Behavioral and Sleep Disorders in Children and Adolescents following COVID-19 Disease: A Case-Control Study
Abstract
:1. Introduction
Neuropsychiatric Aspects of Long COVID in Children: Underlying Mechanisms
2. Materials and Methods
2.1. Assessment Tools
2.1.1. CBCL: Child Behavior Checklist
- -
- Score 0: the item is not true;
- -
- Score 1: the item is somewhat or sometimes true;
- -
- Score 2: the item is very true or often true.
- The main scales:
- -
- Internalizing;
- -
- Externalizing;
- -
- Total problems.
- The syndromic scales:
- -
- Emotionally Reactive;
- -
- Anxious/Depressed;
- -
- Somatic Complaints;
- -
- Withdrawn/Depressed;
- -
- Attention Problems;
- -
- Aggressive Behavior;
- -
- Sleep Problems.
- DSM-oriented scales:
- -
- Affective Problems;
- -
- Anxiety Problems;
- -
- Pervasive Developmental Problems;
- -
- Attention Deficit/Hyperactivity Problems;
- -
- Stress Problems;
- -
- Autism Spectrum Problems;
- -
- Oppositional Defiant Problems.
- N: normal range;
- N–B: borderline clinical range;
- N–C: clinical range.
- 0 = “absent”;
- 1 = “occurs sometimes”;
- 2 = “occurs often”.
| The 2001 revised version of the CBCL is structured around 8 syndromic scales: |
|
|
|
|
|
|
|
|
| Syndromes are further combined into: |
|
|
|
| The 2021 revision of CBCL has a scale showing scores associated with disorders from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) |
|
|
|
|
|
|
- N: normal range;
- N–B: borderline clinical range;
- N–C: clinical range.
2.1.2. SDSC: Sleep Disturbance Scale for Children
- DIMS: disorders of initiating and maintaining sleep (sum the score of the items 1, 2, 3, 4, 5, 10, 11). DIMS is clinically significant if the score is superior to 17.
- SBD: sleep breathing disorders (sum the score of the items 13, 14, 15). SBD is clinically significant if the score is superior to 7.
- DA: disorders of arousal (sum the score of the items 17, 20, 21). DA is clinically significant if the score is superior to 6.
- SWDT: sleep–wake transition disorders (sum the score of the items 6, 7, 8, 12, 18, 19). SWDT is clinically significant if the score is superior to 14.
- DOES: disorders of excessive somnolence (sum the score of the items 22, 23, 24, 25, 26). DOES is clinically significant if the score is superior to 13.
- SHY: sleep hyperhidrosis (sum the score of the items 9, 16). SHY is clinically significant if the score is superior to 7.
- Total score: clinically significant if the score is superior to 71.
2.2. Statistical Analysis
3. Results
3.1. Results of the Whole COVID-19 Group
3.1.1. 1.5–5-Year-Old Sample
3.1.2. 6–18-Year-Old Sample
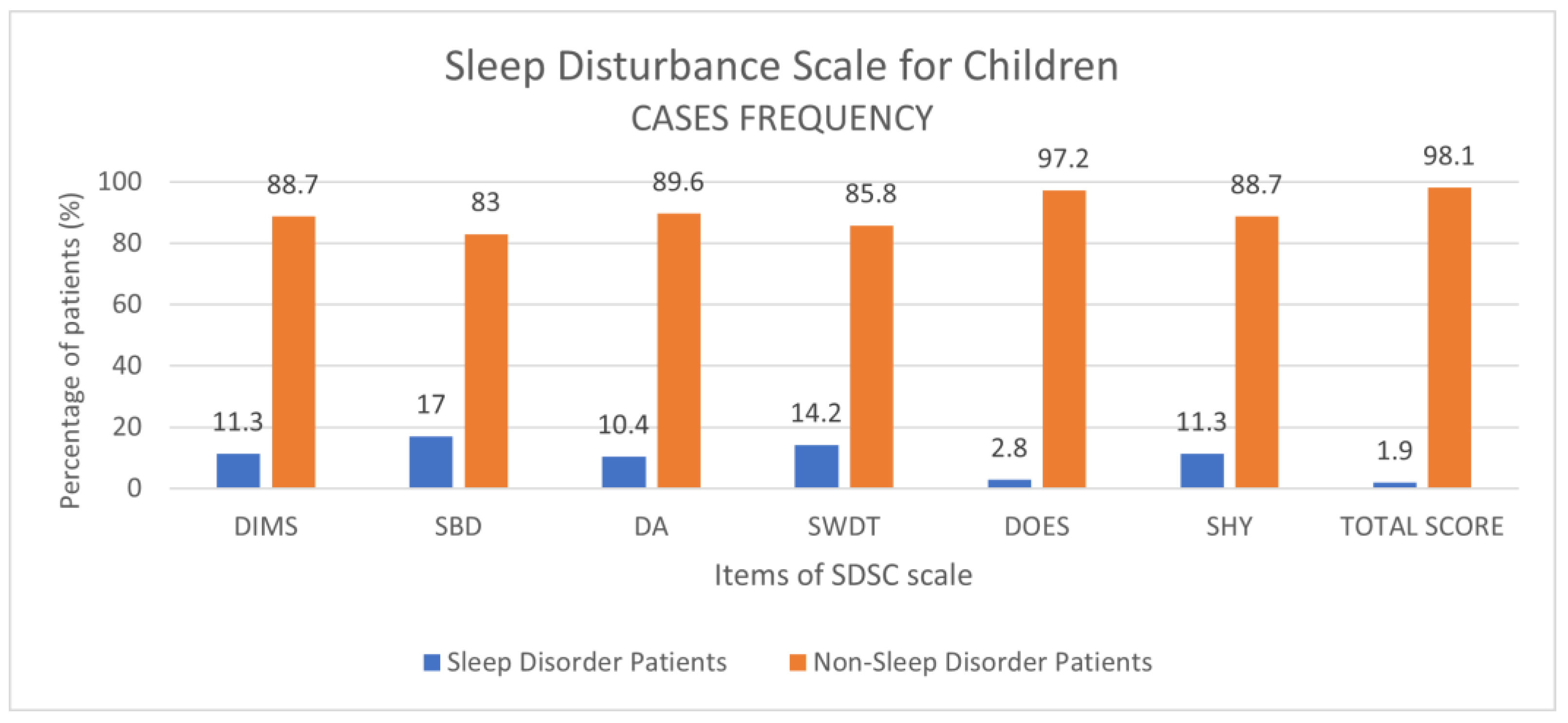
3.2. Control Group: Prevalence of Disorders and Comparison to COVID-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chippa, V.; Aleem, A.; Anjum, F. Post-Acute Coronavirus (COVID-19) Syndrome. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570608/ (accessed on 3 February 2023).
- Miraglia del Giudice, M.; Indolfi, C.; Dinardo, G.; Decimo, F.; Decimo, A.; Klain, A. Vitamin D status can affect COVID-19 outcomes also in pediatric population. PharmaNutrition 2022, 22, 100319. [Google Scholar] [CrossRef]
- Mann, J.A.; Bird, P.W.; Bandi, S.; Tang, J.W. Asymptomatic SARS-CoV-2-infected children attending hospital with non-COVID-19 diagnoses, March 2020–February 2021. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J.; Zhang, J.; Dong, C.; Na, R.; Zheng, L.; et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J. Med. Virol. 2021, 93, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.B.; Fajardo, T.C.G.; De Oliveira, L.B.; De Quadros Junior, A.C.; Catalan, D.T.; Piovesan, K.C.; Garcia, M.E.D.D.; Da Silva, M.F.; Dezena, R.D.C.D.A.B.; Passos, S.D. Mild and Asymptomatic Coronavirus Disease in Children, Adolescents, and Household Contacts and Prolonged Viral Excretion. Int. J. Microbiol. 2022, 2022, 5625104. [Google Scholar] [CrossRef]
- Parisi, G.F.; Indolfi, C.; Decimo, F.; Leonardi, S.; Miraglia del Giudice, M. COVID-19 Pneumonia in Children: From Etiology to Management. Front. Pediatr. 2020, 8, 616622. [Google Scholar] [CrossRef] [PubMed]
- Howard-Jones, A.R.; Burgner, D.P.; Crawford, N.W.; Goeman, E.; Gray, P.E.; Hsu, P.; Kuek, S.; McMullan, B.J.; Tosif, S.; Wurzel, D.; et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J. Paediatr. Child Health 2022, 58, 46–53. [Google Scholar] [CrossRef]
- Parisi, G.F.; Brindisi, G.; Indolfi, C.; Diaferio, L.; Marchese, G.; Ghiglioni, D.G.; Zicari, A.M.; Miraglia del Giudice, M. Upper airway involvement in pediatric COVID-19. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S26), 85–88. [Google Scholar] [CrossRef]
- Piazza, M.; Di Cicco, M.; Pecoraro, L.; Ghezzi, M.; Peroni, D.; Comberiati, P. Long COVID-19 in Children: From the Pathogenesis to the Biologically Plausible Roots of the Syndrome. Biomolecules 2022, 12, 556. [Google Scholar] [CrossRef]
- Garazzino, S.; Denina, M.; Pruccoli, G.; Funiciello, E.; Ramenghi, U.; Fagioli, F. Long COVID-19/post-COVID condition in children: Do we all speak the same language? Ital. J. Pediatr. 2023, 49, 556. [Google Scholar] [CrossRef]
- Post COVID-19 condition (Long COVID). [Internet]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 1 June 2023).
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet. Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of covid-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Evaluating and Caring for Patients with Post-COVID Conditions: Interim Guidance: Patient History and Physical Exam [Internet]. 2021. Available online: https://stacks.cdc.gov/view/cdc/107148 (accessed on 1 June 2023).
- RKI—Selected Outbreaks—RKI Information Portal on Long COVID [Internet]. Available online: https://www.rki.de/EN/Content/infections/epidemiology/outbreaks/COVID-19/Long-COVID/content-total.html (accessed on 1 June 2023).
- Parisi, G.F.; Diaferio, L.; Brindisi, G.; Indolfi, C.; Umano, G.R.; Klain, A.; Marchese, G.; Ghiglioni, D.G.; Zicari, A.M.; Marseglia, G.L.; et al. Cross-Sectional Survey on Long Term Sequelae of Pediatric COVID-19 among Italian Pediatricians. Children 2021, 8, 769. [Google Scholar] [CrossRef] [PubMed]
- Umano, G.R.; Rondinelli, G.; Rivetti, G.; Klain, A.; Aiello, F.; Miraglia del Giudice, M.; Decimo, F.; Papparella, A.; Miraglia del Giudice, E. Effect of COVID-19 Lockdown on Children’s Eating Behaviours: A Longitudinal Study. Children 2022, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Savino, R.; Polito, A.N.; Arcidiacono, G.; Poliseno, M.; Lo Caputo, S. Neuropsychiatric Disorders in Pediatric Long COVID-19: A Case Series. Brain Sci. 2022, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Ashkenazi-Hoffnung, L.; Greenberg, D.; Dalal, I.; Livni, G.; Chapnick, G.; Stein-Zamir, C.; Ashkenazi, S.; Hecht-Sagie, L.; Grossman, Z. The Burden of COVID-19 in Children and Its Prevention by Vaccination: A Joint Statement of the Israeli Pediatric Association and the Israeli Society for Pediatric Infectious Diseases. Vaccines 2022, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide [Internet]. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 1 June 2023).
- Bera, L.; Souchon, M.; Ladsous, A.; Colin, V.; Lopez-Castroman, J. Emotional and Behavioral Impact of the COVID-19 Epidemic in Adolescents. Curr. Psychiatry Rep. 2022, 24, 37–46. [Google Scholar] [CrossRef]
- de Oliveira, J.M.D.; Butini, L.; Pauletto, P.; Lehmkuhl, K.M.; Stefani, C.M.; Bolan, M.; Guerra, E.; Dick, B.; De Luca Canto, G.; Massignan, C. Mental health effects prevalence in children and adolescents during the COVID-19 pandemic: A systematic review. Worldviews Evid.-Based Nurs. 2022, 19, 130–137. [Google Scholar] [CrossRef]
- Guessoum, S.B.; Lachal, J.; Radjack, R.; Carretier, E.; Minassian, S.; Benoit, L.; Moro, M.R. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatry Res. 2020, 291, 113264. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Thurman, M.; Johnson, S.D.; Acharya, A.; Johnston, M.; Klug, E.A.; Olwenyi, O.A.; Rajaiah, R.; Byrareddy, S.N. Mental Health Issues During and After COVID-19 Vaccine Era. Brain Res. Bull. 2021, 176, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Hassinger, A.B.; Monegro, A.; Perez, G. Parental survey of the sleep patterns and screen time in US school children during the first 6 months of the COVID-19 pandemic. BMC Pediatr. 2023, 23, 65. [Google Scholar] [CrossRef]
- Diaferio, L.; Parisi, G.F.; Brindisi, G.; Indolfi, C.; Marchese, G.; Ghiglioni, D.G.; Zicari, A.M.; Marseglia, G.L.; Miraglia Del Giudice, M. Cross-sectional survey on impact of paediatric COVID-19 among Italian paediatricians: Report from the SIAIP rhino-sinusitis and conjunctivitis committee. Ital. J. Pediatr. 2020, 46, 146. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.T.C.; Ramamurthy, M.B.; Aishworiya, R.; Rajgor, D.D.; Tran, A.P.; Hiriyur, P.; Kunaseelan, S.; Jabri, M.; Goh, D.Y.T. School closure during the coronavirus disease 2019 (COVID-19) pandemic—Impact on children’s sleep. Sleep Med. 2021, 78, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Aggarwal, S.; Madaan, P.; Saini, L.; Bhutani, M. Impact of COVID-19 pandemic on sleep in children and adolescents: A systematic review and meta-analysis. Sleep Med. 2021, 84, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Iwasaki, A. The neurobiology of long COVID. Neuron 2022, 110, 3484. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Balabandian, M.; Sodeifian, F.; Rezaei, N.; Rostami, M.R.; Naser Moghadasi, A. Autoimmune encephalitis associated with COVID-19: A systematic review. Mult. Scler. Relat. Disord. 2022, 62, 103795. [Google Scholar] [CrossRef]
- Simonnet, A.; Engelmann, I.; Moreau, A.S.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now 2021, 51, 296–299. [Google Scholar] [CrossRef]
- Shafiee, A.; Teymouri Athar, M.M.; Amini, M.J.; Hajishah, H.; Siahvoshi, S.; Jalali, M.; Jahanbakhshi, B.; Mozhgani, S. Reactivation of herpesviruses during COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2437. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Siokas, V.; Aloizou, A.M.; Liampas, I.; Mentis, A.F.A.; Tsouris, Z.; Papadimitriou, A.; Mitsias, P.D.; Tsatsakis, A.; Bogdanos, D.P.; et al. Unraveling the Possible Routes of SARS-COV-2 Invasion into the Central Nervous System. Curr. Treat. Options Neurol. 2020, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Lampe, J.; Müller-Fielitz, H.; Schuster, R.; Zille, M.; Müller, K.; Krohn, M.; Körbelin, J.; Zhang, L.; Özorhan, Ü.; et al. The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat. Neurosci. 2021, 24, 1522–1533. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2022, 59, 2101341. [Google Scholar] [CrossRef]
- So, P.; Wierdsma, A.I.; Mulder, C.L.; Vermeiren, R.R.J.M. The impact of the COVID-19 pandemic on psychiatric emergency consultations in adolescents. BMC Psychol. 2023, 11, 101. [Google Scholar] [CrossRef]
- Parisi, G.F.; Brindisi, G.; Indolfi, C.; Diaferio, L.; Marchese, G.; Ghiglioni, D.G.; Zicari, A.M.; Miraglia Del Giudice, M. COVID-19, anosmia, and ageusia in atopic children. Pediatr. Allergy Immunol. 2022, 33 (Suppl. S27), 99–101. [Google Scholar] [CrossRef]
- Guido, C.A.; Lucidi, F.; Midulla, F.; Zicari, A.M.; Bove, E.; Avenoso, F.; Amedeo, I.; Mancino, E.; Nenna, R.; De Castro, G.; et al. Neurological and psychological effects of long COVID in a young population: A cross-sectional study. Front. Neurol. 2022, 13, 925144. [Google Scholar] [CrossRef]
- Jarvers, I.; Ecker, A.; Schleicher, D.; Brunner, R.; Kandsperger, S. Impact of preschool attendance, parental stress, and parental mental health on internalizing and externalizing problems during COVID-19 lockdown measures in preschool children. PLoS ONE 2023, 18, e0281627. [Google Scholar] [CrossRef]
- Schmidt, S.J.; Barblan, L.P.; Lory, I.; Landolt, M.A. Age-related effects of the COVID-19 pandemic on mental health of children and adolescents. Eur. J. Psychotraumatol. 2021, 12, 1901407. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Kikkenborg Berg, S.; Dam Nielsen, S.; Nygaard, U.; Bundgaard, H.; Palm, P.; Rotvig, C.; Vinggaard Christensen, A. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): A national, cross-sectional study. Lancet. Child Adolesc. Health 2022, 6, 240–248. [Google Scholar] [CrossRef]
- Morrow, A.K.; Malone, L.A.; Kokorelis, C.; Petracek, L.S.; Eastin, E.F.; Lobner, K.L.; Neuendorff, L.; Rowe, P.C. Long-Term COVID 19 Sequelae in Adolescents: The Overlap with Orthostatic Intolerance and ME/CFS. Curr. Pediatr. Rep. 2022, 10, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Ohler, A.; Gileles-Hillel, A.; Choshen-Hillel, S.; Gozal, D.; Bruni, O.; Aydinoz, S.; Cortese, R.; Kheirandish-Gozal, L. Effects of the COVID-19 lockdown on sleep duration in children and adolescents: A survey across different continents. Pediatr. Pulmonol. 2021, 56, 2265–2273. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, H.; Jin, Q.; Wang, G.; Yang, Z.; Chen, H.; Yan, H.; Rao, W.; Owens, J. Sleep of preschoolers during the coronavirus disease 2019 (COVID-19) outbreak. J. Sleep Res. 2021, 30, e13142. [Google Scholar] [CrossRef]
- Wong, O.Y.; Au, C.T.; Yuen, H.M.; Yu, K.N.; Lan, Q.Y.; Chan, N.Y.; Tsang, C.C.; Li, A.M.; Chan, K.C. Impact of COVID-19 on the sleep-wake patterns of preschool children. Sleep Med. 2023, 101, 50. [Google Scholar] [CrossRef]







| Definition of Long COVID or Post-COVID Syndrome | |
|---|---|
| National Institute for Clinical Excellence (NICE) [13] | Symptoms of COVID-19 experienced for 12 or more weeks after initial recovery.
|
| Centers for Disease Control and Prevention (CDC) [14] | The post-COVID condition indicates consequences that are present >4 weeks after SARS-CoV-2 infection. This includes both general complications of prolonged acute illness and new, returning, or ongoing health problems as post-acute sequelae of SARS-CoV-2 infection (PASC). |
| Robert Koch Institute (RKI) [15] | Long COVID is a longer-term health impairment following a SARS-CoV-2 infection that is present beyond the acute phase of the sickness of 4 weeks. The symptoms either begin during the acute phase of the disease and persist for a longer period of time, or appear or reoccur in the course of weeks and months after the infection.Post-COVID condition or post-COVID syndrome: symptoms are either still present at least 12 weeks and longer after the acute infection, or appear anew after this period and cannot be explained otherwise. |
| Total Score | Externalizing Scale | |||
|---|---|---|---|---|
| SDSC Scale | p | R | p | R |
| SWDT | <0.001 | 0.58 | <0.001 | 0.59 |
| DOES | <0.05 | 0.50 | <0.05 | 0.53 |
| Total Score | Externalizing | Internalizing | ||||
|---|---|---|---|---|---|---|
| SDSC | p | R | p | R | p | R |
| DIMS | <0.01 | 0.31 | <0.01 | 0.34 | <0.05 | 0.23 |
| SBD | <0.05 | 0.23 | <0.05 | 0.23 | / | / |
| DA | <0.05 | 0.24 | / | / | <0.05 | 0.23 |
| SWDT | <0.001 | 0.35 | =0.001 | 0.34 | <0.01 | 0.31 |
| DOES | <0.01 | 0.39 | <0.05 | 0.24 | <0.05 | 0.24 |
| TOTAL | <0.001 | 0.37 | <0.05 | 0.33 | <0.01 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miraglia del Giudice, M.; Klain, A.; Dinardo, G.; D’Addio, E.; Bencivenga, C.L.; Fontanella, C.; Decimo, F.; Umano, G.R.; Siciliano, M.; Carotenuto, M.; et al. Behavioral and Sleep Disorders in Children and Adolescents following COVID-19 Disease: A Case-Control Study. Children 2023, 10, 1189. https://doi.org/10.3390/children10071189
Miraglia del Giudice M, Klain A, Dinardo G, D’Addio E, Bencivenga CL, Fontanella C, Decimo F, Umano GR, Siciliano M, Carotenuto M, et al. Behavioral and Sleep Disorders in Children and Adolescents following COVID-19 Disease: A Case-Control Study. Children. 2023; 10(7):1189. https://doi.org/10.3390/children10071189
Chicago/Turabian StyleMiraglia del Giudice, Michele, Angela Klain, Giulio Dinardo, Elisabetta D’Addio, Chiara Lucia Bencivenga, Cristina Fontanella, Fabio Decimo, Giuseppina Rosaria Umano, Margherita Siciliano, Marco Carotenuto, and et al. 2023. "Behavioral and Sleep Disorders in Children and Adolescents following COVID-19 Disease: A Case-Control Study" Children 10, no. 7: 1189. https://doi.org/10.3390/children10071189
APA StyleMiraglia del Giudice, M., Klain, A., Dinardo, G., D’Addio, E., Bencivenga, C. L., Fontanella, C., Decimo, F., Umano, G. R., Siciliano, M., Carotenuto, M., & Indolfi, C. (2023). Behavioral and Sleep Disorders in Children and Adolescents following COVID-19 Disease: A Case-Control Study. Children, 10(7), 1189. https://doi.org/10.3390/children10071189







