, K.M.; Piqueras, B.; Banchereau, J.; Palucka, A.K.; et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood 2006, 109, 2066–2077. [Google Scholar] [CrossRef]
- Herberg, J.A.; Kaforou, M.; Wright, V.J.; Shailes, H.; Eleftherohorinou, H.; Hoggart, C.J.; Cebey-López, M.; Carter, M.J.; Janes, V.A.; Gormley, S.; et al. Diagnostic test accuracy of a 2-transcript host RNA signature for discriminating bacterial vs viral infection in febrile children. JAMA 2016, 316, 835–845. [Google Scholar] [CrossRef]
- Wahlund, M.; Sinha, I.; Broliden, K.; Saghafian-Hedengren, S.; Nilsson, A.; Berggren, A. The Feasibility of Host Transcriptome Profiling as a Diagnostic Tool for Microbial Etiology in Childhood Cancer Patients with Febrile Neutropenia. Int. J. Mol. Sci. 2020, 21, 5305. [Google Scholar] [CrossRef]
- Haeusler, G.M.; Garnham, A.L.; Li-Wai-Suen, C.S.; Clark, J.E.; Babl, F.E.; Allaway, Z.; Slavin, M.A.; Mechinaud, F.; Smyth, G.K.; Phillips, B.; et al. Blood transcriptomics identifies immune signatures indicative of infectious complications in childhood cancer patients with febrile neutropenia. Clin. Transl. Immunol. 2022, 11, e1383. [Google Scholar] [CrossRef]
- Kelly, R.S.; Lasky-Su, J.; Yeung, S.-C.J.; Stone, R.M.; Caterino, J.M.; Hagan, S.C.; Lyman, G.H.; Baden, L.R.; Glotzbecker, B.E.; Coyne, C.J.; et al. Integrative omics to detect bacteremia in patients with febrile neutropenia. PLoS ONE 2018, 13, e0197049. [Google Scholar] [CrossRef]
- Kaforou, M.; Wright, V.; Oni, T.; French, N.; Anderson, S.T.; Bangani, N.; Banwell, C.M.; Brent, A.J.; Crampin, A.; Dockrell, H.; et al. Detection of Tuberculosis in HIV-Infected and -Uninfected African Adults Using Whole Blood RNA Expression Signatures: A Case-Control Study. PLOS Med. 2013, 10, e1001538. [Google Scholar] [CrossRef]
- Bruel, A.V.D.; Thompson, M.J.; Haj-Hassan, T.; Stevens, R.; Moll, H.; Lakhanpaul, M.; Mant, D. Diagnostic value of laboratory tests in identifying serious infections in febrile children: Systematic review. BMJ 2011, 342, d3082. [Google Scholar] [CrossRef]
- Kool, M.; Elshout, G.; Koes, B.W.; Bohnen, A.M.; Berger, M.Y. C-Reactive Protein Level as Diagnostic Marker in Young Febrile Children Presenting in a General Practice Out-of-Hours Service. J. Am. Board Fam. Med. 2016, 29, 460–468. [Google Scholar] [CrossRef]
- Martinez-Albarran, M.; de Jesus Perez-Molina, J.; Gallegos-Castorena, S.; Sanchez-Zubieta, F.; Del Toro-Arreola, S.; Troyo-Sanroman, R.; Gonzalez-Ramella, O. Procalcitonin and C-reactive protein serum levels as markers of infection in a pediatric population with febrile neutropenia and cancer. Pediatr. Hematol. Oncol. 2009, 26, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Rhedin, S.; Lindstrand, A.; Rotzén-Östlund, M.; Tolfvenstam, T.; Öhrmalm, L.; Rinder, M.R.; Zweygberg-Wirgart, B.; Ortqvist, A.; Henriques-Normark, B.; Broliden, K.; et al. Clinical Utility of PCR for Common Viruses in Acute Respiratory Illness. Pediatrics 2014, 133, e538–e545. [Google Scholar] [CrossRef] [PubMed]
- Acuña, M.; O’Ryan, M.; Cofré, J.; Alvarez, I.; Benadof, D.; Rodríguez, P.; Torres, M.T.; Aguilera, L.; Santolaya, M.E. Differential Time to Positivity and Quantitative Cultures for Noninvasive Diagnosis of Catheter-Related Blood Stream Infection in Children. Pediatr. Infect. Dis. J. 2008, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Batard, E.; Gastinne, T.; Potel, G.; de La Cochetière, M.F. Recent changes in bacteremia in patients with cancer: A systematic review of epidemiology and antibiotic resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 841–850. [Google Scholar] [CrossRef]
- Long, E.; Babl, F.E.; Phillips, N.; Craig, S.; Zhang, M.; Kochar, A.; McCaskill, M.; Borland, M.L.; Slavin, M.A.; Phillips, R.; et al. Prevalence and predictors of poor outcome in children with febrile neutropaenia presenting to the emergency department. Emerg. Med. Australas. 2022, 34, 786–793. [Google Scholar] [CrossRef]
- Garwicz, D.; Lennartsson, A.; Jacobsen, S.E.W.; Gullberg, U.; Lindmark, A. Biosynthetic profiles of neutrophil serine proteases in a human bone marrow-derived cellular myeloid differentiation model. Haematologica 2005, 90, 38–44. [Google Scholar]
- Standish, A.J.; Weiser, J.N. Human Neutrophils Kill Streptococcus pneumoniae via Serine Proteases. J. Immunol. 2009, 183, 2602–2609. [Google Scholar] [CrossRef] [PubMed]


| Probable Viral (n = 13) | Probable Bacterial (n = 17) | Fever of Unknown Origin (n = 13) | Control (n = 12) | |
|---|---|---|---|---|
| Age (years) (median, range) | 4.6 (0.6–15.6) | 9.4 (0.5–16.1) | 9.7 (0.5–15.7) | 9.7 (0.6–15.8) |
| Gender (n female) (%) | 8 (62) | 10 (59) | 5 (38) | 8 (67) |
| Peak CRP (mg/L) * (median, range) | 25 (4–97) | 141 (22–412) | 59 (28–90) | N/A |
| WBC count (109/L) (median, range) | 2.1 (0.2–5.6) | 1.3 (0.3–14.6) | 1.8 (0.2–8.1) | 3.7 (0.7–6.6) |
| ANC (109/L) (median, range) | 0.2 (0.1–0.6) | 0.1 (0.1–0.5) | 0.1 (0.1–0.4) | 2.1 (0.1–5.6) |
| Days with neutropenia (median, range) | 10 (5–37) | 6 (1–>30) | 9 (5–20) | N/A |
| Peak temperature (°C) (median, range) | 38.9 (38.1–40.5) | 39.2 (38.1–40.3) | 39.3 (38.5–40.2) | N/A |
| Days with fever (median, range) | 2 (1–6) | 2 (1–16) | 2 (1–4) | N/A |
| Days hospitalized † (median, range) | 4 (0–8) | 6 (3–>30) | 4 (3–10) | N/A |
| Days with antibiotics (median, range) | 5 (0–10) | 8 † (5–30) | 7 (3–10) | N/A |
| Respiratory symptoms, n (%) ‡ | 12 (92) | 14 (82) | 6 (46) | N/A |
| Gastrointestinal symptoms, n (%) | 2 (15) | 3 (18) | 2 (15) | N/A |
| Local symptoms, n (%) | 1 (8) | 4 (24) | 2 (15) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aasa, J.; Tiselius, E.; Sinha, I.; Edman, G.; Wahlund, M.; Hedengren, S.S.; Nilsson, A.; Berggren, A. The Applicability of a 2-Transcript Signature to Identify Bacterial Infections in Children with Febrile Neutropenia. Children 2023, 10, 966. https://doi.org/10.3390/children10060966
Aasa J, Tiselius E, Sinha I, Edman G, Wahlund M, Hedengren SS, Nilsson A, Berggren A. The Applicability of a 2-Transcript Signature to Identify Bacterial Infections in Children with Febrile Neutropenia. Children. 2023; 10(6):966. https://doi.org/10.3390/children10060966
Chicago/Turabian StyleAasa, Johannes, Eva Tiselius, Indranil Sinha, Gunnar Edman, Martina Wahlund, Shanie Saghafian Hedengren, Anna Nilsson, and Anna Berggren. 2023. "The Applicability of a 2-Transcript Signature to Identify Bacterial Infections in Children with Febrile Neutropenia" Children 10, no. 6: 966. https://doi.org/10.3390/children10060966






