The Audiological Follow-Up of Children with Symptomatic Congenital Cytomegalovirus Infection: An Experience in Two Italian Centers
Abstract
:1. Introduction
2. Materials and Methods
2.1. CMV Testing
2.2. NHS Program
2.3. Audiological Evaluation and Follow-Up
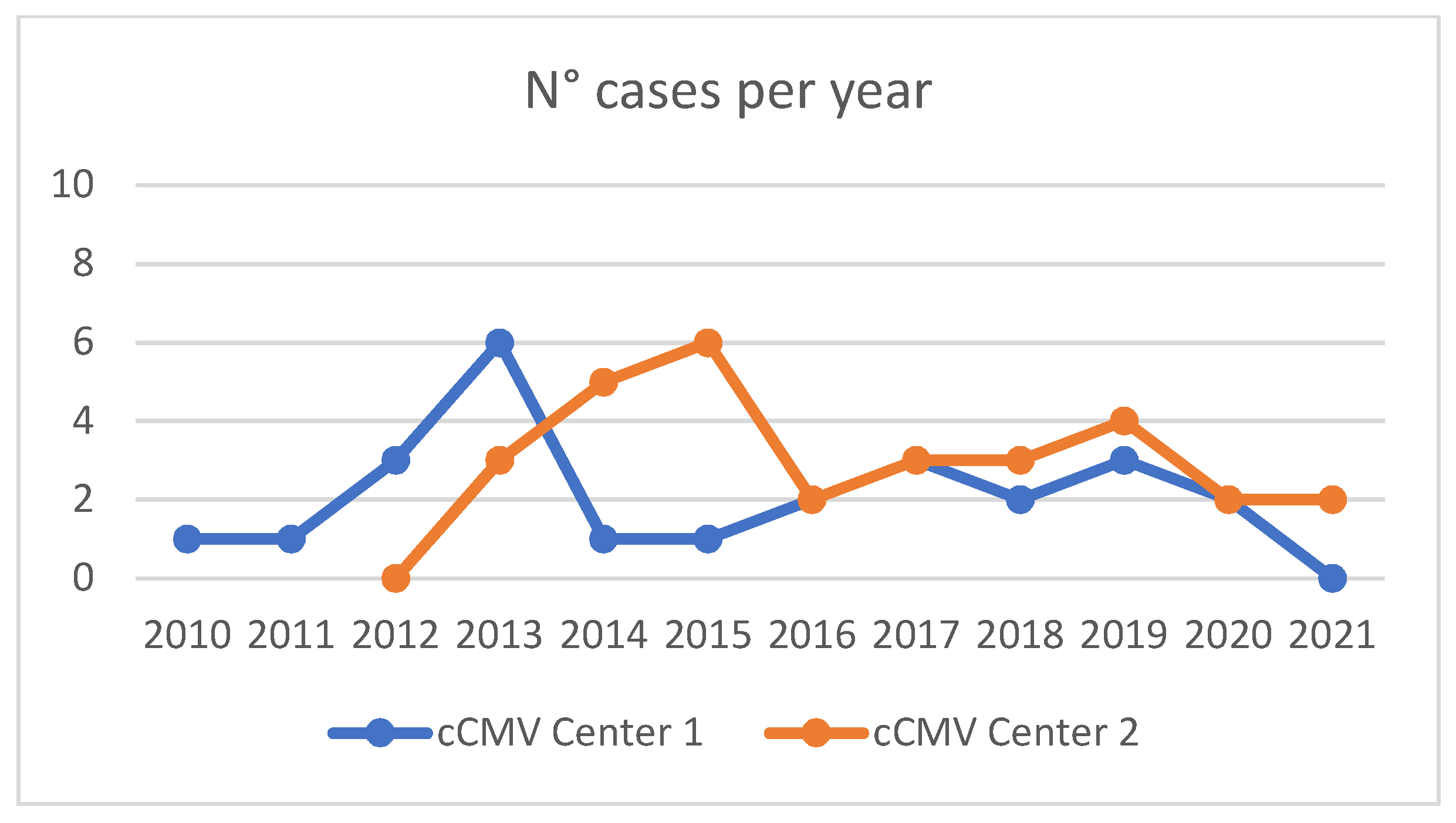
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanzieri, T.M.; Dollard, S.C.; Bialek, S.R.; Grosse, S.D. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int. J. Infect. Dis. 2014, 22, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- De Cuyper, E.; Acke, F.; Keymeulen, A.; De Leenheer, E.M.R.; Van Hoecke, H.; Padalko, E.; Boudewyns, A.; Gilles, A.; Muylle, M.; Kuhweide, R.; et al. Risk Factors for Hearing Loss at Birth in Newborns with Congenital Cytomegalovirus Infection. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Pass, R.F.; Britt, W.J.; Stagno, S.; Alford, C.A. Symptomatic congenital cytomegalovirus infection: Neonatal morbidity and mortality. Pediatr. Infect. Dis. J. 1992, 11, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.W.; McMullan, B.; Rawlinson, W.D.; Palasanthiran, P. Hearing and neurodevelopmental outcomes for children with asymptomatic congenital cytomegalovirus infection: A systematic review. Rev. Med. Virol. 2017, 27, e1938. [Google Scholar] [CrossRef]
- Marsico, C.; Kimberlin, D.W. Congenital Cytomegalovirus infection: Advances and challenges in diagnosis, prevention and treatment. Ital. J. Pediatr. 2017, 43, 38. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, K.T.; Horrell, E.M.W.; Ayugi, J.; Irungu, C.; Muthoka, M.; Creel, L.M.; Lester, C.; Bush, M.L. The natural history and rehabilitative outcomes of hearing loss in congenital cytomegalovirus: A systematic review. Otol. Neurotol. 2018, 39, 854–864. [Google Scholar] [CrossRef]
- Cushing, S.L.; Purcell, P.L.; Papaiaonnou, V.; Neghandi, J.; Daien, M.; Blaser, S.I.; Ertl-Wagner, B.; Wagner, M.; Sheng, M.; James, A.L.; et al. Hearing Instability in Children with Congenital Cytomegalovirus: Evidence and Neural Consequences. Laryngoscope 2022, 132 (Suppl. S11), S1–S24. [Google Scholar] [CrossRef]
- Tomblin, J.B.; Oleson, J.J.; Ambrose, S.E.; Walker, E.; Moeller, M.P. The influence of hearing aids on the speech and language development of children with hearing loss. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 403–409. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, K.; Nielson, C.; Graham, E.M.; Price, M.S.; Haller, T.J.; Carraro, M.; Firpo, M.A.; Park, A.H.; Harrison, R.V. Hearing loss caused by CMV infection is correlated with reduced endocochlear potentials caused by strial damage in murine models. Hear. Res. 2022, 417, 108454. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Grosse, S.D.; Miller, J.A.; Demmler-Harrison, G. Congenital Cytomegalovirus Longitudinal Study Group. Hearing Loss in Children with Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 2017, 139, e20162610. [Google Scholar] [CrossRef] [Green Version]
- Gugliesi, F.; Coscia, A.; Griffante, G.; Galitska, G.; Pasquero, S.; Albano, C.; Biolatti, M. Where do we stand after decades of studying human cytomegalovirus? Microorganisms 2020, 8, 685. [Google Scholar] [CrossRef]
- Linee Guida SIN. Available online: https://www.neonatologia.it/upload/725_CITOMEGALOVIRUS_Aprile_2012.pdf (accessed on 20 March 2023).
- Forli, F.; Lazzerini, F.; Canelli, R.; Lorenzoni, F.; Franciosi, B.; Berrettini, S.; Bruschini, L. Extended-hearing targeted screening for congenital cytomegalovirus infection. Minerva Pediatr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bianchin, G.; Palma, S.; Polizzi, V.; Kaleci, S.; Stagi, P.; Cappai, M.; Baiocchi, M.P.; Benincasa, P.; Brandolini, C.; Casadio, L.; et al. A regional-based newborn hearing screening program: The Emilia-Romagna model after ten years of legislation. Ann. Ig. 2023, 35, 297–307. [Google Scholar]
- Berrettini, S.; Ghirri, P.; Lazzerini, F.; Lenzi, G.; Forli, F. Newborn hearing screening protocol in tuscany region. Ital. J. Pediatr. 2017, 43, 82. [Google Scholar] [CrossRef] [Green Version]
- The Joint Committee on Infant Hearing. Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. J. Early Hear. Detect. Interv. 2019, 4, 1–44. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Caviness, A.C.; Blum, P.; Demmler-Harrison, G.; Ahmed, S.; Baer, H.; Bhatt, A.R.; Brown, F.; Catlin, F.; Coats, D.K.; et al. Progressive, Long-Term Hearing Loss in Congenital CMV Disease after Ganciclovir Therapy. J. Pediatr. Infect. Dis. Soc. 2022, 11, 16–23. [Google Scholar] [CrossRef]
- WHO. Classification of Hearing Impairment. 2013. Available online: http://www.who.int/pbd/deafness/hearing_impairment_grades/ (accessed on 20 May 2023).
- Liao, E.N.; Stephans, J.; Taketa, E.; Mohamad, N.I.; Khalsa, I.K.; Naugle, K.; Chan, D.K. Factors associated with congenital cytomegalovirus infection detected by dried blood spot testing in children with hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2023, 165, 111446. [Google Scholar] [CrossRef]
- Pesch, M.H.; Schleiss, M.R. Emerging Concepts in Congenital Cytomegalovirus. Pediatrics 2022, 150, e2021055896. [Google Scholar] [CrossRef] [PubMed]
- Tapasak, B.; Cronkite, D.A.; Hustedt-Mai, A.R.; Morlet, T.M.; Parkes, W.J.; Maul, T.M.; Pritchett, C.V. Hearing outcomes in children with Congenital Cytomegalovirus: A multi-center, single-enterprise experience. Int. J. Pediatr. Otorhinolaryngol. 2022, 163, 111376. [Google Scholar] [CrossRef] [PubMed]
- Kettler, M.; Shoup, A.; Moats, S.; Steuerwald, W.; Jones, S.; Stiell, S.C.; Chappetto, J. American Academy of Audiology Position Statement on Early Identification of Cytomegalovirus in Newborns. J. Am. Acad. Audiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Palma, S.; Roversi, M.F.; Bettini, M.; Mazzoni, S.; Pietrosemoli, P.; Lucaccioni, L.; Berardi, A.; Genovese, E. Hearing loss in children with congenital cytomegalovirus infection: An 11-year retrospective study based on laboratory database of a tertiary paediatric hospital. Acta Otorhinolaryngol. Ital. 2019, 39, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: A systematic review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.M.; Rana, P.S.J.B.; Jaeger, H.K.; O’dowd, J.M.; Balemba, O.B.; Fortunato, E.A. Human Cytomegalovirus Compromises Development of Cerebral Organoids. J. Virol. 2019, 93, e00957-19. [Google Scholar] [CrossRef] [Green Version]
- Ciuman, R.R. Communication routes between intracranial spaces and inner ear: Function, pathophysiologic importance and relations with inner ear diseases. Am. J. Otolaryngol. 2009, 30, 193–202. [Google Scholar] [CrossRef]
- Pinninti, S.G.; Rodgers, M.D.; Novak, Z.; Britt, W.J.; Fowler, K.B.; Boppana, S.B.; Ross, S.A. Clinical Predictors of Sensorineural Hearing Loss and Cognitive Outcome in Infants with Symptomatic Congenital Cytomegalovirus Infection. Pediatr. Infect. Dis. J. 2016, 35, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Craeghs, L.; Goderis, J.; Acke, F.; Keymeulen, A.; Smets, K.; Van Hoecke, H.; De Leenheer, E.; Boudewyns, A.; Desloovere, C.; Kuhweide, R.; et al. Congenital CMV-Associated Hearing Loss: Can Brain Imaging Predict Hearing Outcome? Ear. Hear. 2021, 42, 373–380. [Google Scholar] [CrossRef]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing Loss in Children: A Review. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef]
- Chant, K.; BitnerGlindzicz, M.; Marlow, N. Cumulative risk factors contributing to hearing loss in preterm infants. Arch. Dis. Child Fetal. Neonatal. Ed. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Novelli, M.; Natale, F.; Di Norcia, A.; Boiani, A.; Temofonte, S.; Calandriello, F.; Zitarelli, C.; Caravale, B. Early neurodevelopmental outcomes in children with asymptomatic congenital CMV infection. Ital. J. Pediatr. 2022, 48, 203. [Google Scholar] [CrossRef]
- Chiopris, G.; Veronese, P.; Cusenza, F.; Procaccianti, M.; Perrone, S.; Daccò, V.; Colombo, C.; Esposito, S. Congenital Cytomegalovirus Infection: Update on Diagnosis and Treatment. Microorganisms 2020, 8, 1516. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- De Cuyper, E.; Acke, F.; Keymeulen, A.; Dhooge, I. The Effect of (Val)ganciclovir on Hearing in Congenital Cytomegalovirus: A Systematic Review. Laryngoscope 2022, 132, 2241–2250. [Google Scholar] [CrossRef]
- Wills, M.R.; Poole, E.; Lau, B.; Krishna, B.; Sinclair, J.H. The immunology of human cytomegalovirus latency: Could latent infection be cleared by novel immunotherapeutic strategies? Cell. Mol. Immunol. 2015, 12, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Renzette, N.; Gibson, L.; Jensen, J.D.; Kowalik, T.F. Human cytomegalovirus intrahost evolution-a new avenue for understanding and controlling herpesvirus infections. Curr. Opin. Virol. 2014, 8, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Landolfo, S.; Gariglio, M.; Gribaudo, G.; Lembo, D. The human cytomegalovirus. Pharmacol. Ther. 2003, 98, 269–297. [Google Scholar] [CrossRef]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay with the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef]
- Griffiths, P. The direct and indirect consequences of cytomegalovirus infection and potential benefits of vaccination. Antivir. Res. 2020, 176, 104732. [Google Scholar] [CrossRef]
- Puhakka, L.; Lappalainen, M.; Lönnqvist, T.; Nieminen, T.; Boppana, S.; Saxen, H.; Niemensivu, R. Hearing outcome in congenitally CMV infected children in Finland—Results from follow-up after three years age. Int. J. Pediatr. Otorhinolaryngol. 2022, 156, 111099. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, E.; Niemensivu, R.; Lappalainen, M.; Palomäki, M.; Nieminen, T.; Boppana, S.; Saxèn, H.; Puhakka, L. Long-term outcome of vestibular function and hearing in children with congenital cytomegalovirus infection: A prospective cohort study. Eur. Arch. Otorhinolaryngol. 2023, 280, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Aldè, M.; Caputo, E.; Di Berardino, F.; Ambrosetti, U.; Barozzi, S.; Piatti, G.; Zanetti, D.; Pignataro, L.; Cantarella, G. Hearing outcomes in children with congenital cytomegalovirus infection: From management controversies to lack of parents’ knowledge. Int. J. Pediatr. Otorhinolaryngol. 2023, 164, 111420. [Google Scholar] [CrossRef] [PubMed]
| Clinical Features | Delivered in Center 1 Hospital N = 23 | Delivered Outside Center 1 n = 39 | Delivered in Center 2 Hospital N = 30 | Delivered Outside Center 2 n = 11 | Total n = 103 |
|---|---|---|---|---|---|
| Gestational age at birth | |||||
| Premature (<36th week) | 12 (52%) | 11 (28%) | 20 (66%) | 3 (27%) | 46 (45%) |
| Birth weight | |||||
| SGA (<3° percentile) | 3 | - | 3 | 2 | 8 (8%) |
| Hearing Screening | |||||
| Pass bilaterally | 17 (74%) | 19 (48.7%) | 25 (83%) | 6 (54%) | 67 (65%) |
| Refer bilaterally | 2 (8.7%) | 16 (41%) | 4 (13%) | 4 (36%) | 26 (25%) |
| Refer unilateral | 4 (17.3%) | 4 (10%) | 1 (3%) | 1 (3%) | 10 (10%) |
| Familiarity for hearing loss/syndromes | - | 3(8%) | 2 (18%) | 5 (5%) | |
| Symptomatic * | 6 (31%) | 21 (53.8%) | 14 (46%) | 4(36%) | 45 (43%) |
| Antiviral Therapy | 2 (8.6%) | 10 (25.6%) | 13 (43.3%) | 4 (36.3%) | 29 (28%) |
| Tot 28 (27%) | Outside Center 2 n = 4/11 (36%) | Center 2 Hospital n = 7/30 (23.3%) | Outside Center 1 n = 13/39 (33.3%) | Center 1 Hospital n = 4/23 (17.3%) | N. Subjects with Hearing Impairment |
| N. Subjects with Hearing Impairment | Center 1 n = 17 | Center 2 n = 11 | Tot 28/103 |
|---|---|---|---|
| Bilateral Hearing Impairment | 10 | 8 | 18/28 = 64.3% |
| Mild | |||
| Moderate | 2 | 3 | 5 |
| Severe/Profound | 8 | 5 | 13 |
| Unilateral Hearing Impairment | 7 | 3 | 10/28 = 35.7% |
| Mild | 1 | 1 | |
| Moderate | 1 | 1 | 2 |
| Severe/Profound | 5 | 2 | 7 |
| Hearing Loss | Subjects | Degree | Timing (Years) * | |||||
|---|---|---|---|---|---|---|---|---|
| N° | Mild | Moderate | Severe-Deep | <1 Year | <2 Years | <3 Years | 3–6 Years | |
| Progressive | 6 | - | ||||||
| Bilateral HL | 2 | - | 1 | 1 | 1 | 1 | ||
| Unilateral HL | 4 | - | - | 4 | 1 | 1 | 1 | 1 |
| Late onset | 4 | - | ||||||
| Bilateral HL | 1 | - | 1 | - | - | - | - | 1 |
| Unilateral HL | 3 | 1 | 2 | - | 2 | - | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, S.; Forli, F.; Rossi, C.; Filice, R.; D’adamo, C.; Roversi, M.F.; Monzani, D.; Lorenzoni, F.; Botti, C.; Berrettini, S.; et al. The Audiological Follow-Up of Children with Symptomatic Congenital Cytomegalovirus Infection: An Experience in Two Italian Centers. Children 2023, 10, 1136. https://doi.org/10.3390/children10071136
Palma S, Forli F, Rossi C, Filice R, D’adamo C, Roversi MF, Monzani D, Lorenzoni F, Botti C, Berrettini S, et al. The Audiological Follow-Up of Children with Symptomatic Congenital Cytomegalovirus Infection: An Experience in Two Italian Centers. Children. 2023; 10(7):1136. https://doi.org/10.3390/children10071136
Chicago/Turabian StylePalma, Silvia, Francesca Forli, Cecilia Rossi, Riccardo Filice, Concetta D’adamo, Maria Federica Roversi, Daniele Monzani, Francesca Lorenzoni, Cecilia Botti, Stefano Berrettini, and et al. 2023. "The Audiological Follow-Up of Children with Symptomatic Congenital Cytomegalovirus Infection: An Experience in Two Italian Centers" Children 10, no. 7: 1136. https://doi.org/10.3390/children10071136





