Preoperative Spinal Angiography for Thoracic Neuroblastoma: Impact of Identification of the Adamkiewicz Artery on Gross Total Resection and Neurological Sequelae
Abstract
:Simple Summary
Abstract
1. Introduction
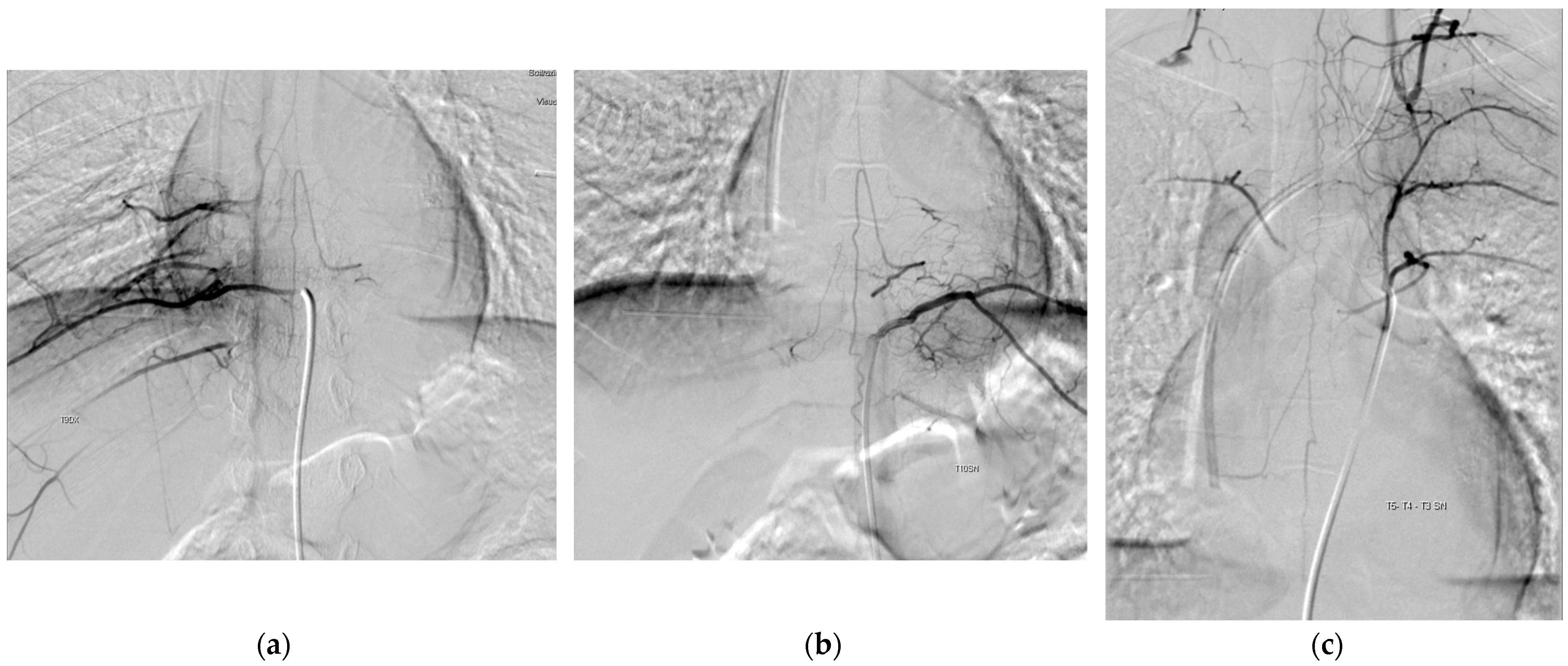
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthyssens, L.E.; Nuchtern, J.G.; Van De Ven, C.P.; Gabra, H.O.S.; Bjornland, K.; Irtan, S.; Stenman, J.; Pio, L.; Cross, K.M.; Avanzini, S.; et al. A Novel Standard for Systematic Reporting of Neuroblastoma Surgery: The International Neuroblastoma Surgical Report Form (INSRF): A Joint Initiative by the Pediatric Oncological Cooperative Groups SIOPEN∗, COG∗∗, and GPOH∗∗∗. Ann. Surg. 2022, 275, e575–e585. [Google Scholar] [CrossRef]
- Chen, A.M.; Trout, A.T.; Towbin, A.J. A review of neuroblastoma image-defined risk factors on magnetic resonance imaging. Pediatr. Radiol. 2018, 48, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Parhar, D.; Joharifard, S.; Lo, A.C.; Schlosser, M.-P.; Daodu, O.O. How well do image-defined risk factors (IDRFs) predict surgical outcomes and survival in patients with neuroblastoma? A systematic review and meta-analysis. Pediatr. Surg. Int. 2020, 36, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Pio, L.; Blanc, T.; Denis, T.D.S.; Irtan, S.; Valteau-Couanet, M.; Michon, J.; Brisse, H.; Galmiche-Rolland, L.; Joyeux, L.; Odent, T.; et al. Multidisciplinary surgical strategy for dumbbell neuroblastoma: A single-center experience of 32 cases. Pediatr. Blood Cancer 2019, 66 (Suppl. S3), e27670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boglino, C.; Martins, A.G.; Ciprandi, G.; Sousinha, M.; Inserra, A. Spinal cord vascular injuries following surgery of advanced thoracic neuroblastoma: An unusual catastrophic complication. Med. Pediatr. Oncol. 1999, 32, 349–352. [Google Scholar] [CrossRef]
- Schmidt, A.; Hempel, J.-M.; Ellerkamp, V.; Warmann, S.W.; Ernemann, U.; Fuchs, J. The Relevance of Preoperative Identification of the Adamkiewicz Artery in Posterior Mediastinal Pediatric Tumors. Ann. Surg. Oncol. 2022, 29, 493–499. [Google Scholar] [CrossRef]
- Nordin, A.B.; Fallon, S.C.; Jea, A.; Kim, E.S. The use of spinal angiography in the management of posterior mediastinal tumors: Case series and review of the literature. J. Pediatr. Surg. 2013, 48, 1871–1877. [Google Scholar] [CrossRef]
- Clark, R.A.; Jacobson, J.C.; Murphy, J.T. Preoperative spinal angiography decreases risk of spinal ischemia in pediatric posterior thoracic tumor resection. Pediatr. Surg. Int. 2022, 38, 1427–1434. [Google Scholar] [CrossRef]
- Hino, T.; Kamitani, T.; Sagiyama, K.; Yamasaki, Y.; Matsuura, Y.; Tsutsui, S.; Sakai, Y.; Furuyama, T.; Yabuuchi, H. Detectability of the artery of Adamkiewicz on computed tomography angiography of the aorta by using ultra-high-resolution computed tomography. Jpn. J. Radiol. 2020, 38, 658–665. [Google Scholar] [CrossRef]
- Englum, B.R.; Rialon, K.L.; Speicher, P.J.; Gulack, B.; Driscoll, T.A.; Kreissman, S.G.; Rice, H.E. Value of surgical resection in children with high-risk neuroblastoma. Pediatr. Blood Cancer 2015, 62, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
- La Quaglia, M.P. State of the art in oncology: High risk neuroblastoma, alveolar rhabdomyosarcoma, desmoplastic small round cell tumor, and POST-TEXT 3 and 4 hepatoblastoma. J. Pediatr. Surg. 2014, 49, 233–240. [Google Scholar] [CrossRef] [PubMed]
- von Allmen, D.; Davidoff, A.M.; London, W.B.; Van Ryn, C.; Haas-Kogan, D.A.; Kreissman, S.G.; Khanna, G.; Rosen, N.; Park, J.R.; La Quaglia, M.P. Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.; Pötschger, U.; Pearson, A.D.J.; Sarnacki, S.; Cecchetto, G.; Gomez-Chacon, J.; Squire, R.; Freud, E.; Bysiek, A.; Matthyssens, L.; et al. Influence of Surgical Excision on the Survival of Patients With Stage 4 High-Risk Neuroblastoma: A Report From the HR-NBL1/SIOPEN Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2902–2915. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, E.; Fukui, S.; Chiras, J.; Koskas, F.; Bahnini, A.; Cormier, E. Spinal cord arteriography: A safe adjunct before descending thoracic or thoracoabdominal aortic aneurysmectomy. J. Vasc. Surg. 2002, 35, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madafferi, S.; Accinni, A.; Martucci, C.; Voglino, V.; Frediani, S.; Picardo, S.; Inserra, A. Paraplegia after thoracotomy: A single center experience with pediatric patients and a review of the literature. Ann. Ital. Chir. 2022, 92, 27–32. [Google Scholar] [PubMed]
- Nijenhuis, R.J.; Mull, M.; Wilmink, J.T.; Thron, A.K.; Backes, W.H. MR angiography of the great anterior radiculomedullary artery (Adamkiewicz artery) validated by digital subtraction angiography. AJNR Am. J. Neuroradiol. 2006, 27, 1565–1572. [Google Scholar]
- Ou, P.; Schmit, P.; Layouss, W.; Sidi, D.; Bonnet, D.; Brunelle, F. CT angiography of the artery of Adamkiewicz with 64-section technology: First experience in children. AJNR Am. J. Neuroradiol. 2007, 28, 216–219. [Google Scholar]
- Uotani, K.; Yamada, N.; Kono, A.; Taniguchi, T.; Sugimoto, K.; Fujii, M.; Kitagawa, A.; Okita, Y.; Naito, H.; Sugimura, K. Preoperative visualization of the artery of Adamkiewicz by intra-arterial CT angiography. AJNR Am. J. Neuroradiol. 2008, 29, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Taterra, D.; Skinningsrud, B.; Pękala, P.A.; Hsieh, W.C.; Cirocchi, R.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A.; Henry, B.M. Artery of Adamkiewicz: A meta-analysis of anatomical characteristics. Neuroradiology 2019, 61, 869–880. [Google Scholar] [CrossRef] [Green Version]
- Biglioli, P.; Spirito, R.; Roberto, M.; Grillo, F.; Cannata, A.; Parolari, A.; Maggioni, M.; Coggi, G. The anterior spinal artery: The main arterial supply of the human spinal cord—A preliminary anatomic study. J. Thorac. Cardiovasc. Surg. 2000, 119, 376–379. [Google Scholar] [CrossRef] [Green Version]
- Lo, D.; Valleé, J.N.; Spelle, L.; Cormier, E.; Saillant, G.; Rancurel, G.; Chiras, J. Unusual origin of the artery of Adamkiewicz from the fourth lumbar artery. Neuroradiology 2002, 44, 153–157. [Google Scholar] [CrossRef]
- Murthy, N.S.; Maus, T.P.; Behrns, C.L. Intraforaminal location of the great anterior radiculomedullary artery (artery of Adamkiewicz): A retrospective review. Pain Med. 2010, 11, 1756–1764. [Google Scholar] [CrossRef] [Green Version]
- Shlobin, N.A.; Raz, E.; Shapiro, M.; Clark, J.R.; Hoffman, S.C.; Shaibani, A.; Hurley, M.C.; Ansari, S.A.; Jahromi, B.S.; Dahdaleh, N.S.; et al. Spinal neurovascular complications with anterior thoracolumbar spine surgery: A systematic review and review of thoracolumbar vascular anatomy. Neurosurg. Focus 2020, 49, E9. [Google Scholar] [CrossRef] [PubMed]
- Champlin, A.M.; Rael, J.; Benzel, E.C.; Kesterson, L.; King, J.N.; Orrison, W.W.; Mirfakhraee, M. Preoperative spinal angiography for lateral extracavitary approach to thoracic and lumbar spine. AJNR Am. J. Neuroradiol. 1994, 15, 73–77. [Google Scholar] [PubMed]
- Charles, Y.P.; Barbe, B.; Beaujeux, R.; Boujan, F.; Steib, J.-P. Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg. Radiol. Anat. 2011, 33, 3–9. [Google Scholar] [CrossRef]
- Fanous, A.A.; Lipinski, L.J.; Krishna, C.; Roger, E.P.M.; Siddiqui, A.H.M.; Levy, E.I.M.; Leonardo, J.; Pollina, J.M. The Impact of Preoperative Angiographic Identification of the Artery of Adamkiewicz on Surgical Decision Making in Patients Undergoing Thoracolumbar Corpectomy. Spine 2015, 40, 1194–1199. [Google Scholar] [CrossRef]
- Savader, S.J.; Williams, G.M.; Trerotola, S.O.; Perler, B.A.; Wang, M.C.; Venbrux, A.C.; Lund, G.B.; Osterman, F.A. Preoperative spinal artery localization and its relationship to postoperative neurologic complications. Radiology 1993, 189, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gailloud, P. Safety of spinal angiography: Complication rate analysis in 302 diagnostic angiograms. Neurology 2011, 77, 1235–1240. [Google Scholar] [CrossRef]
| Patient Nr | Sex | Age at Diagnosis (y) | Side | Thoracoabdominal | Number of IDRFs | n-MYC Amplified | D Max (cm) | Stage Risk | Protocol |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 16.4 | L | No | 1 | Yes | 18 × 8.5 × 8 | High (M) | COJEC |
| 2 | F | 1.9 | R | No | 1 | No | 3.5 × 5.1 × 6 | Intermediate (L2) | LINES |
| 3 | F | 0.5 | L | Yes | 8 | No | 5 × 3.8 × 7 | Intermediate (L2) | LINES |
| 4 | M | 3.5 | L | No | 2 | Yes | 14 × 6 × 6.6 | High (M) | COJEC |
| 5 | F | 1.7 | L | Yes | 4 | No | 5 × 5.5 × 10 | Intermediate (L2) | LINES |
| 6 | M | 3.9 | L | Yes | DU | No | 11 × 7 × 20 | High (M) | COJEC |
| Patient Nr | Diagnosis–POSA (Months) | Nr IDRFs Pre-POSA | Age at POSA (y) | Complications | AKA Origin | AKA-TNB Position | Management Post-POSA |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 1 | 16,6 | No | Left intercostal artery T11 | Homolateral | Surgery |
| 2 | 4 | 1 | 2,2 | No | Left intercostal artery T5 | Contralateral | Surgery |
| 3 | 13 | 4 | 1,6 | No | Left intercostal artery T10 | Homolateral | No surgery |
| 4 | 3 | 2 | 3,7 | No | Left intercostal artery T10 | Homolateral | Surgery |
| 5 | 6 | 3 | 2,2 | No | Right intercostal artery T9 | Contralateral | Surgery |
| 6 | 30 | 6 | 1,5 | No | Left intercostal artery T9 | Homolateral | Surgery |
| Patient Nr | Access | Intraoperative Lesions | Gross Total Resection | FU Since Diagnosis (Years) | Relapse | Neurologic Complications | Status at Last FU |
|---|---|---|---|---|---|---|---|
| 1 | Median sternotomy | No | Yes | 3.9 | Yes | No | Alive and well |
| 2 | Posterolateral thoracotomy | Lung | Yes | 3.6 | No | No | Alive and well |
| 3 | No surgery | NS | NS | 8.0 | No | No | Alive and well |
| 4 | Posterolateral thoracotomy | No | No | 2.6 | No | No | Alive and well |
| 5 | Posterolateral thoracotomy | No | Yes | 4.3 | No | No | Alive and well |
| 6 | Posterolateral thoracotomy | Aorta | No | 6.5 | Yes | No | Deceased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarfati, A.; Martucci, C.; Persano, G.; Cassanelli, G.; Crocoli, A.; Madafferi, S.; Natali, G.L.; De Ioris, M.A.; Inserra, A. Preoperative Spinal Angiography for Thoracic Neuroblastoma: Impact of Identification of the Adamkiewicz Artery on Gross Total Resection and Neurological Sequelae. Children 2023, 10, 1116. https://doi.org/10.3390/children10071116
Zarfati A, Martucci C, Persano G, Cassanelli G, Crocoli A, Madafferi S, Natali GL, De Ioris MA, Inserra A. Preoperative Spinal Angiography for Thoracic Neuroblastoma: Impact of Identification of the Adamkiewicz Artery on Gross Total Resection and Neurological Sequelae. Children. 2023; 10(7):1116. https://doi.org/10.3390/children10071116
Chicago/Turabian StyleZarfati, Angelo, Cristina Martucci, Giorgio Persano, Giulia Cassanelli, Alessandro Crocoli, Silvia Madafferi, Gian Luigi Natali, Maria Antonietta De Ioris, and Alessandro Inserra. 2023. "Preoperative Spinal Angiography for Thoracic Neuroblastoma: Impact of Identification of the Adamkiewicz Artery on Gross Total Resection and Neurological Sequelae" Children 10, no. 7: 1116. https://doi.org/10.3390/children10071116
APA StyleZarfati, A., Martucci, C., Persano, G., Cassanelli, G., Crocoli, A., Madafferi, S., Natali, G. L., De Ioris, M. A., & Inserra, A. (2023). Preoperative Spinal Angiography for Thoracic Neuroblastoma: Impact of Identification of the Adamkiewicz Artery on Gross Total Resection and Neurological Sequelae. Children, 10(7), 1116. https://doi.org/10.3390/children10071116






