Fetal Congenital Complete Heart Block: A Rare Case with an Extremely Low Ventricular Rate and Review of Current Management Strategies
Abstract
:1. Introduction
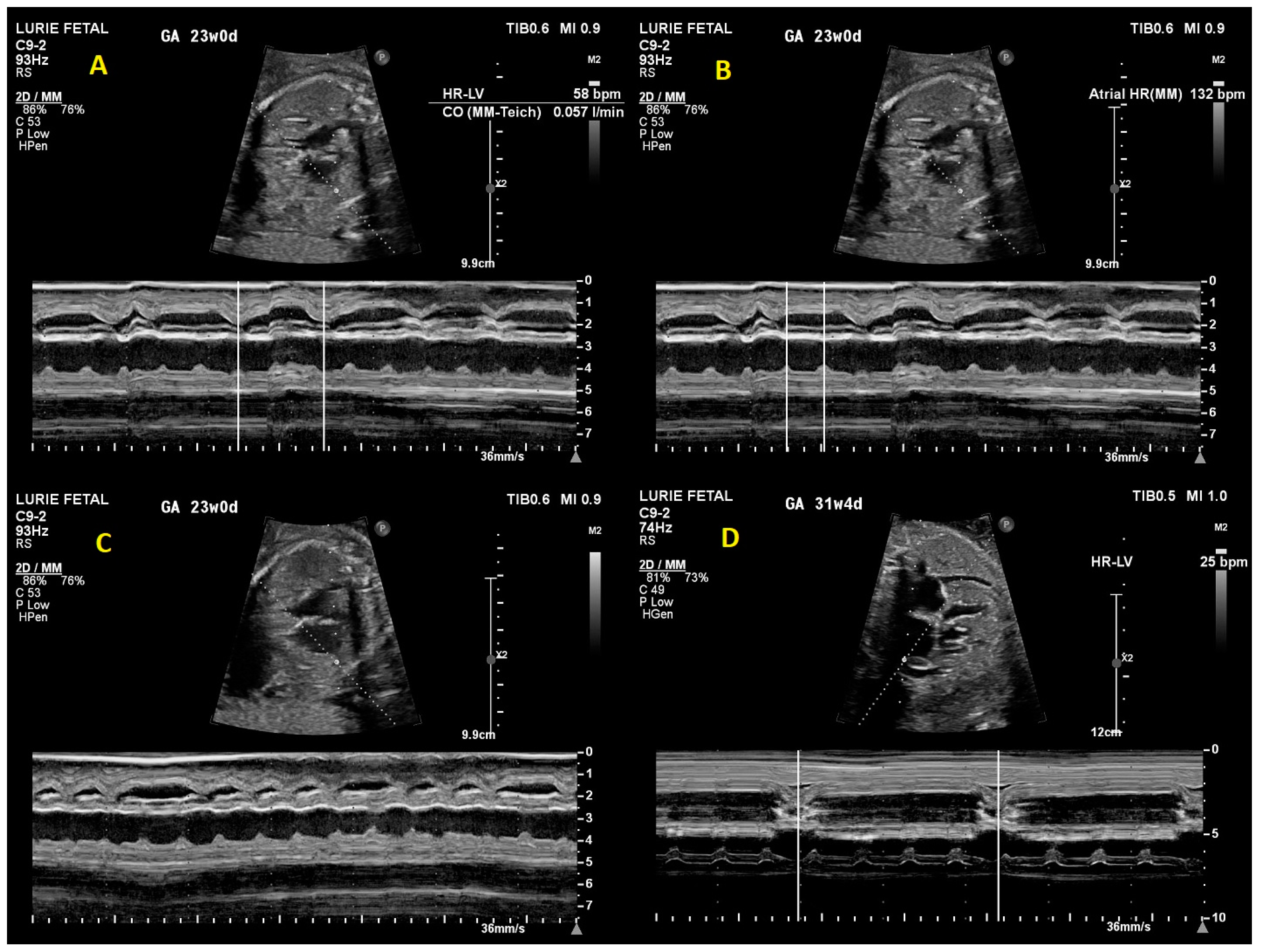
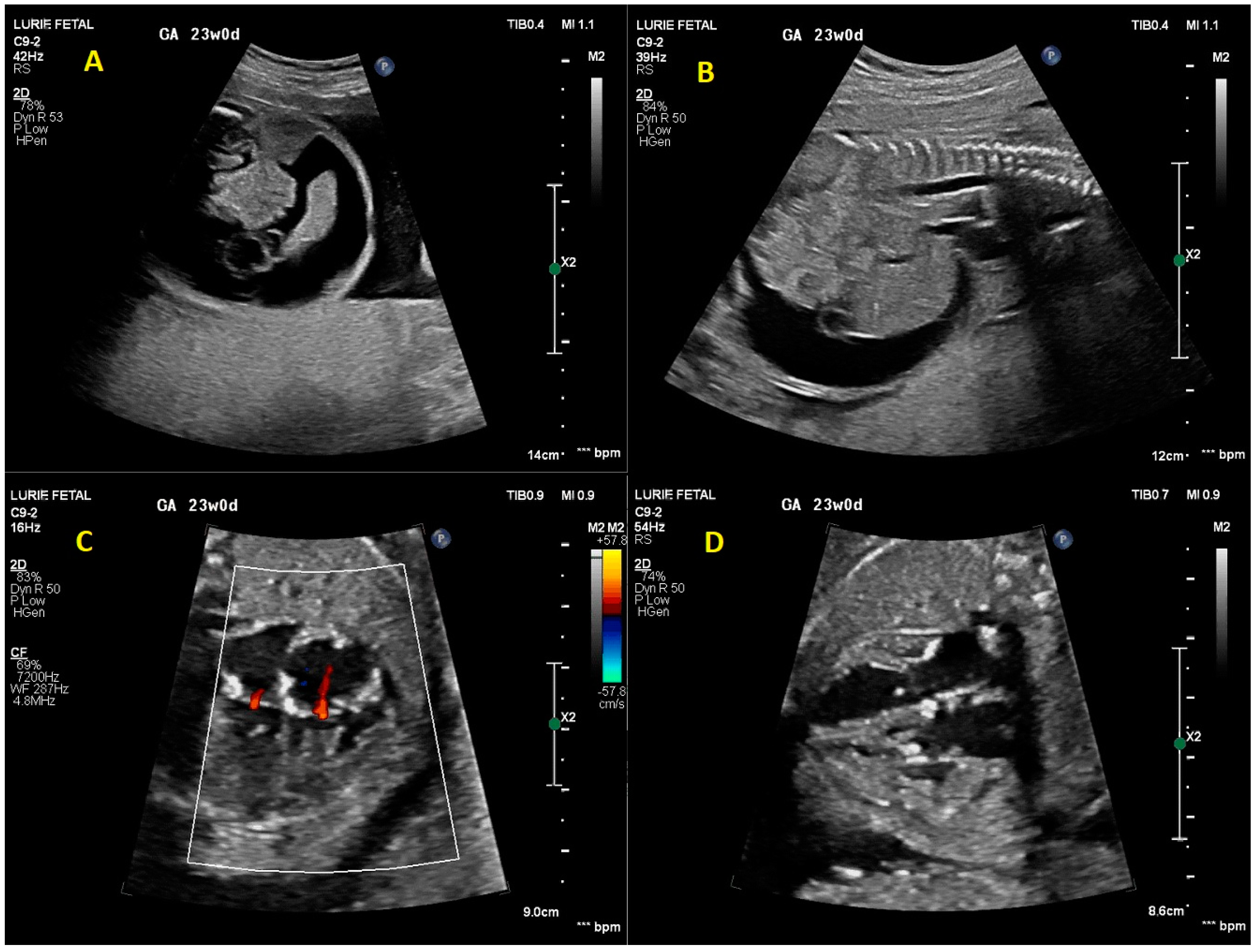
2. Case Presentation
3. Discussion
3.1. Etiology and Expected Outcomes
3.2. Prenatal Cardiac Evaluation
3.3. Prenatal Management
3.4. Delivery Room and Post-Natal Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaëlsson, M.; Engle, M.A. Congenital complete heart block: An international study of the natural history. Cardiovasc. Clin. 1972, 4, 85–101. [Google Scholar] [PubMed]
- Strasburger, J.F. Prenatal Diagnosis of Fetal Arrhythmias. Clin. Perinatol. 2005, 32, 891–912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cuneo, B.F.; Strasburger, J.F.; Huhta, J.C.; Gotteiner, N.L.; Wakai, R.T. Electrophysiological Characteristics of Fetal Atrioventricular Block. J. Am. Coll. Cardiol. 2008, 51, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuneo, B.F.; Zhao, H.; Strasburger, J.F.; Ovadia, M.; Huhta, J.C.; Wakai, R.T. Atrial and ventricular rate response and patterns of heart rate acceleration during maternal–fetal terbutaline treatment of fetal complete heart block. Am. J. Cardiol. 2007, 100, 661–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordachar, P.; Zachary, W.; Ploux, S.; Labrousse, L.; Haissaguerre, M.; Thambo, J.-B. Pathophysiology, clinical course, and management of congenital complete atrioventricular block. Heart Rhythm. 2013, 10, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Matta, M.J.; Cuneo, B.F. Doppler echocardiography for managing fetal cardiac arrhythmia. Clin. Obstet. Gynecol. 2010, 53, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Eronen, M.; Sirèn, M.-K.; Ekblad, H.; Tikanoja, T.; Julkunen, H.; Paavilainen, T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics 2000, 106 Pt 1, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hernstadt, H.; Regan, W.; Bhatt, H.; Rosenthal, E.; Meau-Petit, V. Cohort study of congenital complete heart block among preterm neonates: A single-center experience over a 15-year period. Eur. J. Pediatr. 2021, 181, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Eronen, M.; Heikkilä, P.; Teramo, K. Congenital Complete Heart Block in the Fetus: Hemodynamic Features, Antenatal Treatment, and Outcome in Six Cases. Pediatr. Cardiol. 2001, 22, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Vesel, S.; Završnik, T.; Podnar, T. Successful outcome in a fetus with an extremely low heart rate due to isolated complete congenital heart block. Ultrasound Obstet. Gynecol. 2003, 21, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Pruetz, J.D.; Miller, J.C.; Loeb, G.E.; Silka, M.J.; Bar-Cohen, Y.; Chmait, R.H. Prenatal diagnosis and management of congenital complete heart block. Birth Defects Res. 2019, 111, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Wacker-Gussmann, A.; Cuneo, B.; Wakai, R.; Strasburger, J. Diagnosis and Treatment of Fetal Arrhythmia. Am. J. Perinatol. 2014, 31, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasburger, J.F.; Eckstein, G.; Butler, M.; Noffke, P.; Wacker-Gussmann, A. Fetal Arrhythmia Diagnosis and Pharmacologic Management. J. Clin. Pharmacol. 2022, 62 (Suppl. S1), S53–S66. [Google Scholar] [CrossRef] [PubMed]
- Wacker-Gussmann, A.; Strasburger, J.F.; Wakai, R.T. Contribution of Fetal Magnetocardiography to Diagnosis, Risk Assessment, and Treatment of Fetal Arrhythmia. J. Am. Heart Assoc. 2022, 11, e025224. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.S.; Wagman, A.J.; Cabaniss, M.L. Ascites not due to congestive heart failure in a fetus with lupus-induced heart block. Obstet. Gynecol. 1990, 76 Pt 2, 957–959. [Google Scholar]
- Ciardulli, A.; D’Antonio, F.; Magro-Malosso, E.R.; Manzoli, L.; Anisman, P.; Saccone, G.; Berghella, V. Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: A systematic review and meta-analysis. Acta Obstet. et Gynecol. Scand. 2018, 97, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuneo, B.F.; Ambrose, S.E.; Tworetzky, W. Detection and successful treatment of emergent anti-SSA mediated fetal atrioventricular block. Am. J. Obstet. Gynecol. 2016, 215, 527–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donofrio, M.T. Predicting the Future: Delivery Room Planning of Congenital Heart Disease Diagnosed by Fetal Echocardiography. Am. J. Perinatol. 2018, 35, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, J.; Zhou, K.; Wang, C.; Hua, Y.; Shi, X.; Mu, D. Prediction of fetal outcome without intrauterine intervention using a cardiovascular profile score: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2015, 28, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, M.T.; Gullquist, S.D.; Mehta, I.D.; Moskowitz, W.B.; Faap; Facc; Moskowitz, F.W.B. Congenital complete heart block: Fetal management protocol, review of the literature, and report of the smallest successful pacemaker implantation. J. Perinatol. 2004, 24, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samples, S.; Fitt, C.; Satzer, M.; Wakai, R.; Strasburger, J.; Patel, S. Fetal Congenital Complete Heart Block: A Rare Case with an Extremely Low Ventricular Rate and Review of Current Management Strategies. Children 2023, 10, 1132. https://doi.org/10.3390/children10071132
Samples S, Fitt C, Satzer M, Wakai R, Strasburger J, Patel S. Fetal Congenital Complete Heart Block: A Rare Case with an Extremely Low Ventricular Rate and Review of Current Management Strategies. Children. 2023; 10(7):1132. https://doi.org/10.3390/children10071132
Chicago/Turabian StyleSamples, Stefani, Catherine Fitt, Michael Satzer, Ronald Wakai, Janette Strasburger, and Sheetal Patel. 2023. "Fetal Congenital Complete Heart Block: A Rare Case with an Extremely Low Ventricular Rate and Review of Current Management Strategies" Children 10, no. 7: 1132. https://doi.org/10.3390/children10071132
APA StyleSamples, S., Fitt, C., Satzer, M., Wakai, R., Strasburger, J., & Patel, S. (2023). Fetal Congenital Complete Heart Block: A Rare Case with an Extremely Low Ventricular Rate and Review of Current Management Strategies. Children, 10(7), 1132. https://doi.org/10.3390/children10071132





